Lifestyle Modification in Prediabetes and Diabetes: A Large Population Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects and Study Design
2.2. Laboratory Measurements
2.3. Statistical Analysis
3. Results
3.1. Comparison of Healthy, Prediabetic, and Diabetic Subjects—Age and Weight
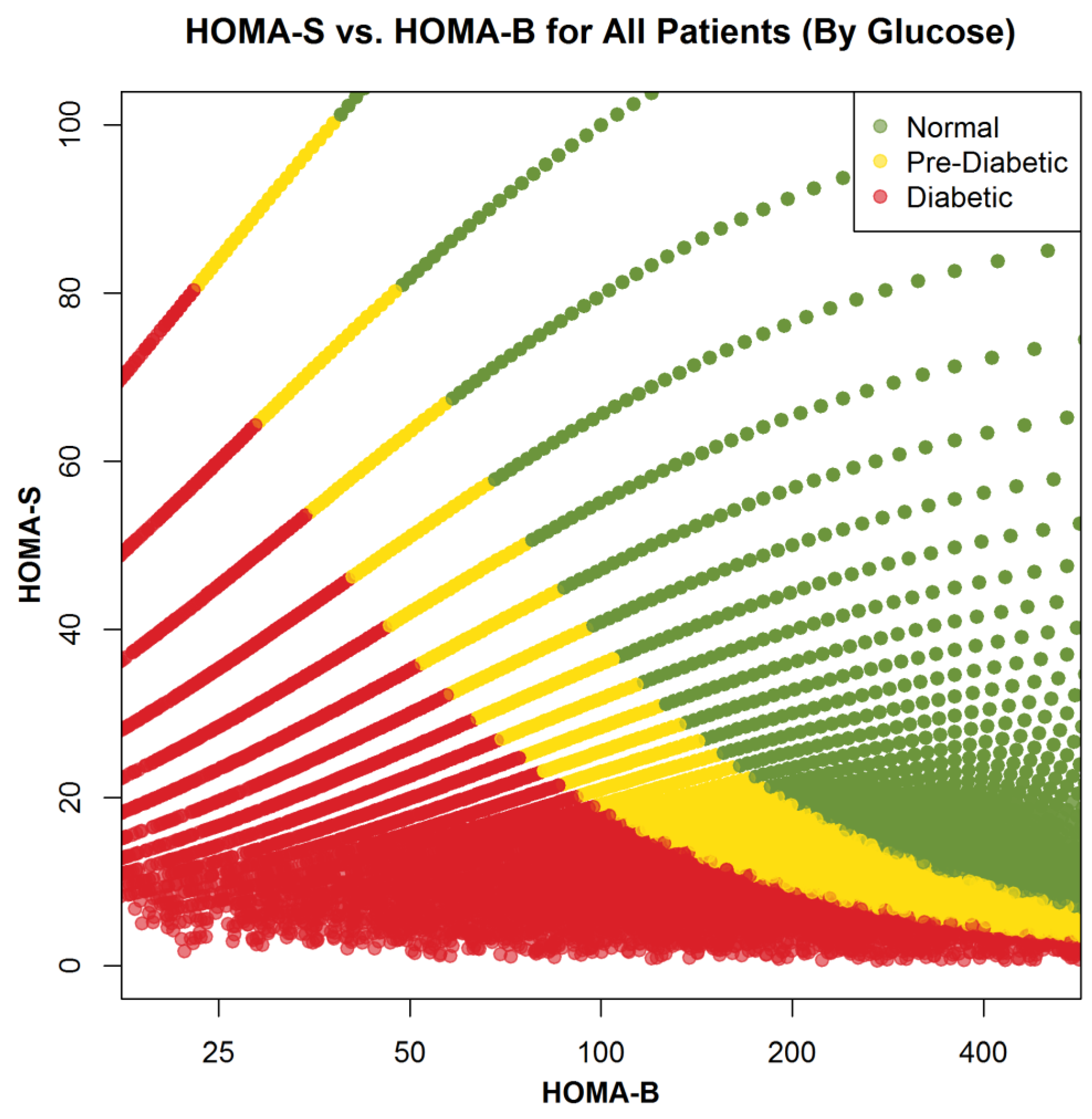
3.2. Comparison of Healthy, Prediabetic, and Diabetic Subjects—Glucose Homeostasis
3.3. Comparison of Healthy, Prediabetic, and Diabetic Subjects—Inflammation
3.4. Comparison of Healthy, Prediabetic, and Diabetic Subjects—Lipid Parameters
3.5. Effects of Lifestyle Modification in Prediabetes
3.6. Effects of Lifestyle Modification in Diabetes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASCVD | atherosclerotic cardiovascular disease |
| apo | apolipoprotein |
| BMI | body mass index |
| GLP-1RA | glucagon-like peptide 1 receptor agonist |
| GSP | glycated serum protein |
| HbA1c | glycosylated hemoglobin |
| HDL-C | high-density lipoprotein cholesterol |
| HOMAβ | homeostasis model of insulin production |
| HOMAIR | homeostasis model of insulin resistance |
| HOMAs | homeostasis model of insulin sensitivity |
| hs-CRP | high sensitivity C-reactive protein |
| LDL-C | low-density lipoprotein cholesterol |
| LpPLA2 | lipoprotein-associated phospholipase A2 |
| MPO | myeloperoxidase |
| sdLDL-C | small dense low-density lipoprotein cholesterol |
References
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. S1), S27–S49. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Fact Sheet on Diabetes and Prediabetes. Available online: https://www.cdc.gov/diabetes/index.html (accessed on 12 January 2025).
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. S1), S158–S178, Erratum in Diabetes Care 2024, 47, 1238. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [PubMed]
- Steven, S.; Hollingsworth, K.G.; Al-Mrabeh, A.; Avery, L.; Aribisala, B.; Caslake, M.; Taylor, R. Very Low-Calorie Diet and 6 Months of Weight Stability in Type 2 Diabetes: Pathophysiological Changes in Responders and Nonresponders. Diabetes Care 2016, 39, 808–815, Erratum in Diabetes Care 2018, 41, 1321. [Google Scholar] [CrossRef]
- Hemmingsen, B.; Gimenez-Perez, G.; Mauricio, D.; Roqué I Figuls, M.; Metzendorf, M.I.; Richter, B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2017, 12, CD003054. [Google Scholar] [CrossRef]
- Stern, M.P.; Williams, K.; Haffner, S.M. Identification of persons at high risk for type 2 diabetes mellitus: Do we need the oral glucose tolerance test? Ann. Intern. Med. 2002, 136, 575–581. [Google Scholar] [CrossRef]
- Wilson, P.W.; Meigs, J.B.; Sullivan, L.; Fox, C.S.; Nathan, D.M.; D’Agostino, R.B., Sr. Prediction of incident diabetes mellitus in middle-aged adults: The Framingham Offspring Study. Arch. Intern. Med. 2007, 167, 1068–1074. [Google Scholar] [CrossRef]
- Noble, D.; Mathur, R.; Dent, T.; Meads, C.; Greenhalgh, T. Risk models and scores for type 2 diabetes: Systematic review. BMJ 2011, 343, 1–31. [Google Scholar] [CrossRef]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS ONE 2018, 13, e0194127. [Google Scholar] [CrossRef]
- Richter, B.; Hemmingsen, B.; Metzendorf, M.I.; Takwoingi, Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst. Rev. 2018, 10, CD012661. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Feng, W.; Mo, H.; Hua, Y.; Zhang, M.; Zhu, Z.; Zhang, X.; Wu, Z.; Zheng, L.; et al. A meta-analysis of diabetes risk prediction models applied to prediabetes screening. Diabetes Obes. Metab. 2024, 26, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Ai, M.; Otokozawa, S.; Asztalos, B.F.; Maddalena, J.; Diffenderfer, M.R.; Russo, G.; Thongtang, N.; Liu, C.-T.; Dansinger, M.L. Diabetes mellitus risk prediction in the Framingham Offspring Study and large population analysis. Nutrients 2025, 17, 1117. [Google Scholar] [CrossRef]
- Gill, R.; Superko, H.R.; McCarthy, M.M.; Jack, K.; Jones, B.; Ghosh, D.; Richards, S.; Gleason, J.A.; Williams, P.T.; Dansinger, M. Cardiovascular risk factor reduction in first responders resulting from an individualized lifestyle and blood test program: A randomized controlled trial. J. Occup. Environ. Med. 2019, 61, 183–189. [Google Scholar] [CrossRef]
- Ikezaki, H.; Fisher, V.A.; Lim, E.; Ai, M.; Liu, C.-T.; Cupples, L.A.; Nakajima, K.; Asztalos, B.F.; Furusyo, N.; Schaefer, E.J. Direct versus calculated LDL cholesterol and C-reactive protein in cardiovascular disease risk assessment in the Framingham Offspring Study. Clin. Chem. 2019, 65, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, H.; Lim, E.; Cupples, L.A.; Liu, C.-T.; Asztalos, B.F.; Schaefer, E.J. Small dense low-density lipoprotein cholesterol is the most atherogenic lipoprotein parameter in the prospective Framingham Offspring Study. J. Am. Heart Assoc. 2021, 10, e019140. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Ikezaki, H.; Diffenderfer, M.R.; Lim, E.; Liu, C.-T.; Hoogeveen, R.C.; Guan, W.; Tsai, M.Y.; Ballantyne, C.M. Atherosclerotic cardiovascular disease risk and small dense low-density lipoprotein cholesterol in men, women, African Americans and non-African Americans: The pooling project. Atherosclerosis 2023, 367, 15–23. [Google Scholar] [CrossRef]
- Schaefer, E.J.; Tsunoda, F.; Diffenderfer, M.; Polisecki, E.; Thai, N.; Asztalos, B. The Measurement of lipids, lipoproteins, apolipoproteins, fatty acids, and sterols, and next generation sequencing for the diagnosis and treatment of lipid disorders. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ai, M.; Otokozawa, S.; Asztalos, B.F.; White, C.C.; Cupples, L.A.; Nakajima, K.; Lamon-Fava, S.; Wilson, P.W.; Matsuzawa, Y.; Schaefer, E.J. Adiponectin: An independent risk factor for coronary heart disease in men in the Framingham Offspring Study. Atherosclerosis 2011, 217, 543–548. [Google Scholar] [CrossRef]
- Ai, M.; Otokozawa, S.; Schaefer, E.J.; Asztalos, B.F.; Nakajima, K.; Shrader, P.; Kathiresan, S.; Meigs, J.B.; Williams, G.; Nathan, D.M. Glycated albumin and direct low density lipoprotein cholesterol levels in type 2 diabetes mellitus. Clin. Chim. Acta 2009, 406, 71–74. [Google Scholar] [CrossRef]
- Rutter, M.K.; Meigs, J.B.; Sullivan, L.M.; D’Agostino, R.B., Sr.; Wilson, P.W. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation 2004, 110, 380–385. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Buckley, D.I.; Fu, R.; Freeman, M.; Rogers, K.; Helfand, M. C-reactive protein as a risk factor for coronary heart disease: A systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2009, 151, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, R.C.; Gaubatz, J.W.; Sun, W.; Dodge, R.C.; Crosby, J.R.; Jiang, J.; Couper, D.; Virani, S.S.; Kathiresan, S.; Boerwinkle, E.; et al. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: The Atherosclerosis Risk In Communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Yalow, R.S.; Berson, S.A. Immunoassay of endogenous plasma insulin in man. J. Clin. Investig. 1960, 39, 1157–1175. [Google Scholar] [CrossRef]
- Turner, R.C.; Holman, R.R.; Matthews, D.; Hockaday, T.D.; Peto, J. Insulin deficiency and insulin resistance interaction in diabetes: Estimation of their relative contribution by feedback analysis from basal plasma insulin and glucose concentrations. Metabolism 1979, 28, 1086–1096. [Google Scholar] [CrossRef]
- Rudenski, A.S.; Hadden, D.R.; Atkinson, A.B.; Kennedy, L.; Matthews, D.R.; Merrett, J.D.; Pockaj, B.; Turner, R.C. Natural history of pancreatic islet B-cell function in type 2 diabetes mellitus studied over six years by homeostasis model assessment. Diabet. Med. 1988, 5, 36–41. [Google Scholar] [CrossRef]
- Rudenski, A.S.; Matthews, D.R.; Levy, J.C.; Turner, R.C. Understanding “insulin resistance”: Both glucose resistance and insulin resistance are required to model human diabetes. Metabolism 1991, 40, 908–917. [Google Scholar] [CrossRef]
- Levy, J.C.; Matthews, D.R.; Hermans, M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998, 21, 2191–2192. [Google Scholar] [CrossRef]
- Sacks, D.B.; Arnold, M.; Bakris, G.L.; Bruns, D.E.; Horvath, A.R.; Lernmark, Å.; Metzger, B.E.; Nathan, D.M.; Kirkman, M.S. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care 2023, 46, e151–e199. [Google Scholar] [CrossRef]
- Taylor, R. Understanding the cause of type 2 diabetes. Lancet Diabetes Endocrinol. 2024, 12, 664–673, Erratum in Lancet Diabetes Endocrinol. 2024, 12, e18. [Google Scholar] [CrossRef]
- Horikawa, Y.; Hosomichi, K.; Yabe, D. Monogenic diabetes. Diabetol. Int. 2024, 15, 679–687. [Google Scholar] [CrossRef]
- Fredensborg Holm, T.; Udsen, F.W.; Giese, I.E.; Færch, K.; Jensen, M.H.; von Scholten, B.J.; Hangaard, S. The effectiveness of digital health lifestyle interventions on weight loss in people with prediabetes: A systematic review, meta-analysis, and meta-regression. J. Diabetes Sci. Technol. 2024, 19322968241292646. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chai, X.; Wang, Y.; Yin, X.; Huang, X.; Gong, Q.; Zhang, J.; Shao, R.; Li, G. Effectiveness of different intervention modes in lifestyle intervention for the prevention of type 2 diabetes and the reversion to normoglycemia in adults with prediabetes: Systematic review and meta-analysis of randomized controlled trials. J. Med. Internet. Res. 2025, 27, e63975. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, J.K.; Imes, C.C.; Engberg, S.J.; Scott, P.W.; Klem, M.L.; Cortes, Y.I. Impact of lifestyle-based interventions on absolute cardiovascular disease risk: A systematic review and meta-analysis. JBI Evid. Synth. 2024, 22, 4–65. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, H.; Ai, M.; Schaefer, E.J.; Otokozawa, S.; Asztalos, B.F.; Nakajima, K.; Zhou, Y.; Liu, C.-T.; Jacques, P.F.; Cupples, L.A.; et al. Ethnic differences in glucose homeostasis markers between the Kyushu-Okinawa Population Study and the Framingham Offspring Study. Sci. Rep. 2016, 6, 36725. [Google Scholar] [CrossRef][Green Version]
- Yoshinari, M.; Hirakawa, Y.; Hata, J.; Higashioka, M.; Honda, T.; Yoshida, D.; Mukai, N.; Nakamura, U.; Kitazono, T.; Ninomiya, T. Comparison of the contributions of impaired beta cell function and insulin resistance to the development of type 2 diabetes in a Japanese community: The Hisayama Study. Diabetologia 2021, 64, 1775–1784. [Google Scholar] [CrossRef]
- Wing, R.R.; Phelan, S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 2005, 82 (Suppl. S1), 222S–225S. [Google Scholar] [CrossRef]
- Schaefer, E.J. Nutrition in the prevention of coronary heart disease and the management of lipoprotein disorders. In Modern Nutrition in Health and Disease, 12th ed.; Tucker, K.L., Duggan, C.P., Jensen, G.L., Peterson, K.E., Ross, C.A., Touger-Decker, R., Eds.; Jones & Bartlett Learning: Burlington, MA, USA, 2024; pp. 941–956. [Google Scholar]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef]
- Turner, R.C.; Cull, C.A.; Frighi, V.; Holman, R.R.; the UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: Progressive requirement for multiple therapies (UKPDS 49). JAMA 1999, 281, 2005–2012. [Google Scholar] [CrossRef]
- Echouffo-Tcheugul, J.B.; Perreault, L.; Dagogo-Jack, S. Diagnosis and management of prediabetes. A review. JAMA 2023, 329, 1206–1216. [Google Scholar] [CrossRef]
- Drucker, D.J. Efficacy and safety of GLP-1 medicines for type 2 diabetes and obesity. Diabetes Care 2024, 47, 1873–1888. [Google Scholar] [CrossRef]
- Kim, H.S. Blood Glucose Measurement: Is Serum Equal to Plasma? Diabetes Metab. J. 2016, 40, 365–366. [Google Scholar] [CrossRef]
| Parameter | Healthy n = 75,271 (56.3%) | Prediabetic n = 48,455 (36.2%) | Diabetic n = 10,038 (7.5%) | % Difference, vs. Healthy Subjects | |||
|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | ||
| Demographics | |||||||
| Age (years) | 75,271 | 52.0 (22.0) | 48,455 | 59.0 (17.0) | 10,038 | 60.0 (17.0) * | +15.4% |
| Females | 48,476 | 52.0 (22.0) | 22,977 | 59.0 (17.0) | 4175 | 60.0 (18.0) * | +15.4% |
| Males | 26,795 | 52.0 (23.0) | 25,478 | 58.0 (18.0) | 5863 | 60.0 (17.0) * | +15.4% |
| Sex | |||||||
| Females | 48,476 | 64.4% | 22,977 | 47.4% | 4175 | 41.6% * | −35.4% |
| Males | 26,795 | 35.6% | 25,478 | 52.6% | 5863 | 58.4% * | +64.0% |
| BMI (kg/m2) | 12,794 | 27.0 (7.0) | 11,758 | 30.0 (8.0) | 801 | 32.0 (9.0) * | +18.5% |
| Females | 8575 | 26.0 (8.0) | 5605 | 30.0 (10.0) | 331 | 34.0 (10.0) * | +30.8% |
| Males | 4219 | 28.0 (7.0) | 6153 | 30.0 (6.0) | 470 | 31.0 (7.0) * | +10.7% |
| Weight (pounds) | 34,298 | 169.0 (58.0) | 22,078 | 191.0 (59.0) | 4432 | 204.0 (65.0) * | +20.7% |
| Females | 22,311 | 153.0 (49.0) | 10,394 | 172.0 (57.0) | 1850 | 188.0 (63.0) * | +22.9% |
| Males | 11,987 | 195.0 (49.0) | 11,684 | 205.0 (51.0) | 2582 | 214.0 (60.0) * | +9.7% |
| Metabolism | |||||||
| Glucose (mg/dL) | 75,271 | 90.0 (10.0) | 48,455 | 106.0 (10.0) | 10,038 | 154.0 (57.0) * | +71.1% |
| Females | 48,476 | 90.0 (9.0) | 22,977 | 105.0 (9.0) | 4175 | 153.0 (56.0) * | +70.0% |
| Males | 26,795 | 92.0 (9.0) | 25,478 | 106.0 (10.0) | 5863 | 155.0 (57.0) * | +68.5% |
| Adiponectin (µg/mL) | 75,271 | 12.6 (9.3) | 48,455 | 10.1 (7.6) | 10,038 | 8.3 (6.3) * | −34.1% |
| Females | 48,476 | 14.6 (9.6) | 22,977 | 12.1 (8.6) | 4175 | 9.7 (7.4) * | −33.6% |
| Males | 26,795 | 9.4 (6.4) | 25,478 | 8.6 (5.9) | 5863 | 7.5 (5.4) * | −20.2% |
| GSP (µmol/L) | 75,269 | 199 (53) | 48,454 | 205 (59) | 10,038 | 299 (62) * | +50.3% |
| Females | 48,476 | 198 (55) | 22,976 | 200 (60) | 4175 | 285 (98) * | +43.9% |
| Males | 26,793 | 202 (52) | 25,478 | 210 (58) | 5863 | 308 (63) * | +52.5% |
| 10 yr Diabetes Risk (%) | 75,271 | 0.4 (0.6) | 48,455 | 5.5 (12.1) | 10,038 | 100.0 | +250% |
| Females | 48,476 | 0.3 (0.5) | 22,977 | 4.2 (8.9) | 4175 | 100.0 | +333% |
| Males | 26,795 | 0.6 (1.0) | 25,478 | 7.0 (14.7) | 5863 | 100.0 | +167% |
| Parameter | Healthy n = 75,271 (56.3%) | Prediabetic n = 48,455 (36.2%) | Diabetic n = 10,038 (7.5%) | % Difference, vs. Healthy Subjects | |||
|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | ||
| Metabolism | |||||||
| HbA1c (%) | 72,980 | 5.5 (0.5) | 45,176 | 5.7 (0.5) | 9599 | 7.2 (1.9) * | +30.9% |
| Females | 47,206 | 5.4 (0.5) | 21,540 | 5.7 (0.5) | 3989 | 7.2 (1.8) * | +33.3% |
| Males | 25,774 | 5.5 (0.4) | 23,636 | 5.7 (0.6) | 5610 | 7.2 (1.9) * | +30.9% |
| Insulin (µU/mL) | 73,624 | 8.0 (8.0) | 45,176 | 13.0 (12.0) | 9940 | 17.0 (18.0) * | +112.5% |
| Females | 47,420 | 8.0 (7.0) | 21,477 | 13.0 (12.0) | 4130 | 18.0 (18.0) * | +125.0% |
| Males | 26,204 | 9.0 (8.0) | 23,699 | 13.0 (12.0) | 5810 | 17.0 (19.0) * | +88.9% |
| HOMAIR | 73,460 | 1.8 (1.8) | 45,069 | 3.5 (3.3) | 9781 | 7.3 (8.1) * | +305.6% |
| Females | 47,331 | 1.7 (1.6) | 21,421 | 3.6 (3.3) | 4042 | 7.6 (8.0) * | +347.1% |
| Males | 26,129 | 2.0 (2.0) | 23,648 | 3.5 (3.3) | 5739 | 7.2 (8.2) * | +260.0% |
| HOMAβ | 73,572 | 111 (102) | 45,176 | 108 (95) | 9940 | 64 (81) * | −42.1% |
| Females | 47,390 | 109 (98) | 21,477 | 110 (96) | 4130 | 68 (83) * | −37.9% |
| Males | 26,182 | 115 (111) | 23,699 | 107 (96) | 5810 | 62 (80) * | −46.2% |
| C-Peptide (ng/mL) | 10,322 | 2.1 (1.3) | 4897 | 3.2 (1.8) | 1394 | 3.8 (2.4) * | +81.0% |
| Females | 6597 | 2.0 (1.1) | 2310 | 3.2 (1.8) | 531 | 3.8 (2.5) * | +90.0% |
| Males | 3725 | 2.3 (1.5) | 2587 | 3.2 (1.8) | 863 | 3.8 (2.3) * | +65.2% |
| Inflammation | |||||||
| hs-CRP (mg/L) | 72,717 | 1.1 (2.4) | 45,569 | 1.6 (3.1) | 9765 | 2.5 (4.4) * | +127.3% |
| Females | 46,937 | 1.2 (2.7) | 21,723 | 2.2 (4.1) | 4052 | 3.6 (6.0) * | +200.0% |
| Males | 25,780 | 1.0 (1.9) | 23,846 | 1.2 (2.3) | 5713 | 1.9 (3.3) * | +90.0% |
| Fibrinogen (mg/dL) | 56,241 | 356 (108) | 29,643 | 386 (113) | 7621 | 433 (139) * | +21.6% |
| Females | 36,635 | 356 (107) | 13,976 | 397 (112) | 3187 | 443 (132) * | +24.4% |
| Males | 19,606 | 354 (108) | 15,667 | 377 (113) | 4434 | 425 (141) * | +20.2% |
| MPO (pmol/L) | 58,752 | 263 (146) | 34,592 | 274.0 (151) | 8538 | 317 (180) * | +20.5% |
| Females | 37,830 | 267 (149) | 16,407 | 291 (160) | 3547 | 342 (189) * | +28.2% |
| Males | 20,922 | 256 (139) | 18,185 | 261 (140) | 4991 | 300 (170) * | +17.2% |
| LpPLA2 (nmol/min/mL) | 71,783 | 184 (58) | 44,587 | 180 (59) | 9489 | 166 (61) * | −9.8% |
| Females | 46,307 | 185 (57) | 21,280 | 182 (60) | 3946 | 167 (60) * | −9.7% |
| Males | 25,476 | 182 (60) | 23,307 | 179 (60) | 5543 | 165 (63) * | −9.3% |
| Parameter | Healthy n = 75,271 (56.3%) | Prediabetic n = 48,455 (36.2%) | Diabetic n = 10,038 (7.5%) | % Difference, vs. Healthy Subjects | |||
|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | vs. Healthy Subjects | N | Median (IQR) | ||
| LDL-C (mg/dL) | 73,824 | 117.0 (51.0) | 46,550 | 117.0 (54.0) | 9966 | 107.0 (57.0) * | −8.5% |
| Females | 47,539 | 117.0 (49.0) | 22,127 | 121.0 (53.0) | 4144 | 114.0 (59.0) * | −2.6% |
| Males | 26,285 | 117.0 (54.0) | 24,423 | 113.0 (55.0) | 5822 | 102.0 (55.0) * | −12.8% |
| sdLDL-C (mg/dL) | 73,572 | 24.0 (15.0) | 46,258 | 27.0 (20.0) | 9938 | 31.0 (25.0) * | +29.2% |
| Females | 47,431 | 23.0 (14.0) | 22,036 | 27.0 (18.0) | 4135 | 31.0 (25.0) * | +34.8% |
| Males | 26,141 | 26.0 (19.0) | 24,222 | 28.0 (21.0) | 5803 | 31.0 (25.0) * | +19.2% |
| HDL-C (mg/dL) | 74,272 | 58.0 (24.0) | 46,978 | 51.0 (22.0) | 10,017 | 43.0 (17.0) * | −25.9% |
| Females | 47,793 | 64.0 (25.0) | 22,306 | 57.0 (22.0) | 4161 | 49.0 (19.0) * | −23.4% |
| Males | 26,479 | 49.0 (19.0) | 24,672 | 46.0 (18.0) | 5856 | 40.0 (15.0) * | −18.4% |
| Triglycerides (mg/dL) | 75,271 | 93.0 (67.0) | 48,455 | 116.0 (80.0) | 10,038 | 150 (114) * | +61.3% |
| Females | 48,476 | 89.0 (60.0) | 22,977 | 115.0 (77.0) | 4175 | 151 (107) * | +69.7% |
| Males | 26,795 | 103.0 (75.0) | 25,478 | 116.0 (83.0) | 5863 | 149 (118) * | +44.7% |
| ApoA-I (mg/dL) | 74,089 | 160.6 (44.4) | 46,701 | 152.7 (40.6) | 9994 | 142 (36.5) * | −11.8% |
| Females | 47,705 | 170.5 (43.0) | 22,166 | 165.0 (41.6) | 4156 | 155 (38.8) * | −9.1% |
| Males | 26,384 | 144.0 (34.7) | 24,535 | 142.9 (33.8) | 5838 | 135 (31.1) * | −6.3% |
| ApoB (mg/dL) | 72,409 | 94.0 (36.0) | 44,843 | 97.0 (38.0) | 9715 | 96.0 (42.0) * | +2.1% |
| Females | 46,616 | 93.0 (35.0) | 21,338 | 98.0 (37.0) | 4030 | 100.0 (44.0) * | +7.5% |
| Males | 25,793 | 95.0 (38.0) | 23,505 | 95.0 (39.0) | 5685 | 93.0 (41.0) * | −2.1% |
| Parameter * | Testing Only (N = 8559) | Testing and Life Plan (N = 1179) | p Value § | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline | Most Recent | % Change † | p Value ‡ | N | Baseline | Most Recent | % Change † | p Value ‡ | ||
| Age (years) | 61.0 (17.0) | 60.0 (16.5) | |||||||||
| Females, % | 3988 (46.6%) | 646 (54.8%) | |||||||||
| Weight (pounds) | 2988 | 190.0 (60.0) | 188.0 (60.0) | −1.7% | 0.004 | 569 | 189.0 (58.0) | 187.0 (57.0) | −2.0% | <0.001 | 0.592 |
| HbA1c (%) | 7909 | 5.8 (0.5) | 5.7 (0.5) | −0.0% | <0.001 | 1140 | 5.8 (0.5) | 5.7 (0.5) | −0.1% | <0.001 | <0.001 |
| Glucose (mg/dL) | 8559 | 106.0 (10.0) | 104.0 (14.0) | −1.9% | <0.001 | 1179 | 107.0 (10.0) | 104.0 (12.0) | −2.7% | <0.001 | 0.067 |
| Insulin (µU/mL) | 8098 | 13.0 (11.0) | 12.0 (11.0) | −0.5% | <0.001 | 1150 | 13.0 (11.0) | 12.0 (11.0) | −1.4% | <0.001 | 0.014 |
| HOMA-IR | 8081 | 3.5 (3.2) | 3.2 (3.2) | −0.1% | 0.004 | 1150 | 3.4 (3.1) | 3.0 (3.0) | −0.4% | <0.001 | 0.023 |
| HOMA-B | 8095 | 106.4 (90.6) | 110.0 (96.0) | +5.8% | <0.001 | 1150 | 102.9 (88.6) | 105.4 (88.9) | +2.5% | 0.542 | 0.252 |
| hs-CRP (mg/L) | 7941 | 1.5 (2.9) | 1.4 (2.9) | −0.1% | 0.145 | 1171 | 1.5 (3.0) | 1.4 (2.7) | −0.2% | 0.249 | 0.171 |
| Adiponectin (µg/mL) | 8559 | 9.8 (7.6) | 10.7 (8.5) | +0.9% | <0.001 | 1179 | 9.5 (7.9) | 11.1 (9.1) | +1.4% | <0.001 | <0.001 |
| GSP (µmol/L) | 8559 | 211.0 (59.0) | 202.0 (58.0) | −7.7% | <0.001 | 1179 | 207.0 (59.5) | 199.0 (57.0) | −7.2% | <0.001 | 0.433 |
| Fibrinogen (mg/dL) | 5548 | 390.0 (109.2) | 385.0 (113.0) | −2.6% | 0.019 | 826 | 388.0 (110.0) | 380.0 (109.0) | −7.6% | 0.004 | 0.041 |
| MPO (pmol/L) | 5775 | 272.0 (144.5) | 272.0 (150.0) | +2.2% | 0.401 | 908 | 271.0 (149.0) | 261.0 (144.2) | −11.0% | 0.041 | 0.043 |
| LpPLA2 nmol/min/mL | 7758 | 179.0 (60.0) | 172.0 (59.0) | −6.5% | <0.001 | 1110 | 183.0 (59.0) | 170.5 (62.8) | −10.8% | <0.001 | 0.002 |
| 10 yr Diabetes Risk | 8559 | 6.3 (14.1) | 6.2 (12.1) | −1.6% | 0.945 | 1179 | 5.7 (13.1) | 3.1 (8.7) | −45.6% | <0.001 | <0.001 |
| LDL-C (mg/dL) | 8157 | 112.0 (56.0) | 105.0 (55.0) | −6.2% | <0.001 | 1178 | 117.0 (55.0) | 107.0 (57.0) | −11.1% | <0.001 | <0.001 |
| sdLDL-C (mg/dL) | 8071 | 27.0 (19.0) | 25.0 (17.0) | −3.0% | <0.001 | 1176 | 28.0 (21.0) | 24.0 (16.0) | −5.4% | <0.001 | <0.001 |
| HDL-C (mg/dL) | 8316 | 51.0 (22.0) | 51.0 (22.0) | +0.6% | <0.001 | 1178 | 52.0 (21.8) | 53.0 (23.0) | +1.0% | <0.001 | 0.066 |
| Triglycerides (mg/dL) | 8559 | 116.0 (80.0) | 109.0 (74.0) | −7.7% | <0.001 | 1179 | 113.0 (82.0) | 101.0 (70.0) | −15.4% | <0.001 | <0.001 |
| ApoA-1 (mg/dL) | 8286 | 153.9 (41.1) | 152.2 (41.5) | −2.1% | <0.001 | 1172 | 157.1 (42.3) | 153.0 (40.8) | −4.5% | <0.001 | 0.003 |
| ApoB (mg/dL) | 7895 | 94.0 (39.0) | 90.0 (37.0) | −4.0% | <0.001 | 1120 | 97.0 (40.0) | 89.0 (37.0) | −8.1% | <0.001 | <0.001 |
| Parameter | Testing Only (n = 2017) | Testing and Life Plan (n = 216) | p Value § | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Baseline * | Most Recent * | % Change † | p Value ‡ | N | Baseline * | Most Recent * | % Change † | p Value ‡ | ||
| Age (years) | 63.0 (17.0) | 62.0 (16.0) | |||||||||
| Females, % | 773 (38.3%) | 99 (45.8%) | |||||||||
| Weight (pounds) | 719 | 205.0 (65.5) | 203.0 (65.0) | −2.0% | <0.001 | 110 | 204.5 (65.0) | 198.0 (60.5) | −6.2% | <0.001 | 0.004 |
| HbA1c (%) | 1867 | 7.2 (1.8) | 6.9 (1.7) | −0.3% | <0.001 | 212 | 7.1 (1.5) | 6.8 (1.4) | −0.5% | <0.001 | 0.001 |
| Glucose (mg/dL) | 2017 | 153.0 (54.0) | 141.0 (57.0) | −17.1% | <0.001 | 216 | 152.0 (50.0) | 136.5 (49.5) | −24.7% | <0.001 | 0.028 |
| Insulin (µU/mL) | 1997 | 17.0 (18.0) | 15.0 (15.0) | −4.9% | <0.001 | 215 | 18.0 (19.0) | 16.0 (13.0) | −4.3% | 0.278 | 0.139 |
| HOMA-IR | 1980 | 7.0 (7.3) | 5.4 (6.4) | −2.9% | <0.001 | 212 | 7.8 (8.2) | 5.5 (5.6) | −1.1% | 0.751 | 0.048 |
| HOMA-B | 1997 | 62.9 (79.1) | 68.2 (83.4) | +4.3% | 0.167 | 215 | 70.2 (78.1) | 73.8 (76.3) | −7.4% | 0.491 | 0.414 |
| hs-CRP (mg/L) | 1902 | 2.3 (4.1) | 2.0 (3.8) | −0.5% | 0.014 | 216 | 2.6 (4.1) | 1.8 (3.4) | −0.5% | 0.422 | 0.625 |
| Adiponectin (µg/mL) | 2017 | 8.0 (6.1) | 9.0 (6.8) | +0.9% | <0.001 | 216 | 7.2 (5.0) | 8.1 (6.0) | +1.1% | <0.001 | 0.425 |
| GSP (µmol/L) | 2017 | 301.0 (151.0) | 277.0 (144.0) | −27.5% | <0.001 | 216 | 292.0 (127.8) | 255.5 (110.5) | −50.6% | <0.001 | <0.001 |
| Fibrinogen (mg/dL) | 1546 | 435.5 (142.8) | 436.0 (134.0) | +2.8% | 0.301 | 172 | 433.5 (128.8) | 435.0 (129.0) | −0.5% | 0.952 | 0.623 |
| MPO (pmol/L) | 1567 | 313.0 (174.0) | 303.0 (170.5) | −6.6% | 0.205 | 185 | 303.0 (192.0) | 287.0 (178.0) | −20.9% | 0.010 | 0.285 |
| LpPLA2 nmol/min/mL | 1840 | 168.0 (60.0) | 157.0 (59.2) | −8.5% | <0.001 | 203 | 167.0 (64.5) | 154.0 (59.0) | −14.6% | <0.001 | 0.076 |
| Diabetes, % | 2017 | 100.0 | 96.6 | −3.4% | <0.001 | 216 | 100.0 | 91.8 | −8.2% | <0.001 | 0.012 |
| LDL-C (mg/dL) | 2000 | 103.0 (59.0) | 94.0 (53.0) | −7.1% | <0.001 | 216 | 108.0 (55.8) | 87.0 (56.5) | −12.4% | <0.001 | 0.037 |
| sdLDL-C (mg/dL) | 1989 | 31.0 (26.0) | 26.0 (21.0) | −4.3% | <0.001 | 216 | 32.0 (27.2) | 24.0 (18.0) | −8.0% | <0.001 | 0.005 |
| HDL-C (mg/dL) | 2016 | 43.0 (17.0) | 44.0 (18.0) | +0.6% | 0.001 | 216 | 43.0 (15.5) | 43.5 (18.2) | +0.9% | 0.110 | 0.755 |
| Triglycerides (mg/dL) | 2017 | 150.0 (108.0) | 137.0 (100.0) | −16.4% | <0.001 | 216 | 154.0 (114.2) | 130.5 (88.0) | −30.7% | 0.004 | 0.174 |
| ApoA-I (mg/dL) | 2007 | 143.0 (37.3) | 141.0 (36.9) | −2.7% | <0.001 | 216 | 144.3 (36.1) | 140.5 (34.1) | −6.3% | <0.001 | 0.008 |
| ApoB (mg/dL) | 1984 | 93.0 (43.0) | 87.0 (38.0) | −5.2% | <0.001 | 212 | 96.0 (42.0) | 81.5 (35.2) | −11.5% | <0.001 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dansinger, M.L.; Gleason, J.A.; Maddalena, J.; Asztalos, B.F.; Diffenderfer, M.R. Lifestyle Modification in Prediabetes and Diabetes: A Large Population Analysis. Nutrients 2025, 17, 1333. https://doi.org/10.3390/nu17081333
Dansinger ML, Gleason JA, Maddalena J, Asztalos BF, Diffenderfer MR. Lifestyle Modification in Prediabetes and Diabetes: A Large Population Analysis. Nutrients. 2025; 17(8):1333. https://doi.org/10.3390/nu17081333
Chicago/Turabian StyleDansinger, Michael L., Joi A. Gleason, Julia Maddalena, Bela F. Asztalos, and Margaret R. Diffenderfer. 2025. "Lifestyle Modification in Prediabetes and Diabetes: A Large Population Analysis" Nutrients 17, no. 8: 1333. https://doi.org/10.3390/nu17081333
APA StyleDansinger, M. L., Gleason, J. A., Maddalena, J., Asztalos, B. F., & Diffenderfer, M. R. (2025). Lifestyle Modification in Prediabetes and Diabetes: A Large Population Analysis. Nutrients, 17(8), 1333. https://doi.org/10.3390/nu17081333





