Blood DNA Methylation in Nuclear and Mitochondrial Sequences Links to Malnutrition and Poor Prognosis in ALS: A Longitudinal Study
Abstract
:1. Introduction
2. Materials and Methods
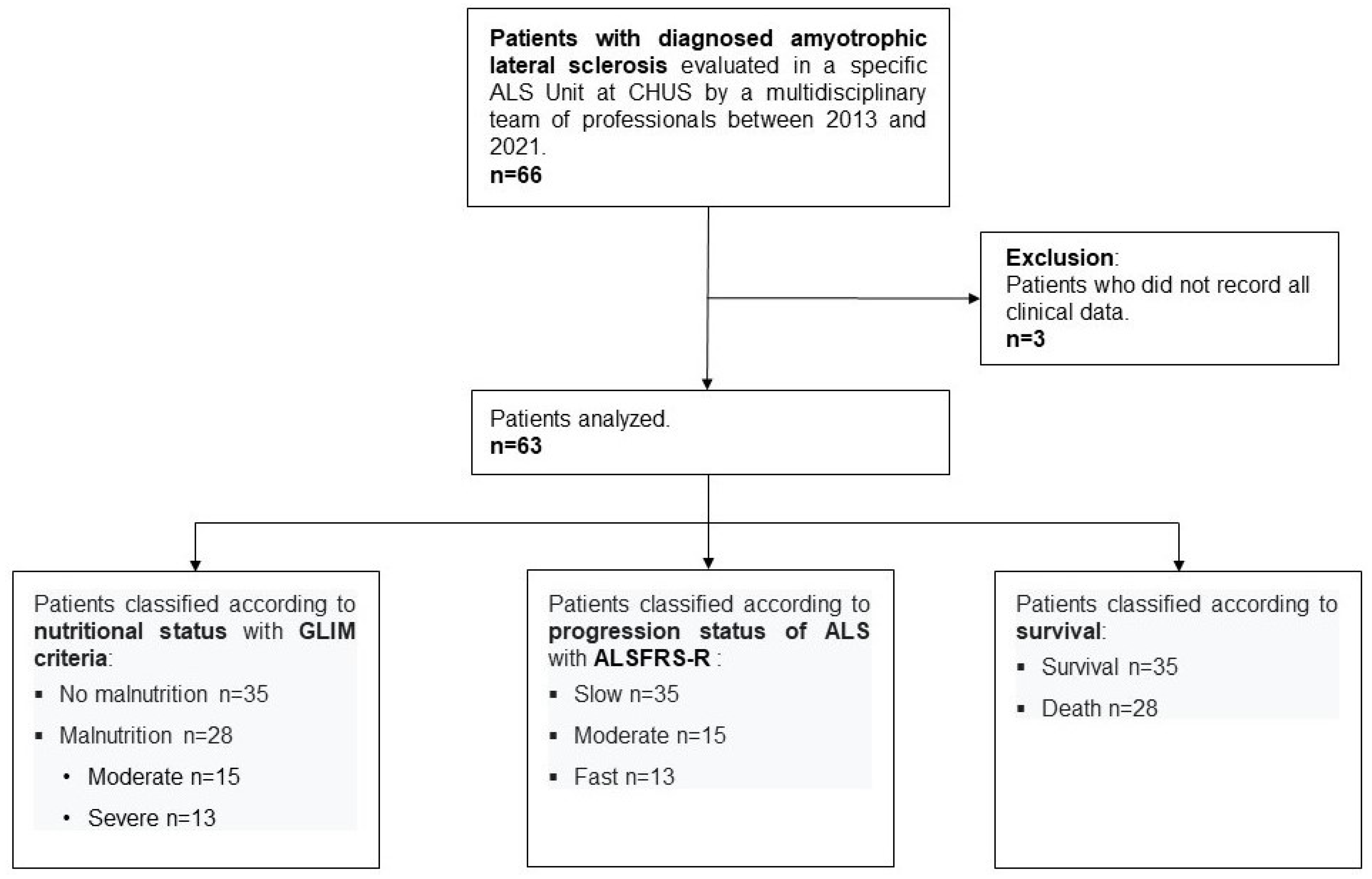
2.1. Study Design and Population
2.2. Nutritional Assessment and Support
2.3. DNA Methylation Analysis
2.3.1. DNA Preparation and Bisulfite Conversion
2.3.2. Pyrosequencing Analysis
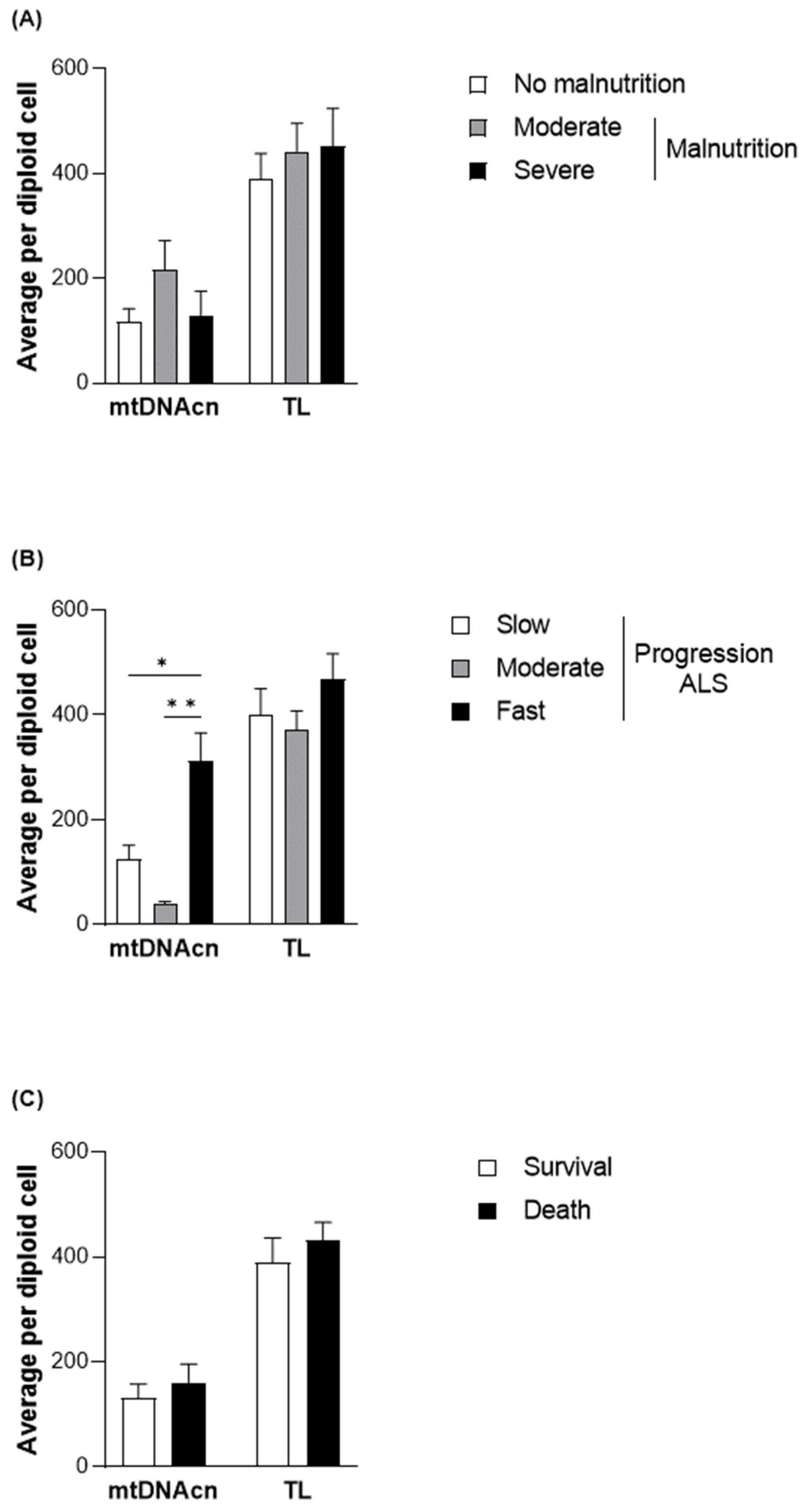
2.4. Assessment of the Mitochondrial DNA Copy Number and Telomere Length
2.5. Statistical Analysis
3. Results
3.1. Clinical and Nutritional Evaluation
3.1.1. Clinical and Nutritional Data at Diagnosis
3.1.2. Clinical and Nutritional Data During Follow-Up
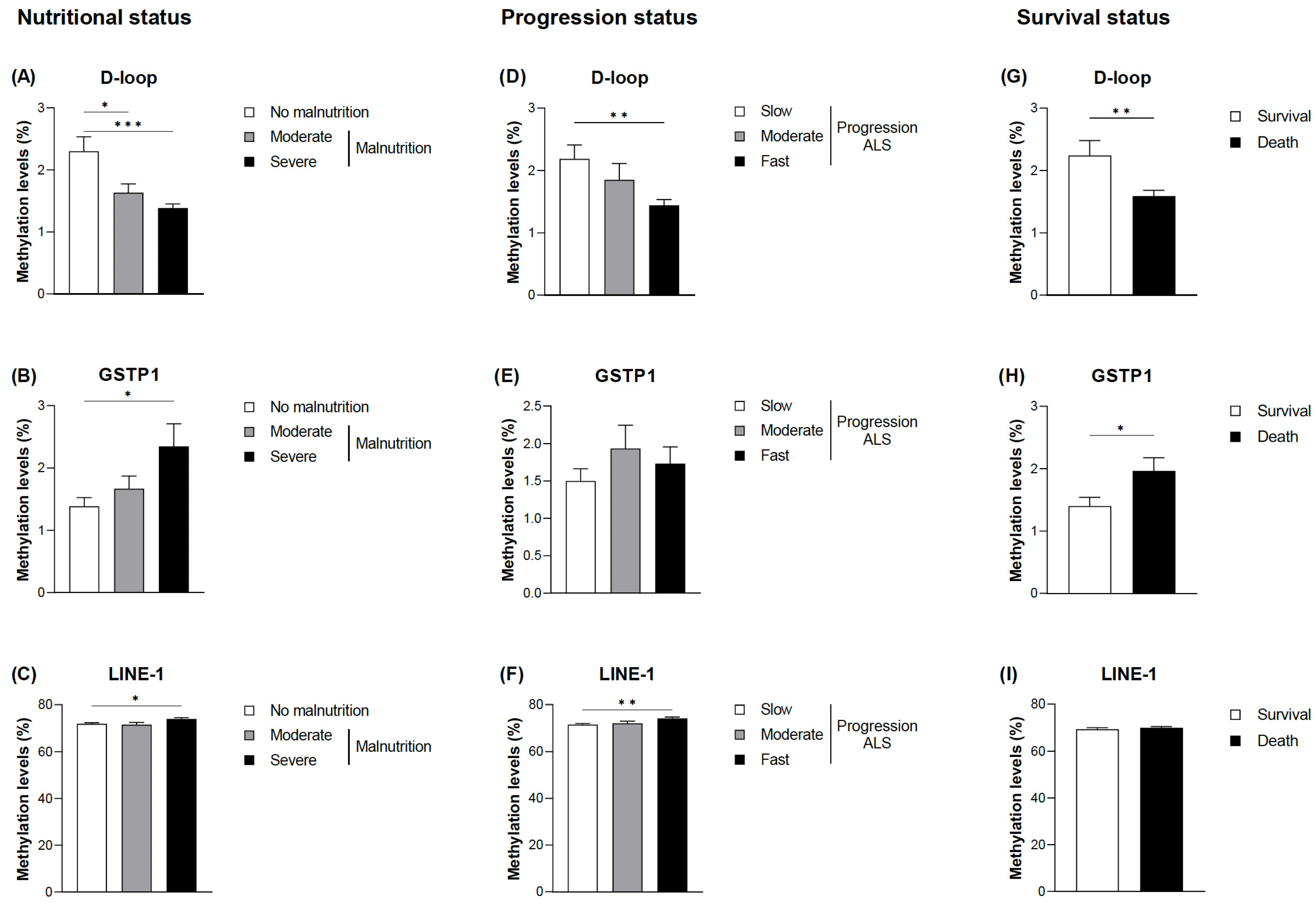
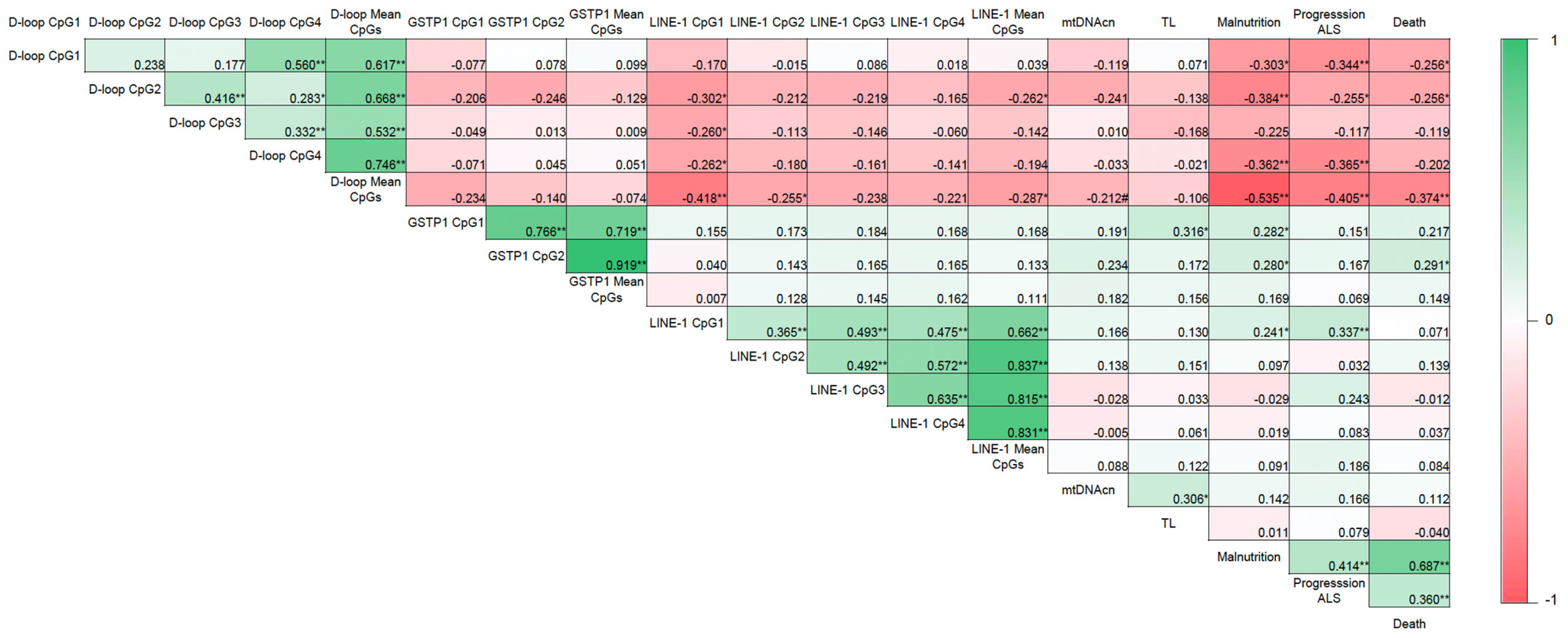
3.2. Evaluation of DNA Methylation Levels in Relation to the Nutritional and Clinical Outcomes
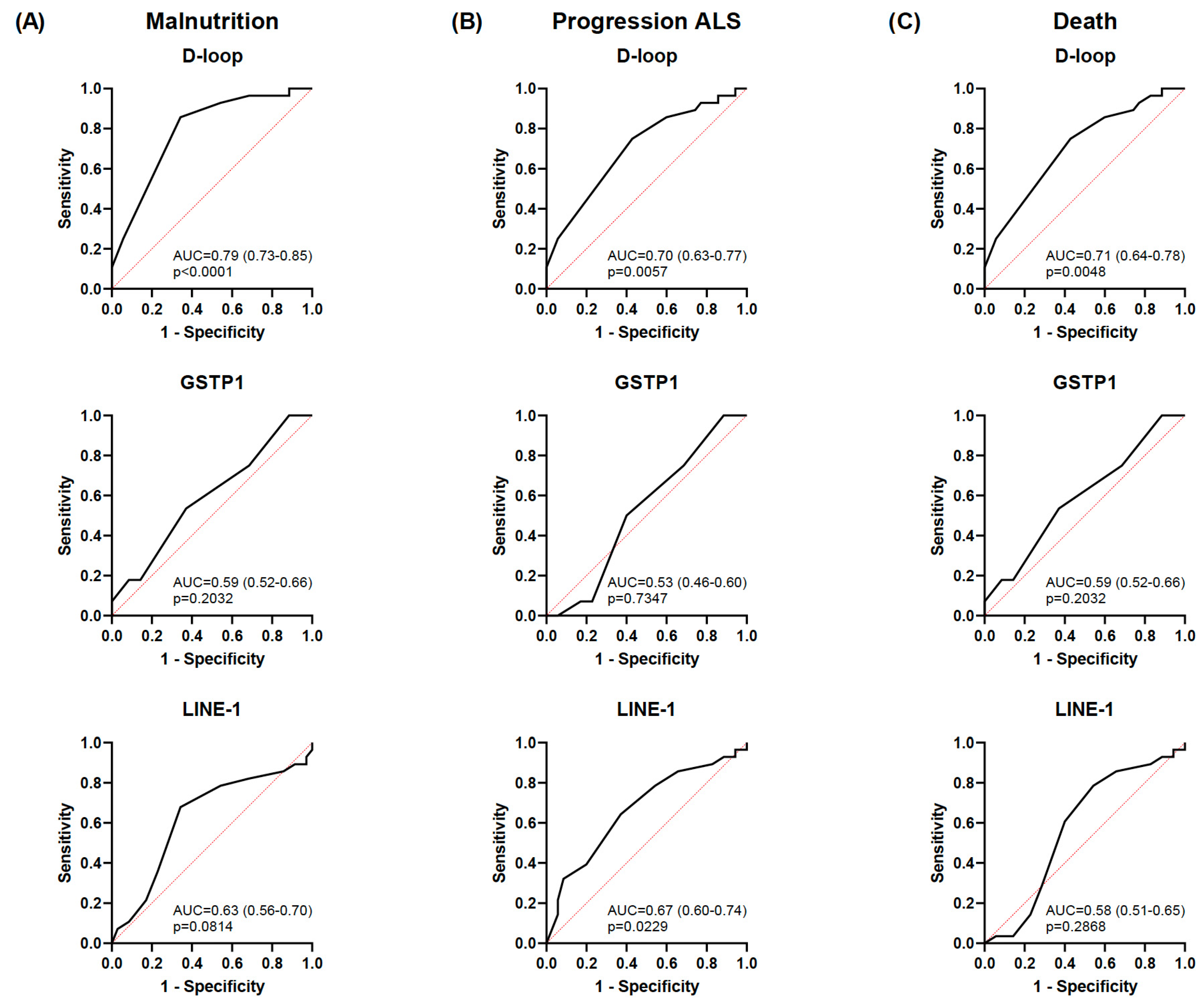
3.3. Diagnostic Accuracy of the Selected Epigenetic Biomarkers in Relation to Nutritional and Clinical Outcomes
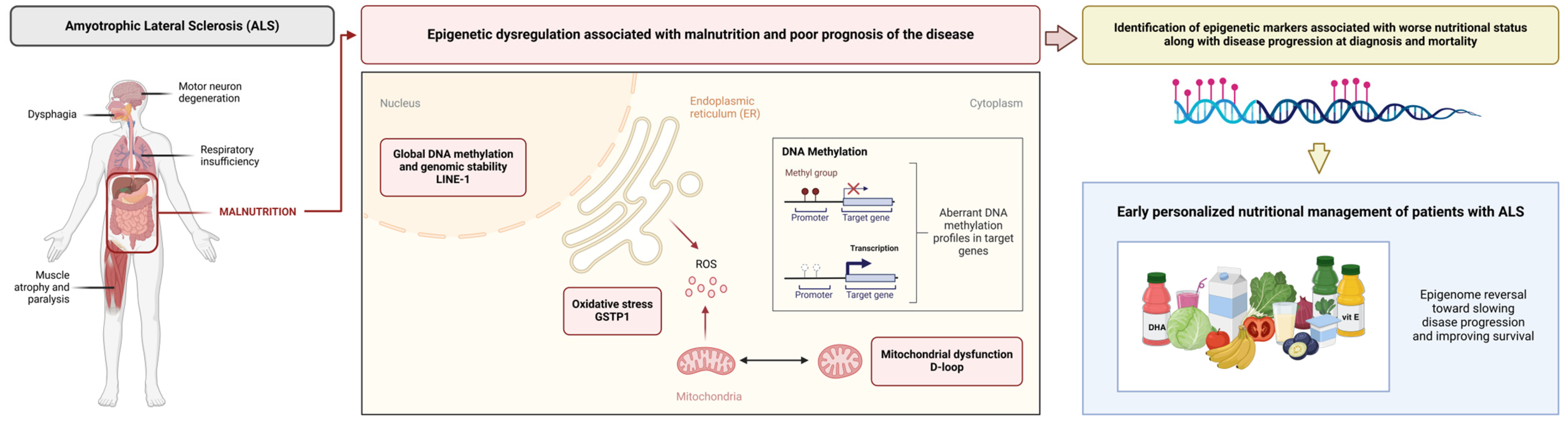
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, J.D.; Borasio, G.D. Amyotrophic lateral sclerosis. Lancet 2007, 369, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Kaye, W.; Raymond, J.; Punjani, R.; Larson, T.; Cohen, J.; Muravov, O.; Horton, K. Prevalence of Amyotrophic Lateral Sclerosis—United States, 2015. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1285–1289. [Google Scholar] [PubMed]
- Zou, Z.Y.; Zhou, Z.R.; Che, C.H.; Liu, C.Y.; He, R.L.; Huang, H.P. Genetic epidemiology of amyotrophic lateral sclerosis: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Ajroud-Driss, S.; Siddique, T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochim. Biophys. Acta 2015, 1852, 679–684. [Google Scholar]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2021, 24, 810–834. [Google Scholar] [CrossRef]
- Ferraiuolo, L.; Kirby, J.; Grierson, A.J.; Sendtner, M.; Shaw, P.J. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 616–630. [Google Scholar]
- Marin, B.; Arcuti, S.; Jesus, P.; Logroscino, G.; Copetti, M.; Fontana, A.; Nicol, M.; Raymondeau, M.; Desport, J.C.; Preux, P.M.; et al. Population-Based Evidence that Survival in Amyotrophic Lateral Sclerosis is Related to Weight Loss at Diagnosis. Neurodegener. Dis. 2016, 16, 225–234. [Google Scholar] [CrossRef]
- López-Gómez, J.J.; Ballesteros-Pomar, M.D.; Torres-Torres, B.; De la Maza, B.P.; Penacho-Lázaro, M.Á.; Palacio-Mures, J.M.; Abreu-Padín, C.; López-Guzmán, A.; De Luis-Román, D.A. Malnutrition at diagnosis in amyotrophic lateral sclerosis (als) and its influence on survival: Using glim criteria. Clin. Nutr. 2021, 40, 237–244. [Google Scholar]
- FOOD Trial Collaboration. Poor nutritional status on admission predicts poor outcomes after stroke: Observational data from the FOOD trial. Stroke 2003, 34, 1450–1456. [Google Scholar]
- Ticinesi, A.; Nouvenne, A.; Lauretani, F.; Prati, B.; Cerundolo, N.; Maggio, M.; Meschi, T. Survival in older adults with dementia and eating problems: To PEG or not to PEG? Clin. Nutr. 2016, 35, 1512–1516. [Google Scholar]
- Nieves, J.W.; Gennings, C.; Factor-Litvak, P.; Hupf, J.; Singleton, J.; Sharf, V.; Oskarsson, B.; Fernandes Filho, J.A.; Sorenson, E.J.; D’Amico, E.; et al. Association Between Dietary Intake and Function in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2016, 73, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.J.; Ravasco, P.; Machado, M.; Pinto, A.; Pinto, S.; Rocha, L.; de Carvalho, M.; Pinto, H.C. What is the relevance of percutaneous endoscopic gastrostomy on the survival of patients with amyotrophic lateral sclerosis? Amyotroph. Lateral Scler. 2012, 13, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.K. Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs. Med. Res. Rev. 2019, 39, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Calió, M.L.; Henriques, E.; Siena, A.; Bertoncini, C.R.A.; Gil-Mohapel, J.; Rosenstock, T.R. Mitochondrial Dysfunction, Neurogenesis, and Epigenetics: Putative Implications for Amyotrophic Lateral Sclerosis Neurodegeneration and Treatment. Front. Neurosci. 2020, 14, 679. [Google Scholar] [CrossRef]
- Swinnen, B.; Robberecht, W. The phenotypic variability of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2014, 10, 661–670. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Lorenzo, P.M.; Crujeiras, A.B. Chapter 11—Epigenetics and precision medicine in diabetes and obesity prevention and management. In Translational Epigenetics. Epigenetics in Precision Medicine; García-Giménez, J.L., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 30, pp. 327–346. [Google Scholar]
- Bennett, D.A.; Yu, L.; Yang, J.; Srivastava, G.P.; Aubin, C.; De Jager, P.L. Epigenomics of Alzheimer’s disease. Transl. Res. 2015, 165, 200–220. [Google Scholar] [CrossRef]
- Stoccoro, A.; Smith, A.R.; Mosca, L.; Marocchi, A.; Gerardi, F.; Lunetta, C.; Lunnon, K.; Migliore, L.; Coppedè, F. Mitochondrial D-loop methylation levels inversely correlate with disease duration in amyotrophic lateral sclerosis. Epigenomics 2024, 16, 203–214. [Google Scholar] [CrossRef]
- Bennett, S.A.; Tanaz, R.; Cobos, S.N.; Torrente, M.P. Epigenetics in amyotrophic lateral sclerosis: A role for histone post-translational modifications in neurodegenerative disease. Transl. Res. 2019, 204, 19–30. [Google Scholar] [CrossRef]
- Abdelhamid, R.F.; Nagano, S. Crosstalk between Oxidative Stress and Aging in Neurodegeneration Disorders. Cells 2023, 12, 753. [Google Scholar] [CrossRef]
- Coppedè, F.; Stoccoro, A. Mitoepigenetics and Neurodegenerative Diseases. Front. Endocrinol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Fallico, M.; Castellino, N.; Reibaldi, M.; Agodi, A. Characterization of SIRT1/DNMTs Functions and LINE-1 Methylation in Patients with Age-Related Macular Degeneration. J. Clin. Med. 2019, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M.; Mazzone, M.G.; Giuliano, F.; Basile, G.; Agodi, A. Resveratrol Modulates SIRT1 and DNMT Functions and Restores LINE-1 Methylation Levels in ARPE-19 Cells under Oxidative Stress and Inflammation. Int. J. Mol. Sci. 2018, 19, 2118. [Google Scholar] [CrossRef] [PubMed]
- Crujeiras, A.B.; Izquierdo, A.G.; Primo, D.; Milagro, F.I.; Sajoux, I.; Jácome, A.; Fernandez-Quintela, A.; Portillo, M.P.; Martínez, J.A.; Martinez-Olmos, M.A.; et al. Epigenetic landscape in blood leukocytes following ketosis and weight loss induced by a very low calorie ketogenic diet (VLCKD) in patients with obesity. Clin. Nutr. 2021, 40, 3959–3972. [Google Scholar] [PubMed]
- Sistema Público de Saúde de Galicia (Electronic Publication). Memoria. 2018. Available online: https://www.sergas.es/A-nosa-organizacion/Documents/832/MemoriaActividade2018.pdf (accessed on 12 November 2024).
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000, 1, 293–299. [Google Scholar]
- Labra, J.; Menon, P.; Byth, K.; Morrison, S.; Vucic, S. Rate of disease progression: A prognostic biomarker in ALS. J. Neurol. Neurosurg. Psychiatry 2016, 87, 628–632. [Google Scholar]
- Stoccoro, A.; Smith, A.R.; Mosca, L.; Marocchi, A.; Gerardi, F.; Lunetta, C.; Cereda, C.; Gagliardi, S.; Lunnon, K.; Migliore, L.; et al. Reduced mitochondrial D-loop methylation levels in sporadic amyotrophic lateral sclerosis. Clin. Epigenetics 2020, 12, 137. [Google Scholar]
- Yoon, H.Y.; Kim, Y.W.; Kang, H.W.; Kim, W.T.; Yun, S.J.; Lee, S.C.; Kim, W.J.; Kim, Y.J. DNA methylation of GSTP1 in human prostate tissues: Pyrosequencing analysis. Korean J. Urol. 2012, 53, 200–205. [Google Scholar]
- Maugeri, A.; Barchitta, M.; Magnano San Lio, R.; Favara, G.; La Rosa, M.C.; La Mastra, C.; Basile, G.; Agodi, A. Adherence to the Mediterranean diet partially mediates socioeconomic differences in leukocyte LINE-1 methylation: Evidence from a cross-sectional study in Italian women. Sci. Rep. 2020, 10, 14360. [Google Scholar]
- de van der Schueren, M.A.E.; Jager-Wittenaar, H. Malnutrition risk screening: New insights in a new era. Clin. Nutr. 2022, 41, 2163–2168. [Google Scholar]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 207–217. [Google Scholar]
- Iannarelli, N.J.; Wade, T.J.; Dempster, K.S.; Moore, J.; Macneil, A.J.; O’leary, D.D. No Mediation Effect of Telomere Length or Mitochondrial DNA Copy Number on the Association Between Adverse Childhood Experiences (ACEs) and Central Arterial Stiffness. J. Am. Heart Assoc. 2022, 11, e026619. [Google Scholar] [PubMed]
- Park, Y.; Park, J.; Kim, Y.; Baek, H.; Kim, S.H. Association between nutritional status and disease severity using the amyotrophic lateral sclerosis (ALS) functional rating scale in ALS patients. Nutrition 2015, 31, 1362–1367. [Google Scholar] [PubMed]
- Marin, B.; Desport, J.C.; Kajeu, P.; Jesus, P.; Nicolaud, B.; Nicol, M.; Preux, P.M.; Couratier, P. Alteration of nutritional status at diagnosis is a prognostic factor for survival of amyotrophic lateral sclerosis patients. J. Neurol. Neurosurg. Psychiatry 2011, 82, 628–634. [Google Scholar] [PubMed]
- Burgos, R.; Bretón, I.; Cereda, E.; Desport, J.C.; Dziewas, R.; Genton, L.; Gomes, F.; Jésus, P.; Leischker, A.; Muscaritoli, M.; et al. ESPEN guideline clinical nutrition in neurology. Clin. Nutr. 2018, 37, 354–396. [Google Scholar]
- López-Gómez, J.J.; Ballesteros-Pomar, M.D.; Gómez-Hoyos, E.; Pintor de la Maza, B.; Penacho-Lázaro, M.Á.; Palacio-Mures, J.M.; Abreu-Padín, C.; Sanz Gallego, I.; de Luis-Román, D.A. Effect of the type of specialized nutrition support on the course of the patient with amyotrophic lateral sclerosis (ALS). Interhospital registry SCLEDyN. Endocrinol. Diabetes Nutr. 2021, 68, 699–707. [Google Scholar]
- Gregory, J.M.; Fagegaltier, D.; Phatnani, H.; Harms, M.B. Genetics of Amyotrophic Lateral Sclerosis. Curr. Genet. Med. Rep. 2020, 8, 121–131. [Google Scholar]
- Crujeiras, A.B.; Diaz-Lagares, A.; Sandoval, J.; Milagro, F.I.; Navas-Carretero, S.; Carreira, M.C.; Gomez, A.; Hervas, D.; Monteiro, M.P.; Casanueva, F.F.; et al. DNA methylation map in circulating leukocytes mirrors subcutaneous adipose tissue methylation pattern: A genome-wide analysis from non-obese and obese patients. Sci. Rep. 2017, 7, 41903. [Google Scholar]
- Rönn, T.; Volkov, P.; Gillberg, L.; Kokosar, M.; Perfilyev, A.; Jacobsen, A.L.; Jørgensen, S.W.; Brøns, C.; Jansson, P.A.; Eriksson, K.F.; et al. Impact of age, BMI and HbA1c levels on the genome-wide DNA methylation and mRNA expression patterns in human adipose tissue and identification of epigenetic biomarkers in blood. Hum. Mol. Genet. 2015, 24, 3792–3813. [Google Scholar]
- Zhang, F.F.; Santella, R.M.; Wolff, M.; Kappil, M.A.; Markowitz, S.B.; Morabia, A. White blood cell global methylation and IL-6 promoter methylation in association with diet and lifestyle risk factors in a cancer-free population. Epigenetics 2012, 7, 606–614. [Google Scholar]
- Zhang, F.F.; Cardarelli, R.; Carroll, J.; Fulda, K.G.; Kaur, M.; Gonzalez, K.; Vishwanatha, J.K.; Santella, R.M.; Morabia, A. Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. Epigenetics 2011, 6, 623–629. [Google Scholar]
- Boughanem, H.; Martin-Nuñez, G.M.; Torres, E.; Arranz-Salas, I.; Alcaide, J.; Morcillo, S.; Tinahones, F.J.; Crujeiras, A.B.; Macias-Gonzalez, M. Impact of Tumor LINE-1 Methylation Level and Neoadjuvant Treatment and Its Association with Colorectal Cancer Survival. J. Pers. Med. 2020, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Vedin, I.; Levi, Y.F.; Basun, H.; Faxén Irving, G.; Eriksdotter, M.; Wahlund, L.O.; Schultzberg, M.; Hjorth, E.; Cederholm, T.; et al. DHA-rich n-3 fatty acid supplementation decreases DNA methylation in blood leukocytes: The OmegAD study. Am. J. Clin. Nutr. 2017, 106, 1157–1165. [Google Scholar] [PubMed]
- Onyango, I.; Khan, S.; Miller, B.; Swerdlow, R.; Trimmer, P.; Bennett, P., Jr. Mitochondrial genomic contribution to mitochondrial dysfunction in Alzheimer’s disease. J. Alzheimers Dis. 2006, 9, 183–193. [Google Scholar] [PubMed]
- Carrera-Juliá, S.; Estrela, J.M.; Zacarés, M.; Navarro, M.Á.; Vega-Bello, M.; de la Rubia Ortí, J.E.; Moreno, M.L.; Drehmer, E. Effect of the Mediterranean diet supplemented with nicotinamide riboside and pterostilbene and/or coconut oil on anthropometric variables in amyotrophic lateral sclerosis. A pilot study. Front. Nutr. 2023, 10, 1232184. [Google Scholar]
- Caplliure-Llopis, J.; Peralta-Chamba, T.; Carrera-Juliá, S.; Cuerda-Ballester, M.; Drehmer-Rieger, E.; López-Rodriguez, M.M.; de la Rubia Ortí, J.E. Therapeutic alternative of the ketogenic Mediterranean diet to improve mitochondrial activity in Amyotrophic Lateral Sclerosis (ALS): A Comprehensive Review. Food Sci. Nutr. 2019, 8, 23–35. [Google Scholar]
- Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Matsuda, S. Potential Diets to Improve Mitochondrial Activity in Amyotrophic Lateral Sclerosis. Diseases 2022, 10, 117. [Google Scholar] [CrossRef]
- Cuffaro, F.; Lamminpää, I.; Niccolai, E.; Amedei, A. Nutritional and Microbiota-Based Approaches in Amyotrophic Lateral Sclerosis: From Prevention to Treatment. Nutrients 2024, 17, 102. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar]
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020, 21, 9299. [Google Scholar] [CrossRef]
- Villar-Taibo, R.; Vidal-Casariego, A.; Santamaría-Nieto, A.; Cantón-Blanco, A.; Crujeiras, A.B.; Lugo Rodríguez, G.; Rodríguez-Carnero, G.; Pita Gutiérrez, F.; Fernández Pombo, A.; Díaz-López, E.; et al. Efficacy of a new immunonutrition formula with extra virgin olive oil in the reduction of complications in surgeries of upper digestive tract tumors. Front. Nutr. 2024, 11, 1384145. [Google Scholar]
- Longo, G.S.; Pinhel, M.S.; Sado, C.L.; Gregório, M.L.; Amorim, G.S.; Florim, G.S.; Mazeti, C.M.; Martins, D.P.; Oliveira, F.N.; Tognola, W.A.; et al. Exposure to pesticides and heterozygote genotype of GSTP1-Alw26I are associated to Parkinson’s disease. Arq. Neuropsiquiatr. 2013, 71, 446–452. [Google Scholar] [PubMed]
- Eum, K.D.; Seals, R.M.; Taylor, K.M.; Grespin, M.; Umbach, D.M.; Hu, H.; Sandler, D.P.; Kamel, F.; Weisskopf, M.G. Modification of the association between lead exposure and amyotrophic lateral sclerosis by iron and oxidative stress related gene polymorphisms. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 72–79. [Google Scholar]
- Jafarian, Z.; Saliminejad, K.; Kamali, K.; Ohadi, M.; Kowsari, A.; Nasehi, L.; Khorram Khorshid, H.R. Association of glutathione S-transferases M1, P1 and T1 variations and risk of late-onset Alzheimer’s disease. Neurol. Res. 2018, 40, 41–44. [Google Scholar]
- Barros, J.B.d.S.; Santos, K.d.F.; Azevedo, R.M.; de Oliveira, R.P.D.; Leobas, A.C.D.; Bento, D.D.C.P.; Santos, R.D.S.; Reis, A.A.D.S. No association of GSTP1 rs1695 polymorphism with amyotrophic lateral sclerosis: A case-control study in the Brazilian population. PLoS ONE 2021, 16, e0247024. [Google Scholar]
- de Sousa Barros, J.B.; de Faria Santos, K.; da Cruz Pereira Bento, D.; do Prado Assunção, L.; da Silva Santos, R.; da Silva Reis, A.A. Influence of GSTP1 rs1695 polymorphism on survival in male patients’ amyotrophic lateral sclerosis: A genetic association study in Brazilian population. Mol. Biol. Rep. 2022, 49, 1655–1659. [Google Scholar]
- Liu, A.; Li, X.; Hao, Z.; Cao, J.; Li, H.; Sun, M.; Zhang, Z.; Liang, R.; Zhang, H. Alterations of DNA methylation and mRNA levels of CYP1A1, GSTP1, and GSTM1 in human bronchial epithelial cells induced by benzo[a]pyrene. Toxicol. Ind. Health 2022, 38, 127–138. [Google Scholar]
- Wang, Q.; Wang, W.; Zhang, A. TET-mediated DNA demethylation plays an important role in arsenic-induced HBE cells oxidative stress via regulating promoter methylation of OGG1 and GSTP1. Toxicol. In Vitro 2021, 72, 105075. [Google Scholar]
- Liu, E.Y.; Russ, J.; Cali, C.P.; Phan, J.M.; Amlie-Wolf, A.; Lee, E.B. Loss of Nuclear TDP-43 Is Associated with Decondensation of LINE Retrotransposons. Cell Rep. 2019, 27, 1409–1421.e6. [Google Scholar]
- Tam, O.H.; Rozhkov, N.V.; Shaw, R.; Kim, D.; Hubbard, I.; Fennessey, S.; Propp, N.; NYGC ALS Consortium; Fagegaltier, D.; Harris, B.T.; et al. Postmortem Cortex Samples Identify Distinct Molecular Subtypes of ALS: Retrotransposon Activation, Oxidative Stress, and Activated Glia. Cell Rep. 2019, 29, 1164–1177.e5. [Google Scholar]
- Savage, A.L.; Lopez, A.I.; Iacoangeli, A.; Bubb, V.J.; Smith, B.; Troakes, C.; Alahmady, N.; Koks, S.; Schumann, G.G.; Al-Chalabi, A.; et al. Frequency and methylation status of selected retrotransposition competent L1 loci in amyotrophic lateral sclerosis. Mol. Brain 2020, 13, 154. [Google Scholar]
- Maugeri, A.; Barchitta, M.; Magnano San Lio, R.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Agodi, A. The Relationship between Body Mass Index, Obesity, and LINE-1 Methylation: A Cross-Sectional Study on Women from Southern Italy. Dis. Markers 2021, 2021, 9910878. [Google Scholar] [PubMed]
- Requardt, M.V.; Görlich, D.; Grehl, T.; Boentert, M. Clinical Determinants of Disease Progression in Amyotrophic Lateral Sclerosis-A Retrospective Cohort Study. J. Clin. Med. 2021, 10, 1623. [Google Scholar] [CrossRef] [PubMed]
- Sguanci, M.; Mancin, S.; Gazzelloni, A.; Diamanti, O.; Ferrara, G.; Morales Palomares, S.; Parozzi, M.; Petrelli, F.; Cangelosi, G. The Internet of Things in the Nutritional Management of Patients with Chronic Neurological Cognitive Impairment: A Scoping Review. Healthcare 2024, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Oniani, D.; Shao, Z.; Arciero, P.; Sivarajkumar, S.; Hilsman, J.; Mohr, A.E.; Ibe, S.; Moharir, M.; Li, L.J.; et al. A Scoping Review of Artificial Intelligence for Precision Nutrition. Adv. Nutr. 2025, 28, 100398. [Google Scholar]
- García-Herreros, S.; López Gómez, J.J.; Cebria, A.; Izaola, O.; Salvador Coloma, P.; Nozal, S.; Cano, J.; Primo, D.; Godoy, E.J.; de Luis, D. Validation of an Artificial Intelligence-Based Ultrasound Imaging System for Quantifying Muscle Architecture Parameters of the Rectus Femoris in Disease-Related Malnutrition (DRM). Nutrients 2024, 16, 1806. [Google Scholar] [CrossRef]
| Data at diagnosis of ALS | |
| Age, years | 66.5 ± 10.1 |
| Gender, n (%) (men/women) | 35 (53.0)/31 (47.0) |
| ALS origin | |
| Sporadic, n (%) | 59 (89.4) |
| Familial, n (%) | 7 (10.6) |
| ALS onset | |
| Spinal, n (%) | 53 (80.3) |
| Bulbar, n (%) | 13 (19.7) |
| Weight, kg | 70.0 ± 12.5 |
| BMI, kg/m2 | 26.9 ± 4.1 |
| Change of BW, % | −4.77 ± 9.3 |
| Weight loss, % | 9.82 ± 8.6 |
| Nutritional assessment (GLIM) | |
| No malnutrition, n (%) | 37 (56.1) |
| Moderate malnutrition, n (%) | 15 (22.7) |
| Severe malnutrition, n (%) | 14 (21.2) |
| ALSFRS-R | |
| Slow progression, n (%) | 35 (53.0) |
| Moderate progression, n (%) | 15 (22.7) |
| Fast progression, n (%) | 13 (19.7) |
| Data during follow-up | |
| Nutritional support | |
| ONS, n (%) | 31 (46.9) |
| Gastrostomy, n (%) | 14 (21.2) |
| Dysphagia, n (%) | 36 (54.4) |
| Hospitalization, n (%) | 14 (21.2) |
| Death, n (%) | 29 (43.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Pombo, A.; Izquierdo, A.G.; Canton-Blanco, A.; Garcia-Sobrino, T.; Hervás, D.; Martínez-Olmos, M.A.; Pardo, J.; Crujeiras, A.B. Blood DNA Methylation in Nuclear and Mitochondrial Sequences Links to Malnutrition and Poor Prognosis in ALS: A Longitudinal Study. Nutrients 2025, 17, 1295. https://doi.org/10.3390/nu17081295
Fernandez-Pombo A, Izquierdo AG, Canton-Blanco A, Garcia-Sobrino T, Hervás D, Martínez-Olmos MA, Pardo J, Crujeiras AB. Blood DNA Methylation in Nuclear and Mitochondrial Sequences Links to Malnutrition and Poor Prognosis in ALS: A Longitudinal Study. Nutrients. 2025; 17(8):1295. https://doi.org/10.3390/nu17081295
Chicago/Turabian StyleFernandez-Pombo, Antia, Andrea G. Izquierdo, Ana Canton-Blanco, Tania Garcia-Sobrino, David Hervás, Miguel A. Martínez-Olmos, Julio Pardo, and Ana B. Crujeiras. 2025. "Blood DNA Methylation in Nuclear and Mitochondrial Sequences Links to Malnutrition and Poor Prognosis in ALS: A Longitudinal Study" Nutrients 17, no. 8: 1295. https://doi.org/10.3390/nu17081295
APA StyleFernandez-Pombo, A., Izquierdo, A. G., Canton-Blanco, A., Garcia-Sobrino, T., Hervás, D., Martínez-Olmos, M. A., Pardo, J., & Crujeiras, A. B. (2025). Blood DNA Methylation in Nuclear and Mitochondrial Sequences Links to Malnutrition and Poor Prognosis in ALS: A Longitudinal Study. Nutrients, 17(8), 1295. https://doi.org/10.3390/nu17081295







