The Antioxidant and Chemopreventive Activity of a Nutraceutical Derived from Brassicaceae Seed Extracts for Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Functional Extracts and Nutraceutical Formulation
2.3. In Vitro Digestion
2.4. Antioxidant Activity
2.5. Chromatographic Studies via UPLC-QTOF Analysis
2.6. In Vivo Experimental Design
2.6.1. Animals
2.6.2. Experimental Design
2.6.3. Tumor Induction in the Colon
2.6.4. Exercise Protocol
2.6.5. Morphological and Histological Analysis
2.6.6. Gene Expression Assays
2.6.7. Metagenomic Analysis
2.7. Statistical Analysis
3. Results
3.1. Antioxidant Capacity of Brassicaceae Extracts
3.2. In Vitro Digestion
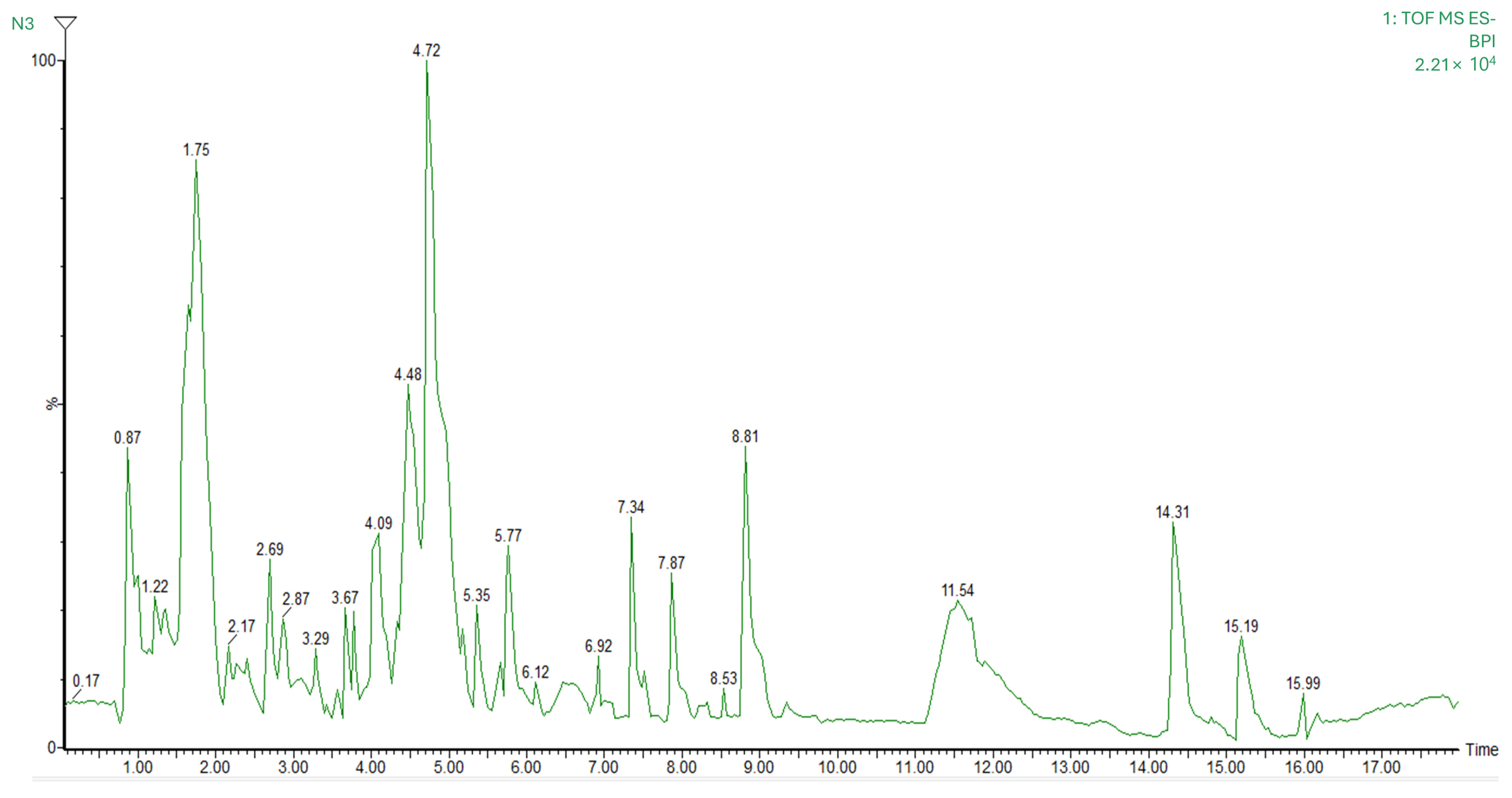
3.3. Mass Spectrometry Identification of Bioactive Compounds
3.4. In Vivo Experimental Model
3.4.1. Body Weight and Food/Water Intake
3.4.2. Tissue and Organ Weights
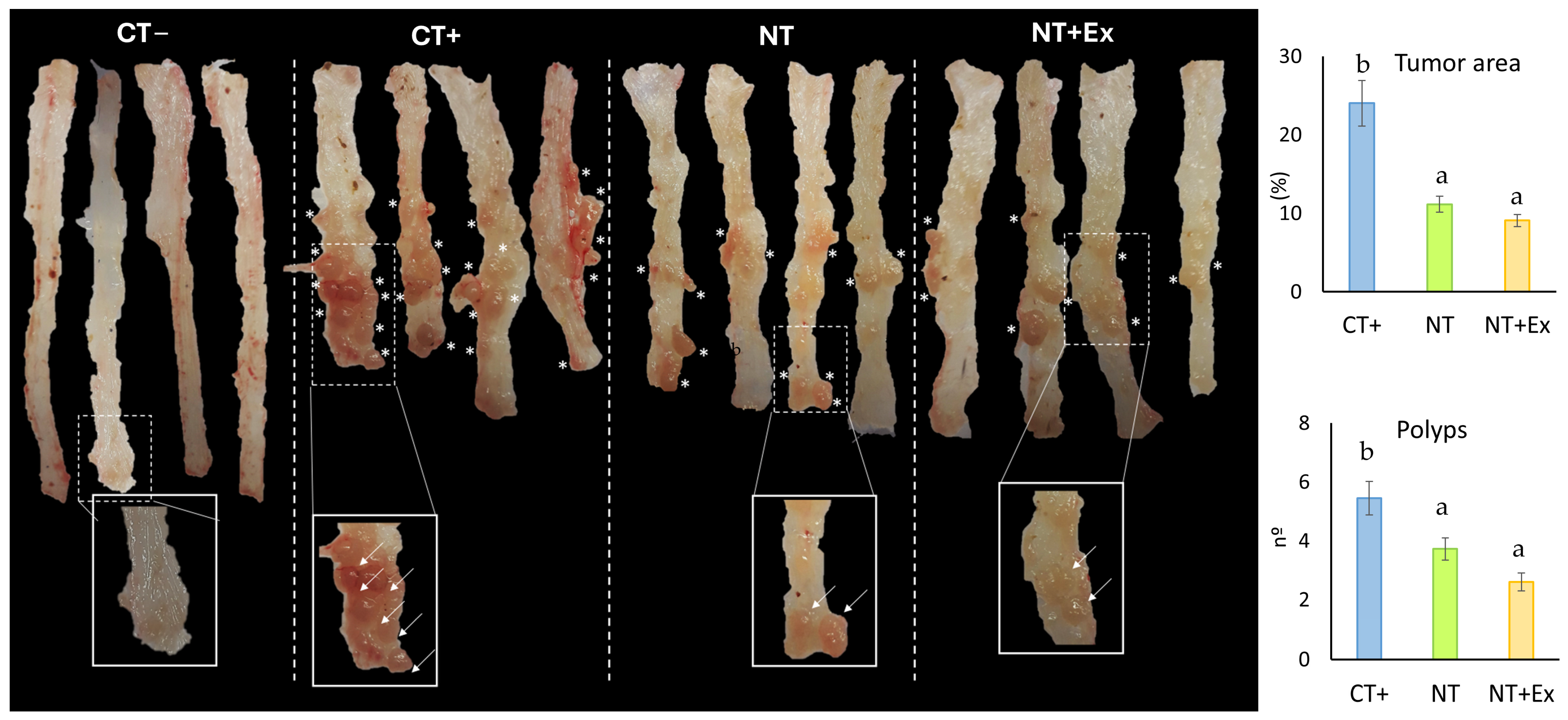
3.4.3. Modulation of Polyp Size and Number
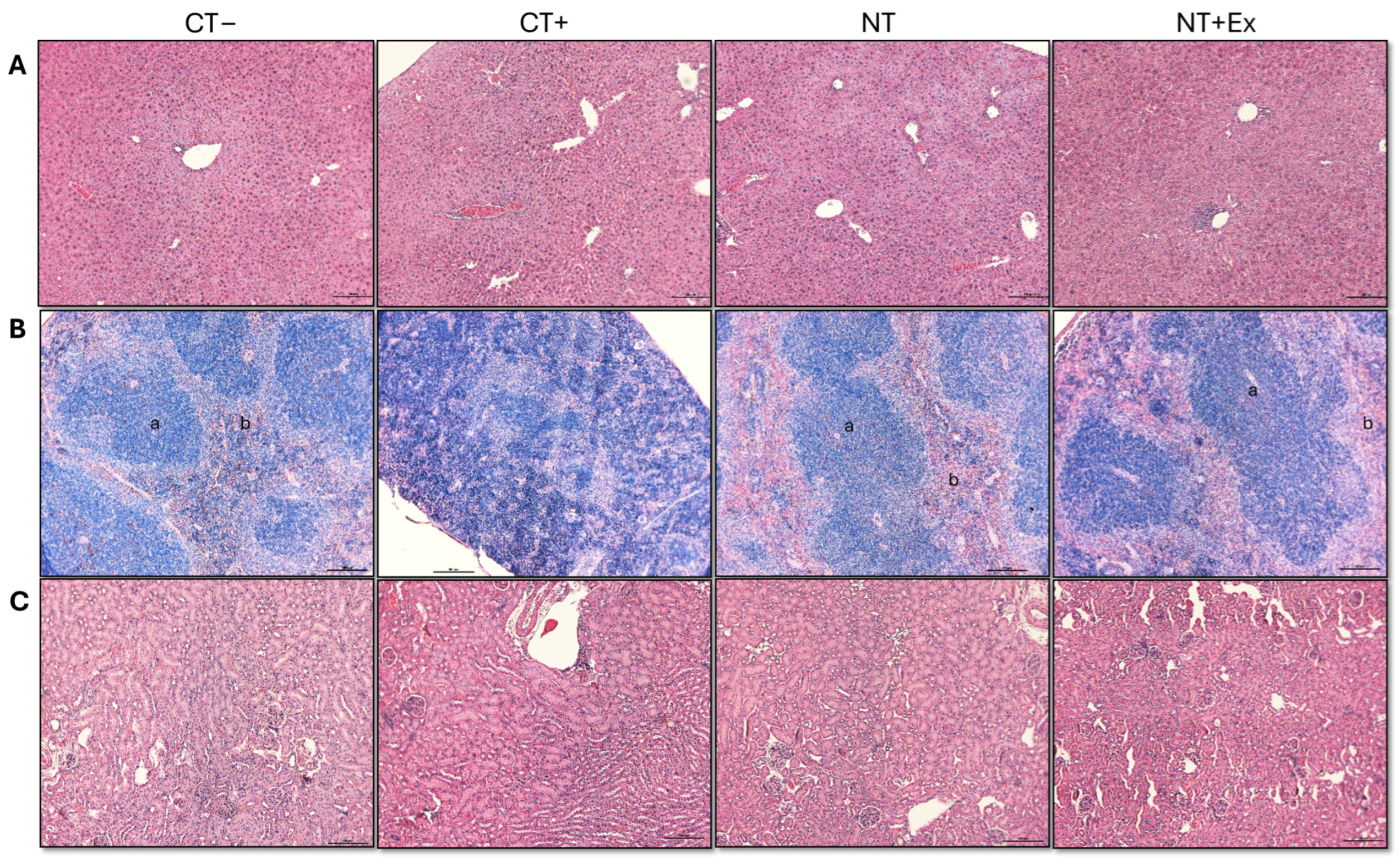
3.4.4. Histological Analysis
3.4.5. Gene Expression
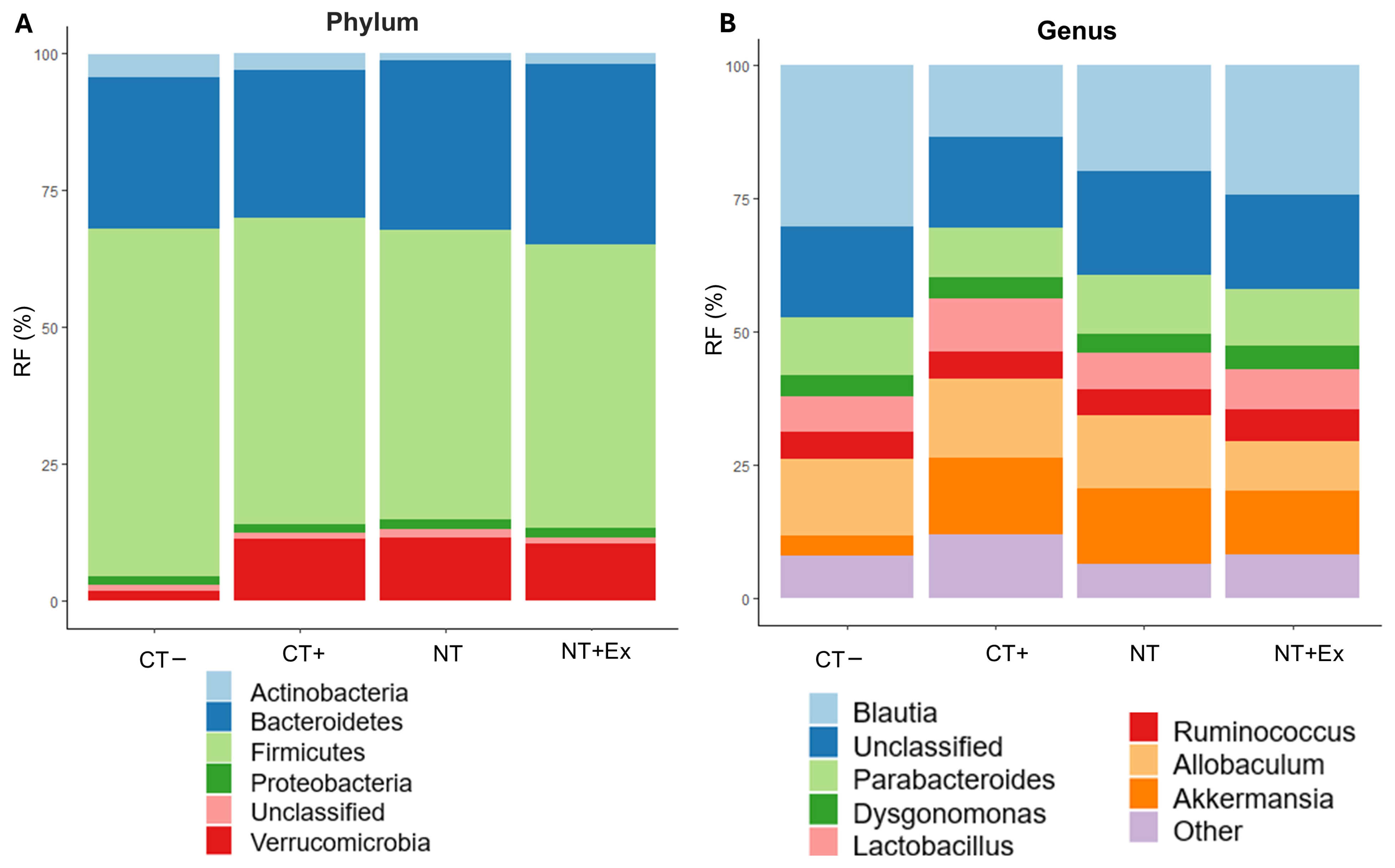
3.4.6. Metagenomic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-España, M.A.; Montes, A.F.; Garcia-Carbonero, R.; Mercadé, T.M.; Maurel, J.; Martín, A.M.; Pazo-Cid, R.; Vera, R.; Carrato, A.; Feliu, J. SEOM Clinical Guidelines for Pancreatic and Biliary Tract Cancer (2020). Clin. Transl. Oncol. 2021, 23, 988–1000. [Google Scholar] [CrossRef]
- WHO. Colorectal Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (accessed on 5 February 2025).
- Santucci, C.; Mignozzi, S.; Malvezzi, M.; Boffetta, P.; Collatuzzo, G.; Levi, F.; Vecchia, C.L.; Negri, E. European Cancer Mortality Predictions for the Year 2024 with Focus on Colorectal Cancer. Ann. Oncol. 2024, 35, 308–316. [Google Scholar] [CrossRef]
- Hardt, L.; Mahamat-Saleh, Y.; Aune, D.; Schlesinger, S. Plant-Based Diets and Cancer Prognosis: A Review of Recent Research. Curr. Nutr. Rep. 2022, 11, 695–716. [Google Scholar] [CrossRef]
- Macharia, J.M.; Mwangi, R.W.; Rozmann, N.; Zsolt, K.; Varjas, T.; Uchechukwu, P.O.; Wagara, I.N.; Raposa, B.L. Medicinal Plants with Anti-Colorectal Cancer Bioactive Compounds: Potential Game-Changers in Colorectal Cancer Management. Biomed. Pharmacother. 2022, 153, 113383. [Google Scholar] [CrossRef]
- Sanchez-Guzman, X.; Alvarez-Domínguez, L.; Ramírez-Torres, M.F.; Montes-Alvarado, J.B.; Garcia-Ibañez, P.; Moreno, D.A.; Domínguez, F.; Maycotte, P. Cruciferous Plant Extracts, Their Isothyocianate or Indol Derivatives, and Their Effect on Cellular Viability of Breast Cancer Cell Lines. J. Med. Food 2024, 27, 1183–1192. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The Value of Bioactive Compounds of Cruciferous Vegetables (Brassica) as Antimicrobials and Antioxidants: A Review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Yang, W.Y.; Lim, K.Y.; Yen, P.L.; Ong, S.H.; Naumovski, N.; Jani, R. The Association between Consumption of Bitter-Taste Vegetables in Asian Culture and Metabolic Syndrome Risk Factors in Children: A Narrative Review. Explor. Res. Hypothesis Med. 2024, 9, 47–54. [Google Scholar] [CrossRef]
- Mohd Nor, N.D.; Houston-Price, C.; Harvey, K.; Methven, L. The Effects of Taste Sensitivity and Repeated Taste Exposure on Children’s Intake and Liking of Turnip (Brassica Rapa Subsp. Rapa); a Bitter Brassica Vegetable. Appetite 2021, 157, 104991. [Google Scholar] [CrossRef]
- Nowak, K.; Rohn, S.; Halagarda, M. Impact of Cooking Techniques on the Dietary Fiber Profile in Selected Cruciferous Vegetables. Molecules 2025, 30, 590. [Google Scholar] [CrossRef]
- Baenas, N.; Marhuenda, J.; García-Viguera, C.; Zafrilla, P.; Moreno, D.A. Influence of Cooking Methods on Glucosinolates and Isothiocyanates Content in Novel Cruciferous Foods. Foods 2019, 8, 257. [Google Scholar] [CrossRef]
- Pereira, S.S.; Guimarães, M.; Monteiro, M.P. Towards Precision Medicine in Bariatric Surgery Prescription. Rev. Endocr. Metab. Disord. 2023, 24, 961–977. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, R.; Gaspar, J.M.; Sargsyan, D.; Su, Z.-Y.; Zhang, C.; Gao, L.; Cheng, D.; Li, W.; Wang, C.; et al. DNA Methylome and Transcriptome Alterations and Cancer Prevention by Curcumin in Colitis-Accelerated Colon Cancer in Mice. Carcinogenesis 2018, 39, 669–680. [Google Scholar] [CrossRef]
- Marino, P.; Pepe, G.; Basilicata, M.G.; Vestuto, V.; Marzocco, S.; Autore, G.; Procino, A.; Gomez-Monterrey, I.M.; Manfra, M.; Campiglia, P. Potential Role of Natural Antioxidant Products in Oncological Diseases. Antioxidants 2023, 12, 704. [Google Scholar] [CrossRef] [PubMed]
- Lučić, D.; Pavlović, I.; Brkljačić, L.; Bogdanović, S.; Farkaš, V.; Cedilak, A.; Nanić, L.; Rubelj, I.; Salopek-Sondi, B. Antioxidant and Antiproliferative Activities of Kale (Brassica oleracea L. Var. Acephala DC.) and Wild Cabbage (Brassica incana Ten.) Polyphenolic Extracts. Molecules 2023, 28, 1840. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, X.; Wang, D.; Zheng, C.; Chen, H.; Yuan, Y.; Wei, F. Metabolomics Combined with Biochemical Analyses Revealed Phenolic Profiles and Antioxidant Properties of Rapeseeds. Food Chem. 2025, 466, 142250. [Google Scholar] [CrossRef]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; Chaves, C.L.; López-Jurado, M.; Aranda, P.; Arrebola, F.; Cañizares, F.J.; Galisteo, M.; Porres, J.M. Improvement of the Antioxidant and Hypolipidaemic Effects of Cowpea Flours (Vigna Unguiculata) by Fermentation: Results of in Vitro and in Vivo Experiments. J. Sci. Food Agric. 2015, 95, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; Sánchez, C.; Chaves, C.L.; López-Jurado, M.; Aranda, P.; Cantarero, S.; Arrebola, F.; Fernández-Segura, E.; et al. Health Promoting Effects of Lupin (Lupinus albus var. multolupa) Protein Hydrolyzate and Insoluble Fiber in a Diet-Induced Animal Experimental Model of Hypercholesterolemia. Food Res. Int. 2013, 54, 1471–1481. [Google Scholar] [CrossRef]
- Porres, J.M.; Aranda, P.; López-Jurado, M.; Urbano, G. Nutritional Evaluation of Protein, Phosphorus, Calcium and Magnesium Bioavailability from Lupin (Lupinus albus var. multolupa)-Based Diets in Growing Rats: Effect of α-Galactoside Oligosaccharide Extraction and Phytase Supplementation. Br. J. Nutr. 2006, 95, 1102–1111. [Google Scholar] [CrossRef]
- Martínez, R.; Mesas, C.; Guzmán, A.; Galisteo, M.; López-Jurado, M.; Prados, J.; Melguizo, C.; Bermúdez, F.; Porres, J.M. Bioavailability and Biotransformation of Linolenic Acid from Basil Seed Oil as a Novel Source of Omega-3 Fatty Acids Tested on a Rat Experimental Model. Food Funct. 2022, 13, 7614–7628. [Google Scholar] [CrossRef]
- Martínez, R.; Kapravelou, G.; Donaire, A.; Lopez-Chaves, C.; Arrebola, F.; Galisteo, M.; Cantarero, S.; Aranda, P.; Porres, J.M.; López-Jurado, M. Effects of a Combined Intervention with a Lentil Protein Hydrolysate and a Mixed Training Protocol on the Lipid Metabolism and Hepatic Markers of NAFLD in Zucker Rats. Food Funct. 2018, 9, 830–850. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and Its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Duh, P.-D.; Tu, Y.-Y.; Yen, G.-C. Antioxidant Activity of Water Extract of Harng Jyur (Chrysanthemum Morifolium Ramat). LWT-Food Sci. Technol. 1999, 32, 269–277. [Google Scholar] [CrossRef]
- Martínez, R.; Kapravelou, G.; Porres, J.M.; Melesio, A.M.; Heras, L.; Cantarero, S.; Gribble, F.M.; Parker, H.; Aranda, P.; López-Jurado, M. Medicago sativa L., a Functional Food to Relieve Hypertension and Metabolic Disorders in a Spontaneously Hypertensive Rat Model. J. Funct. Foods 2016, 26, 470–484. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef]
- Bialkowska, A.B.; Ghaleb, A.M.; Nandan, M.O.; Yang, V.W. Improved Swiss-Rolling Technique for Intestinal Tissue Preparation for Immunohistochemical and Immunofluorescent Analyses. J. Vis. Exp. 2016, 113, e54161. [Google Scholar] [CrossRef]
- Mesas, C.; Martínez, R.; Doello, K.; Ortiz, R.; López-Jurado, M.; Bermúdez, F.; Quiñonero, F.; Prados, J.; Porres, J.M.; Melguizo, C. In Vivo Antitumor Activity of Euphorbia Lathyris Ethanol Extract in Colon Cancer Models. Biomed. Pharmacother. 2022, 149, 112883. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Yang, G.; Meng, B.; Yi, Z.; Yang, G.; Chen, M.; Hou, P.; Wang, H.; Xu, X. Moderate-Intensity Physical Exercise Affects the Exercise Performance and Gut Microbiota of Mice. Front. Cell Infect. Microbiol. 2021, 11, 712381. [Google Scholar] [CrossRef]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Huang, X.-M.; Yang, Z.-J.; Xie, Q.; Zhang, Z.-K.; Zhang, H.; Ma, J.-Y. Natural Products for Treating Colorectal Cancer: A Mechanistic Review. Biomed. Pharmacother. 2019, 117, 109142. [Google Scholar] [CrossRef]
- Peña, M.; Guzmán, A.; Martínez, R.; Mesas, C.; Prados, J.; Porres, J.M.; Melguizo, C. Preventive Effects of Brassicaceae Family for Colon Cancer Prevention: A Focus on in Vitro Studies. Biomed. Pharmacother. 2022, 151, 113145. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Gad, H.A.; Al Musayeib, N.M.; Bishr, M.; Ashour, M.L. Correlation of Glucosinolates and Volatile Constituents of Six Brassicaceae Seeds with Their Antioxidant Activities Based on Partial Least Squares Regression. Plants 2022, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Dzhalilova, D.; Zolotova, N.; Fokichev, N.; Makarova, O. Murine Models of Colorectal Cancer: The Azoxymethane (AOM)/Dextran Sulfate Sodium (DSS) Model of Colitis-Associated Cancer. PeerJ 2023, 11, e16159. [Google Scholar] [CrossRef]
- Robertis, M.D.; Massi, E.; Poeta, M.L.; Carotti, S.; Morini, S.; Cecchetelli, L.; Signori, E.; Fazio, V.M. The AOM/DSS Murine Model for the Study of Colon Carcinogenesis: From Pathways to Diagnosis and Therapy Studies. J. Carcinog. 2011, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Qin, W.; Ketnawa, S. Exploring the Bioaccessibility of Roasted Japanese Green Tea: Impact of Simulated Gastrointestinal Digestion. Foods 2025, 14, 311. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Li, Y.; Yu, Y.; Yu, X.; Liu, H.; Zhang, Q.; Yin, W.; Yu, L.; Ye, T. Natural Product Pectolinarigenin Exhibits Potent Anti-Metastatic Activity in Colorectal Carcinoma Cells in Vitro and in Vivo. Bioorg. Med. Chem. 2019, 27, 115089. [Google Scholar] [CrossRef]
- Deng, Z.; Shen, D.; Yu, M.; Zhou, F.; Shan, D.; Fang, Y.; Jin, W.; Qian, K.; Li, S.; Wang, G.; et al. Pectolinarigenin Inhibits Bladder Urothelial Carcinoma Cell Proliferation by Regulating DNA Damage/Autophagy Pathways. Cell Death Discov. 2023, 9, 214. [Google Scholar] [CrossRef]
- Lee, H.J.; Kwon, Y.S.; Lee, J.H.; Moon, Y.G.; Choi, J.; Hyun, M.; Tak, T.K.; Kim, J.-H.; Heo, J.D. Pectolinarigenin Regulates the Tumor-Associated Proteins in AGS-Xenograft BALB/c Nude Mice. Mol. Biol. Rep. 2024, 51, 305. [Google Scholar] [CrossRef]
- Ding, F.; Yang, S. Epigallocatechin-3-Gallate Inhibits Proliferation and Triggers Apoptosis in Colon Cancer via the Hedgehog/Phosphoinositide 3-Kinase Pathways. Can. J. Physiol. Pharmacol. 2021, 99, 910–920. [Google Scholar] [CrossRef]
- Wubetu, G.Y.; Shimada, M.; Morine, Y.; Ikemoto, T.; Ishikawa, D.; Iwahashi, S.; Yamada, S.; Saito, Y.; Arakawa, Y.; Imura, S. Epigallocatechin Gallate Hinders Human Hepatoma and Colon Cancer Sphere Formation. J. Gastroenterol. Hepatol. 2016, 31, 256–264. [Google Scholar] [CrossRef]
- Lei, J.; Chen, J.; Chen, J.; Fang, J.; Zhou, Z.; Xu, A. Epigallocatechin-3-Gallate Induces Immunogenic Cell Death and Enhances Cancer Immunotherapy in Colorectal Cancer. Biochem. Biophys. Res. Commun. 2024, 736, 150907. [Google Scholar] [CrossRef]
- Costa, E.V.; Soares, L.d.N.; Chaar, J.d.S.; Silva, V.R.; Santos, L.d.S.; Koolen, H.H.F.; da Silva, F.M.A.; Tavares, J.F.; Zengin, G.; Soares, M.B.P.; et al. Benzylated Dihydroflavones and Isoquinoline-Derived Alkaloids from the Bark of Diclinanona Calycina (Annonaceae) and Their Cytotoxicities. Molecules 2021, 26, 3714. [Google Scholar] [CrossRef]
- Huminiecki, L. Evidence for Multilevel Chemopreventive Activities of Natural Phenols from Functional Genomic Studies of Curcumin, Resveratrol, Genistein, Quercetin, and Luteolin. Int. J. Mol. Sci. 2022, 23, 14957. [Google Scholar] [CrossRef] [PubMed]
- Kisacam, M.A. Nobiletin Is Capable of Regulating Certain Anti-Cancer Pathways in a Colon Cancer Cell Line. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Vega-García, A.; Manjarrez-Marmolejo, J.; Moreno, D.A.; Feria-Romero, I.A. The Preventive Effects of Broccoli Bioactives against Cancer: Evidence from a Validated Rat Glioma Model. Biomed. Pharmacother. 2023, 168, 115720. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Trudo, S.P.; Gallaher, D.D. Apiaceous and Cruciferous Vegetables Fed During the Post-Initiation Stage Reduce Colon Cancer Risk Markers in Rats. J. Nutr. 2019, 149, 249–257. [Google Scholar] [CrossRef]
- Tse, G.; Eslick, G.D. Cruciferous Vegetables and Risk of Colorectal Neoplasms: A Systematic Review and Meta-Analysis. Nutr. Cancer 2014, 66, 128–139. [Google Scholar] [CrossRef]
- Mori, N.; Murphy, N.; Sawada, N.; Achaintre, D.; Yamaji, T.; Scalbert, A.; Iwasaki, M.; Inoue, M.; Gunter, M.J.; Tsugane, S. Prediagnostic Plasma Polyphenol Concentrations and Colon Cancer Risk: The JPHC Nested Case–Control Study. Clin. Nutr. 2022, 41, 1950–1960. [Google Scholar] [CrossRef]
- Xian, Y.-F.; Hu, Z.; Ip, S.-P.; Chen, J.-N.; Su, Z.-R.; Lai, X.-P.; Lin, Z.-X. Comparison of the Anti-Inflammatory Effects of Sinapis Alba and Brassica Juncea in Mouse Models of Inflammation. Phytomedicine 2018, 50, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsalam, R.M.; El Badawy, S.A.; Ogaly, H.A.; Ibrahim, F.M.; Farag, O.M.; Ahmed, K.A. Eruca Sativa Seed Extract Modulates Oxidative Stress and Apoptosis and Up-Regulates the Expression of Bcl-2 and Bax Genes in Acrylamide-Induced Testicular Dysfunction in Rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 53249–53266. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, J.; Tian, M.; Sawut, A.; Xiao, D.; Yi, Z.; Chen, L. Circulating Inflammatory Cytokines and Colorectal Cancer: New Insights from Mendelian Randomization. Medicine 2025, 104, e41331. [Google Scholar] [CrossRef]
- Nelson, V.K.; Nuli, M.V.; Mastanaiah, J.; Saleem, T.S.M.; Birudala, G.; Jamous, Y.F.; Alshargi, O.; Kotha, K.K.; Sudhan, H.H.; Mani, R.R.; et al. Reactive Oxygen Species Mediated Apoptotic Death of Colon Cancer Cells: Therapeutic Potential of Plant Derived Alkaloids. Front. Endocrinol. 2023, 14, 1201198. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, J.; Cao, H.; Wang, C.; Shen, C.; Liu, J. Effect and Mechanism of Magnolia Officinalis in Colorectal Cancer: Multi-Component-Multi-Target Approach. J. Ethnopharmacol. 2025, 338, 119007. [Google Scholar] [CrossRef] [PubMed]
- Cid-Gallegos, M.S.; Sánchez-Chino, X.M.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Vásquez-Garzón, V.R.; Baltiérrez-Hoyos, R.; Villa-Treviño, S.; Dávila-Ortíz, G.; Jiménez-Martínez, C. Modification of In Vitro and In Vivo Antioxidant Activity by Consumption of Cooked Chickpea in a Colon Cancer Model. Nutrients 2020, 12, 2572. [Google Scholar] [CrossRef]
- Chen, J.-S.; Huang, J.-Q.; Luo, B.; Dong, S.-H.; Wang, R.-C.; Jiang, Z.-K.; Xie, Y.-K.; Yi, W.; Wen, G.-M.; Zhong, J.-F. PIK3CD Induces Cell Growth and Invasion by Activating AKT/GSK-3β/β-Catenin Signaling in Colorectal Cancer. Cancer Sci. 2019, 110, 997–1011. [Google Scholar] [CrossRef]
- Okamoto, T.; Onaga, C.; Matsuoka, I.; Ozaki, A.; Matsuda, C.; Kasai, T.; Xiong, Y.; Harada, Y.; Sato, T.; Nakano, Y.; et al. High SLC20A1 Expression Indicates Poor Prognosis in Prostate Cancer. Cancer Diagn. Progn. 2023, 3, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Onaga, C.; Tamori, S.; Matsuoka, I.; Ozaki, A.; Motomura, H.; Nagashima, Y.; Sato, T.; Sato, K.; Tahata, K.; Xiong, Y.; et al. High SLC20A1 Expression Is Associated With Poor Prognosis for Radiotherapy of Estrogen Receptor-Positive Breast Cancer. Cancer Diagn. Progn. 2022, 2, 429–442. [Google Scholar] [CrossRef]
- Li, J.; Dong, W.; Li, Z.; Wang, H.; Gao, H.; Zhang, Y. Impact of SLC20A1 on the Wnt/Β-catenin Signaling Pathway in Somatotroph Adenomas. Mol. Med. Rep. 2019, 20, 3276–3284. [Google Scholar] [CrossRef]
- Murphy, N.P.; Binti Ahmad Mokhtar, A.M.; Mott, H.R.; Owen, D. Molecular Subversion of Cdc42 Signalling in Cancer. Biochem. Soc. Trans. 2021, 49, 1425–1442. [Google Scholar] [CrossRef]
- Cai, J.-W.; Huang, X.-M.; Li, X.-L.; Qin, S.; Rong, Y.-M.; Chen, X.; Weng, J.-R.; Zou, Y.-F.; Lin, X.-T. An 11-Gene Signature for the Prediction of Systemic Recurrences in Colon Adenocarcinoma. Gastroenterol. Rep. 2021, 9, 451–460. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Gu, Y.; Shi, J.; Yan, J.; Wang, X.; Li, B.; Wang, B.; Zhong, W.; Cao, H. Dietary Flavonoids-Microbiota Crosstalk in Intestinal Inflammation and Carcinogenesis. J. Nutr. Biochem. 2024, 125, 109494. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-García, L.; Ruiz-Malagon, A.J.; Huertas, F.; Rodríguez-Sojo, M.J.; Molina-Tijeras, J.A.; Diez-Echave, P.; Becerra, P.; Mirón, B.; Morón, R.; Rodríguez-Nogales, A.; et al. Administration of Intestinal Mesenchymal Stromal Cells Reduces Colitis-Associated Cancer in C57BL/6J Mice Modulating the Immune Response and Gut Dysbiosis. Pharmacol. Res. 2023, 195, 106891. [Google Scholar] [CrossRef]
- Song, C.-H.; Kim, N.; Nam, R.H.; Choi, S.I.; Jang, J.Y.; Lee, H.-N. Changes in Gut Microbiome upon Orchiectomy and Testosterone Administration in AOM/DSS-Induced Colon Cancer Mouse Model. Cancer Res. Treat. 2023, 55, 196–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-M.; Yin, D.-K.; Rui, X.-L.; Shao, F.-P.; Li, J.-C.; Xu, L.; Yang, Y. Protective Effect of Pai-Nong-San against AOM/DSS-Induced CAC in Mice through Inhibiting the Wnt Signaling Pathway. Chin. J. Nat. Med. 2021, 19, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Parang, B.; Barret, C.W.; Williams, C.S. AOM/DSS Model of Colitis-Associated Cancer. Methods Mol. Biol. 2016, 1422, 297–307. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, X.; Chen, X.; Wang, M.; Tian, L.; Zhou, H.; Yang, W.; He, F.; Yin, W. Chemopreventive Effects of Polysaccharides and Flavonoids from Okra Flowers in Azomethane/Dextran Sulfate Sodium-Induced Murine Colitis-Associated Cancer. Nutrients 2023, 15, 4820. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, W.; Bian, Z.; Ma, Y.; Kang, Z.; Jin, J.; Li, X.; Ge, S.; Hao, Y.; Zhang, H.; et al. Lactobacillus Plantarum Zhang-LL Inhibits Colitis-Related Tumorigenesis by Regulating Arachidonic Acid Metabolism and CD22-Mediated B-Cell Receptor Regulation. Nutrients 2023, 15, 4512. [Google Scholar] [CrossRef]
- Pal, P.; Shastry, R.P. Exploring the Complex Role of Gut Microbiome in the Development of Precision Medicine Strategies for Targeting Microbial Imbalance-Induced Colon Cancer. Folia Microbiol. 2023, 68, 691–701. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, T.; Tian, Z.; Shi, C.; Yan, C.; Li, H.; Du, Y.; Li, G. Progress in the Investigation of the Firmicutes/Bacteroidetes Ratio as a Potential Pathogenic Factor in Ulcerative Colitis. J. Med. Microbiol. 2025, 74, 001966. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Adomako-Bonsu, A.G.; Wang, M.; Li, J. Three Specific Gut Bacteria in the Occurrence and Development of Colorectal Cancer: A Concerted Effort. PeerJ 2023, 11, e15777. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, D.; Wu, D.; Gao, X.; Shao, F.; Zhao, M.; Wang, J.; Ma, J.; Wang, W.; Qin, X.; et al. Tissue-Resident Lachnospiraceae Family Bacteria Protect against Colorectal Carcinogenesis by Promoting Tumor Immune Surveillance. Cell Host Microbe 2023, 31, 418–432.e8. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, Q.; Chen, Q.; Wei, Z.; Liu, S.; Zhang, L.; Zhang, Y.; Li, Z.; Liu, H.; Sui, H. Akkermansia Muciniphila Inhibits Tryptophan Metabolism via the AhR/β-Catenin Signaling Pathway to Counter the Progression of Colorectal Cancer. Int. J. Biol. Sci. 2023, 19, 4393–4410. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Hao, H.; Liu, Q.; Li, J.; Yao, Y.; Liu, Y.; Zhang, T.; Zhang, Z.; Yi, H. Effect of Extracelluar Vesicles Derived from Akkermansia Muciniphila on Intestinal Barrier in Colitis Mice. Nutrients 2023, 15, 4722. [Google Scholar] [CrossRef]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef]


| Phase | Time | % VO2 Max |
|---|---|---|
| Warm-up | 5′ | 20 |
| Exercise program | ||
| 1× | 1′30″ | 20 → 45 |
| 1′ | 45 | |
| 4× | 2′ | 45 |
| 1′ | 30 | |
| 1′30″ | Rest |
| Function | Genes | Primers |
|---|---|---|
| Oxidative metabolism | cat | Mm00437992 |
| gpx2 | Mm01286848 | |
| sod1 | Mm01344233_g1 | |
| Detoxification pathways | nqo1 | Mm00500822_g1 |
| gsta1 | Mm03019257_g1 | |
| Inflammatory process | IL-1b | Mm00434228_m1 |
| IL-6 | Mm00446190_m1 | |
| Tumor development | cadm1 | Mm00457551_m1 |
| cdc42 | Mm01194005_g1 | |
| pik3cd | Mm00435674_m1 | |
| slc20 | Mm00489378_m1 |
| Yield (mg/g) | TPC (µg GA) | ABTS (µg GA) | ICC (CAU) | IRC (µg GA) | |
|---|---|---|---|---|---|
| EtOH | |||||
| BO var. sabellica | 115.4 ± 2.9 b | 30.9 ± 0.5 b | 11.6 ± 0.4 bc | 1.56 ± 0.11 b | 25.9 ± 1.4 b |
| BO var. italica | 115.3 ± 9.7 b | 31.4 ± 0.9 b | 12.8 ± 0.1 c | 1.29 ± 0.09 b | 27.7 ± 0.3 b |
| BO var. botrytis | 132.1 ± 4.6 b | 27.9 ± 0.4 a | 10.2 ± 0.7 b | 0.87 ± 0.01 a | 20.1 ± 0.6 a |
| Eruca sativa | 86.9 ± 1.9 a | 43.5 ± 0.4 c | 15.0 ± 0.0 d | 3.99 ± 0.06 c | 43.5 ± 1.4 c |
| Sinapis alba | 120.8 ± 1.3 b | 60.5 ± 1.0 d | 7.4 ± 0.1 a | 0.98 ± 0.05 a | 18.8 ± 1.2 a |
| HP | |||||
| BO var. sabellica | 24.4 ± 0.2 b | 28.5 ± 0.3 b | 12.2 ± 0.7 a | 1.00 ± 0.03 a | 13.1 ± 0.3 b |
| BO var. italica | 27.3 ± 0.3 c | 26.2 ± 0.3 ab | 10.2 ± 0.3 a | 1.10 ± 0.02 ab | 12.9 ± 0.2 b |
| BO var. botrytis | 29.3 ± 0.6 cd | 25.1 ± 1.1 a | 11.5 ± 0.7 a | 1.19 ± 0.07 b | 13.0 ± 0.2 b |
| Eruca sativa | 20.3 ± 0.4 a | 33.6 ± 0.4 c | 17.0 ± 0.4 b | 1.34 ± 0.02 c | 12.8 ± 0.1 b |
| Sinapis alba | 31.9 ± 1.1 d | 31.7 ± 0.4 c | 11.1 ± 0.4 a | 0.98 ± 0.02 a | 8.0 ± 0.1 a |
| NT | 32.5 (0.3) | 7.8 (0.1) | 0.93 (0.04) | 9.1 (0.1) | |
| Dialyzed | Retained | |||
|---|---|---|---|---|
| Blank | NT | Blank | NT | |
| Dializability (%) | 73.1 ± 2.3 | |||
| TPC (µg GA) | 7.21 ± 0.23 | 54.5 ± 1.1 * | 8.24 ± 0.4 | 56.8 ± 1.2 * |
| ABTS (µg GA) | 8.96 ± 0.23 | 16.0 ± 0.4 * | 7.05 ± 0.27 | 15.7 ± 0.4 * |
| ICC (CAU) | 0.59 ± 0.03 | 1.44 ± 0.01 * | 0.41 ± 0.02 | 2.48 ± 0.03 * |
| IRC (µg GA) | 0.80 ± 0.11 | 11.1 ± 0.55 * | 1.53 ± 0.16 | 8.9 ± 0.3 * |
| RT | MS | COMPOUND | MF [H−] | % FIT | F1 | F2 | F3 |
|---|---|---|---|---|---|---|---|
| 0.95 | 313.0712 | Pectolinarigenin | C17H13O6 | 96.65 | 225.0631 | 180.0354 | 120.0542 |
| 1.22 | 299.0403 | Mumefural | C12H11O9 | 98.65 | 240.0316 | 225.069 | 220.0431 |
| 2.17 | 379.0818 | Diphyllin | C21H15O7 | 34.36 | 293.0519 | 280.1175 | 105.0269 |
| 2.41 | 471.0927 | Epigallocatechin (1) | C23H19O11 | 99.45 | 331.0149 | 253.0714 | 209.0065 |
| 2.67 | 471.108 | Rubialatin A | C27H19O8 | 69.13 | 347.0891 | 293.041 | 267.1032 |
| 2.69 | 361.0923 | Crotepoxide | C18H17O8 | 99.94 | 220.0375 | 159.0295 | 119.0415 |
| 2.70 | 505.171 | Prunioside A | C25H29O11 | 93.83 | 347.0891 | 293.041 | 267.1032 |
| 3.57 | 305.066 | (+)-Gallocatechin | C15H13O7 | 99.1 | 267.0891 | 239.0623 | 221.0522 |
| 3.78 | 465.1397 | Curculigoside A | C22H25O11 | 99.5 | 347.0685 | 331.0488 | 253.0787 |
| 4.01 | 167.1553 | Cimifugin (2) | C22H27O11 | 99.33 | 227.0043 | 220.0349 | 209.0298 |
| 4.16 | 583.2027 | Lucidumoside C | C27H35O14 | 89.84 | 301.1084 | 262.0572 | 220.0384 |
| 4.19 | 551.1553 | Cucumerin B | C29H27O11 | 2.85 | 315.1268 | 294.0228 | 262.0588 |
| 4.33 | 553.1557 | Ligustrosidic acid | C25H29O14 | 99.07 | 294.0379 | 267.0679 | 253.0775 |
| 4.35 | 553.1557 | Marinoid D | C25H29O14 | 99.07 | 317.038 | 267.0788 | 250.0633 |
| 4.65 | 445.1135 | Glycitin | C22H21O10 | 1.49 | 317.0562 | 267.0958 | 250.0111 |
| 4.68 | 771.1773 | Quercetin (3) | C36H35O19 | 8.75 | 267.0869 | 253.084 | 227.0035 |
| 4.72 | 467.1495 | Dichamanetin | C29H23O6 | 10.39 | 253.0823 | 239.0681 | 195.0144 |
| 5.73 | 401.1236 | Nobiletin | C21H21O8 | 91.69 | 347.0704 | 253.0765 | 239.0632 |
| 6.12 | 709.2285 | Moracenin D | C40H37O12 | 38.63 | 668.2699 | 267.0976 | 239.0707 |
| 6.92 | 551.2856 | Lokundjoside | C29H43O10 | 84.27 | 287.1523 | 267.1325 | 165.0612 |
| 6.95 | 389.1236 | Populin/Populoside | C20H21O8 | 84.36 | 301.1513 | 220.0353 | 195.0285 |
| 7.34 | 323.1495 | Chamissonolide | C17H23O6 | 99.75 | 309.135 | 262.0421 | 221.0459 |
| 7.87 | 251.0919 | Nothoapiole | C13H15O5 | n/a | 220.036 | 207.0571 | 158.9771 |
| 8.32 | 753.2242 | Catharticin | C34H41O19 | 15.22 | 331.0123 | 253.071 | 239.0514 |
| 8.53 | 325.1076 | Eucalyptin | C19H17O5 | 94.85 | 299.1306 | 267.0681 | 220.025 |
| 8.81 | 619.2391 | Hydrangenoside A | C31H39O13 | 54.88 | 556.2574 | 262.052 | 220.054 |
| 9.86 | 339.1232 | 8-Prenylnaringenin | C20H19O5 | 98.19 | 339.1318 | 239.0277 | 105.0285 |
| 11.36 | 533.3631 | Pyrohyperforin | C35H49O4 | 16.01 | 400.2627 | 301.1781 | 159.0122 |
| 12.91 | 433.2015 | Nesodine | C27H29O5 | 25.23 | 317.0427 | 267.1048 | 239.073 |
| 14.31 | 337.2379 | Kirenol | C20H33O4 | n/a | 323.22 | 136.0556 | 119.0428 |
| 15.19 | 339.2535 | Persealide | C20H35O4 | n/a | 325.2375 | 207.0567 | 159.0379 |
| CT− | CT+ | NT | NT + Ex | |
|---|---|---|---|---|
| Liver | 0.798 ± 0.026 a | 0.863 ± 0.026 ab | 0.868 ± 0.014 ab | 0.886 ± 0.017 b |
| Kidneys | 0.108 ± 0.002 a | 0.109 ± 0.002 a | 0.113 ± 0.002 a | 0.109 ± 0.002 a |
| Heart | 0.113 ± 0.002 a | 0.105 ± 0.002 a | 0.109 ± 0.002 a | 0.107 ± 0.003 a |
| Spleen | 0.071 ± 0.001 a | 0.165 ± 0.017 b | 0.132 ± 0.009 b | 0.150 ± 0.016 b |
| Plantaris | 0.013 ± 0.000 a | 0.012 ± 0.001 a | 0.013 ± 0.000 a | 0.013 ± 0.000 a |
| Cecum | 0.096 ± 0.005 a | 0.103 ± 0.004 a | 0.099 ± 0.002 a | 0.096 ± 0.004 a |
| Gastrocnemius | 0.095 ± 0.003 a | 0.095 ± 0.002 a | 0.103 ± 0.002 a | 0.105 ± 0.003 a |
| Colon | 0.119 ± 0.015 a | 0.338 ± 0.031 c | 0.248 ± 0.022 b | 0.262 ± 0.009 bc |
| Colon length | 6.88 ± 0.18 b | 5.99 ± 0.28 a | 6.28 ± 0.15 ab | 6.08 ± 0.13 a |
| CT− | CT+ | NT | NT + Ex | |
|---|---|---|---|---|
| cat | 1.00 ± 0.18 b | 0.77 ± 0.08 ab | 0.93 ± 0.04 ab | 0.75 ± 0.04 a |
| gpx2 | 1.00 ± 0.12 b | 0.64 ± 0.08 a | 1.21 ± 0.09 b | 1.05 ± 0.04 b |
| sod1 | 1.00 ± 0.16 c | 0.38 ± 0.05 a | 0.86 ± 0.06 bc | 0.72 ± 0.03 b |
| nqo1 | 1.00 ± 0.18 a | 0.79 ± 0.14 a | 2.36 ± 0.11 b | 2.23 ± 0.11 b |
| gsta1 | 1.00 ± 0.14 c | 0.65 ± 0.08 b | 0.72 ± 0.08 b | 0.27 ± 0.02 a |
| cadm1 | 1.00 ± 0.16 b | 1.00 ± 0.14 b | 0.75 ± 0.03 a | 0.67 ± 0.03 a |
| cdc42 | 1.00 ± 0.15 b | 1.23 ± 0.23 b | 0.64 ± 0.04 a | 0.55 ± 0.01 a |
| IL-1ß | 1.00 ± 0.11 a | 1.73 ± 0.17 b | 1.56 ± 0.18 b | 1.29 ± 0.09 ab |
| IL-6 | 1.00 ± 0.21 c | 0.54 ± 0.09 b | 0.39 ± 0.03 ab | 0.18 ± 0.01 a |
| pik3cd | 1.00 ± 0.09 b | 2.64 ± 0.28 c | 0.70 ± 0.07 ab | 0.52 ± 0.04 a |
| slc20 | 1.00 ± 0.09 a | 4.49 ± 0.40 b | 0.97 ± 0.08 a | 0.70 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Carrasco, A.; Mesas, C.; Doello, K.; Porres, J.M.; García-Beltrán, A.; Martínez, R.; Bermúdez, F.; Peña, M.; Melguizo, C.; Prados, J. The Antioxidant and Chemopreventive Activity of a Nutraceutical Derived from Brassicaceae Seed Extracts for Colorectal Cancer. Nutrients 2025, 17, 1358. https://doi.org/10.3390/nu17081358
Guzmán-Carrasco A, Mesas C, Doello K, Porres JM, García-Beltrán A, Martínez R, Bermúdez F, Peña M, Melguizo C, Prados J. The Antioxidant and Chemopreventive Activity of a Nutraceutical Derived from Brassicaceae Seed Extracts for Colorectal Cancer. Nutrients. 2025; 17(8):1358. https://doi.org/10.3390/nu17081358
Chicago/Turabian StyleGuzmán-Carrasco, Ana, Cristina Mesas, Kevin Doello, Jesús M. Porres, Alejandro García-Beltrán, Rosario Martínez, Francisco Bermúdez, Mercedes Peña, Consolación Melguizo, and Jose Prados. 2025. "The Antioxidant and Chemopreventive Activity of a Nutraceutical Derived from Brassicaceae Seed Extracts for Colorectal Cancer" Nutrients 17, no. 8: 1358. https://doi.org/10.3390/nu17081358
APA StyleGuzmán-Carrasco, A., Mesas, C., Doello, K., Porres, J. M., García-Beltrán, A., Martínez, R., Bermúdez, F., Peña, M., Melguizo, C., & Prados, J. (2025). The Antioxidant and Chemopreventive Activity of a Nutraceutical Derived from Brassicaceae Seed Extracts for Colorectal Cancer. Nutrients, 17(8), 1358. https://doi.org/10.3390/nu17081358








