The Impact of Macronutrient Intake on Sleep Quality in Female Endurance Athletes: A Pilot Observational Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Statistical Analysis
3. Results
3.1. Participants
3.2. Energy and Macronutrients
3.3. Sleep Parameters
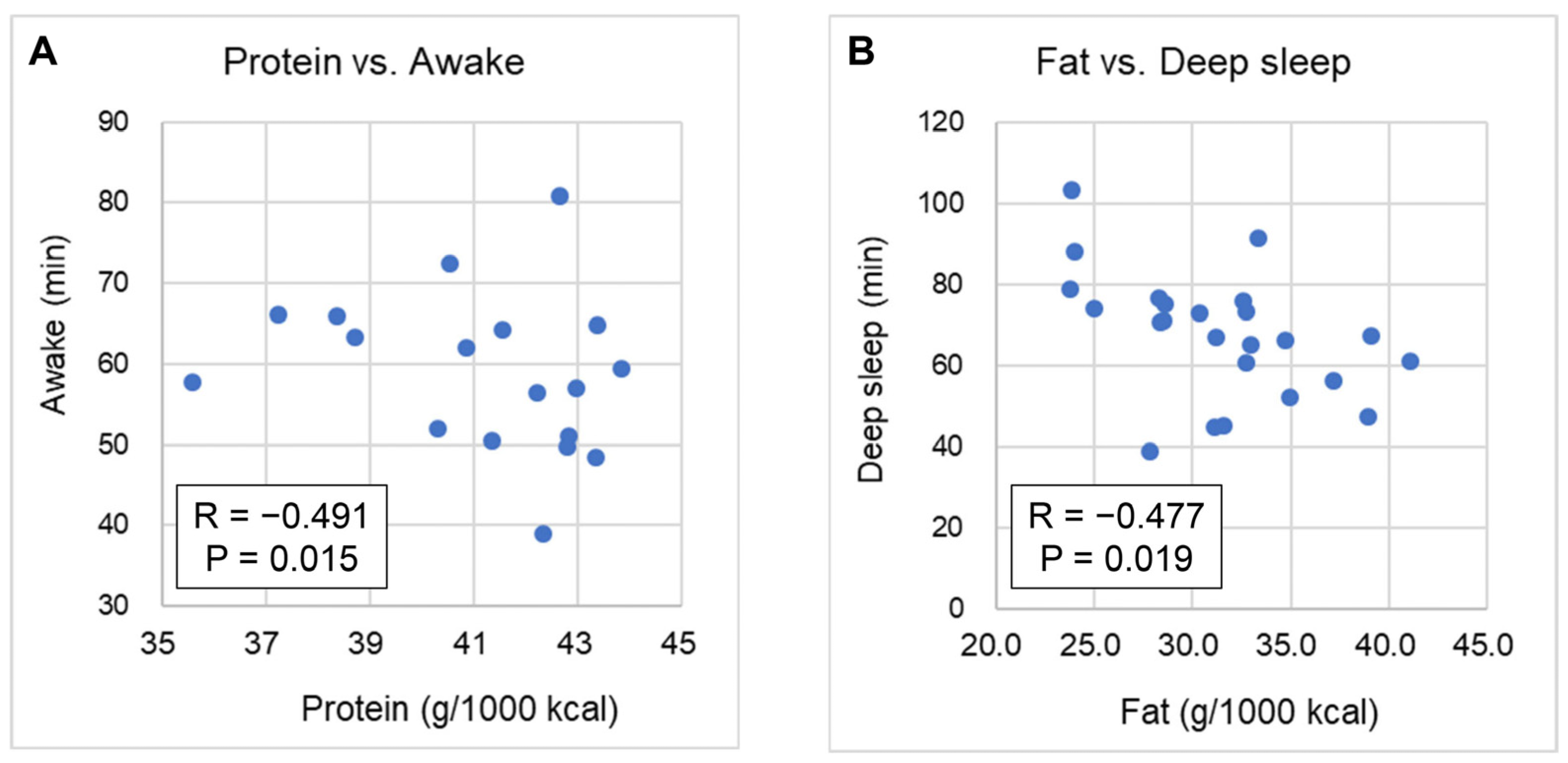
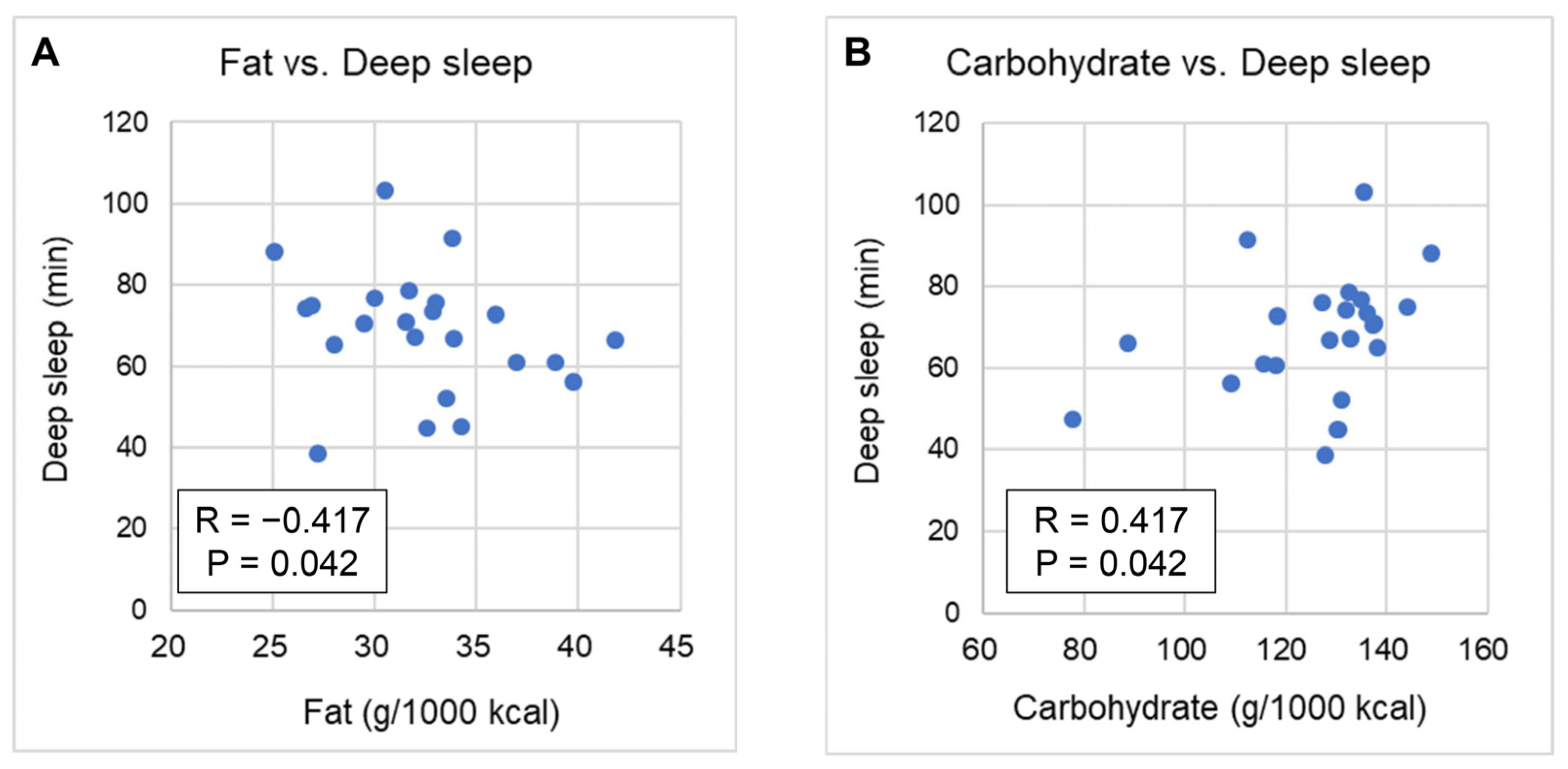
3.4. Correlation of Nutrient Intake with Sleep Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IOC | International Olympic Committee |
| LEA | Low energy availability |
| REM | Rapid eye movement |
| SOL | Sleep onset latency |
References
- Karklin, A.; Driver, H.S.; Buffenstein, R. Restricted Energy Intake Affects Nocturnal Body Temperature and Sleep Patterns. Am. J. Clin. Nutr. 1994, 59, 346–349. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; Roberts, A.; Shechter, A.; Choudhury, A.R. Fiber and Saturated Fat Are Associated with Sleep Arousals and Slow Wave Sleep. J. Clin. Sleep Med. 2016, 12, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Afaghi, A.; O’Connor, H.; Chow, C.M. High-Glycemic-Index Carbohydrate Meals Shorten Sleep Onset. Am. J. Clin. Nutr. 2007, 85, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Mountjoy, M.; Ackerman, K.E.; Bailey, D.M.; Burke, L.M.; Constantini, N.; Hackney, A.C.; Heikura, I.A.; Melin, A.; Pensgaard, A.M.; Stellingwerff, T.; et al. 2023 International Olympic Committee’s (IOC) Consensus Statement on Relative Energy Deficiency in Sport (REDs). Br. J. Sports Med. 2023, 57, 1073–1097. [Google Scholar] [CrossRef] [PubMed]
- Gallant, T.L.; Ong, L.F.; Wong, L.; Sparks, M.; Wilson, E.; Puglisi, J.L.; Gerriets, V.A. Low Energy Availability and Relative Energy Deficiency in Sport: A Systematic Review and Meta-Analysis. Sports Med. 2024, 55, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Suzuki, Y. Identifying and Analyzing Low Energy Availability in Athletes: The Role of Biomarkers and Red Blood Cell Turnover. Nutrients 2024, 16, 2273. [Google Scholar] [CrossRef] [PubMed]
- Saidi, O.; Souabni, M.; Del Sordo, G.C.; Maviel, C.; Peyrel, P.; Maso, F.; Vercruyssen, F.; Duché, P. Association between Low Energy Availability (LEA) and Impaired Sleep Quality in Young Rugby Players. Nutrients 2024, 16, 609. [Google Scholar] [CrossRef] [PubMed]
- Sim, A.; Burns, S.F. Review: Questionnaires as Measures for Low Energy Availability (LEA) and Relative Energy Deficiency in Sport (RED-S) in Athletes. J. Eat. Disord. 2021, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Graybeal, A.J.; Kreutzer, A.; Willis, J.L.; Braun-Trocchio, R.; Moss, K.; Shah, M. The Impact of Dieting Culture Is Different between Sexes in Endurance Athletes: A Cross-Sectional Analysis. BMC Sports Sci. Med. Rehabil. 2022, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Kasai, T.; Sakurama, Y.; Sekiguchi, A.; Kitamura, E.; Midorikawa, I.; Shiroshita, N.; Kawana, F.; Ogasawara, E.; Kitade, M.; et al. Evaluation of Sleep Parameters and Sleep Staging (Slow Wave Sleep) in Athletes by FitBit Alta HR, a Consumer Sleep Tracking Device. Nat. Sci. Sleep 2022, 14, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Kripke, D.F.; Naidoo, N.; Langer, R.D. Relationships among Dietary Nutrients and Subjective Sleep, Objective Sleep, and Napping in Women. Sleep Med. 2010, 11, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Sleep Symptoms Associated with Intake of Specific Dietary Nutrients. J. Sleep Res. 2014, 23, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Zuurbier, L.A.; de Jonge, E.; Voortman, T.; Jacques, P.F.; Lamon-Fava, S.; Scheer, F.A.J.L.; Kiefte-De Jong, J.C.; Hofman, A.; Ordovás, J.M.; et al. Actigraphic Sleep Fragmentation, Efficiency and Duration Associate with Dietary Intake in the Rotterdam Study. J. Sleep Res. 2016, 25, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Crispim, C.A.; Zimberg, I.Z.; dos Reis, B.G.; Diniz, R.M.; Tufik, S.; de Mello, M.T. Relationship between Food Intake and Sleep Pattern in Healthy Individuals. J. Clin. Sleep Med. 2011, 7, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Phillips, F.; Chen, C.N.; Crisp, A.H.; Koval, J.; McGuinness, B.; Kalucy, R.S.; Kalucy, E.C.; Lacey, J.H. Isocaloric Diet Changes and Electroencephalographic Sleep. Lancet 1975, 2, 723–725. [Google Scholar] [CrossRef] [PubMed]
- Lindseth, G.; Lindseth, P.; Thompson, M. Nutritional Effects on Sleep. West. J. Nurs. Res. 2013, 35, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Lindseth, G.; Murray, A. Dietary Macronutrients and Sleep. West. J. Nurs. Res. 2016, 38, 938–958. [Google Scholar] [CrossRef] [PubMed]
- Afaghi, A.; O’Connor, H.; Chow, C.M. Acute Effects of the Very Low Carbohydrate Diet on Sleep Indices. Nutr. Neurosci. 2008, 11, 146–154. [Google Scholar] [CrossRef] [PubMed]
| n = 24 | Mean/Median | SD/IQR | Min. | Max. | |
|---|---|---|---|---|---|
| Age | Years | 19.5 | 0.9 | 18 | 21 |
| Type of endurance sports | |||||
| Long-distance | n, % | 14 (58.3) | |||
| Middle-distance | n, % | 5 (20.8) | |||
| Racewalk | n, % | 5 (20.8) | |||
| Hours of practice per day | h | 3.3 | 0.6 | 2.5 | 4.5 |
| Height | cm | 161.9 | 4.9 | 152.3 | 170.0 |
| Body weight | kg | 51.1 | 5.4 | 40.9 | 61.3 |
| BMI | kg/m2 | 19.4 | 1.2 | 17.1 | 22.8 |
| Fat-free mass | kg | 43.8 | 4.0 | 38.6 | 50.4 |
| Fat | % | 14.2 † | 25.7 † | 5.6 | 17.8 |
| Dairy Intake | Breakfast (%) | Lunch (%) | Dinner (%) | Others (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nutrients | Mean/Median | SD/IQR | Min. | Max. | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Energy | kcal | 1983.0 † | 532.8 † | 1545.3 | 3396.1 | 30.7 | 4.2 | 30.5 | 4.9 | 35.1 | 4.8 | 3.7 | 4.4 |
| Protein | g 1 | 43.1 | 4.3 | 35.6 | 55.7 | 31.9 | 5.3 | 29.5 | 5.3 | 35.9 | 5.8 | 2.7 | 3.4 |
| % | 17.2 | 1.7 | 14.2 | 22.3 | |||||||||
| Fat | g 1 | 31.4 | 4.8 | 23.8 | 41.1 | 30.7 | 5.3 | 29.4 | 6.4 | 37.0 | 5.2 | 2.9 | 5.6 |
| % | 28.2 | 4.3 | 21.4 | 36.9 | |||||||||
| Carbohydrate | g 1 | 132.2 | 11.7 | 110.2 | 152.7 | 30.7 | 5.3 | 31.1 | 5.8 | 33.8 | 6.9 | 4.5 | 6.0 |
| % | 52.9 | 4.7 | 44.1 | 61.1 | |||||||||
| Conditions | Mean/Median | SD/IQR | Min. | Max. | |
|---|---|---|---|---|---|
| Dinner to bedtime | min/day | 222.6 | 41.7 | 127.0 | 321.0 |
| Duration in bed | min/day | 440.7 | 42.2 | 363.8 | 520.2 |
| Awake | min/day | 56.0 | 10.7 | 39.0 | 80.8 |
| % 1 | 14.9 † | 16.4 † | 9.8 | 40.7 | |
| Total sleep time | min/day | 384.6 | 39.1 | 306.0 | 462.2 |
| REM sleep | min/day | 73.9 | 23.5 | 35.7 | 123.3 |
| % 2 | 16.8 | 5.0 | 8.1 | 26.2 | |
| Light sleep | min/day | 243.1 | 31.8 | 188.8 | 307.5 |
| % 2 | 55.3 | 5.6 | 43.3 | 67.6 | |
| Deep sleep | min/day | 67.6 | 15.4 | 38.7 | 103.2 |
| % 2 | 15.3 | 2.9 | 9.8 | 20.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koikawa, N.; Minamino, Y.; Kawasaki, Y.; Kasai, T.; Suzuki, Y. The Impact of Macronutrient Intake on Sleep Quality in Female Endurance Athletes: A Pilot Observational Cross-Sectional Study. Nutrients 2025, 17, 1368. https://doi.org/10.3390/nu17081368
Koikawa N, Minamino Y, Kawasaki Y, Kasai T, Suzuki Y. The Impact of Macronutrient Intake on Sleep Quality in Female Endurance Athletes: A Pilot Observational Cross-Sectional Study. Nutrients. 2025; 17(8):1368. https://doi.org/10.3390/nu17081368
Chicago/Turabian StyleKoikawa, Natsue, Yume Minamino, Yu Kawasaki, Takatoshi Kasai, and Yoshio Suzuki. 2025. "The Impact of Macronutrient Intake on Sleep Quality in Female Endurance Athletes: A Pilot Observational Cross-Sectional Study" Nutrients 17, no. 8: 1368. https://doi.org/10.3390/nu17081368
APA StyleKoikawa, N., Minamino, Y., Kawasaki, Y., Kasai, T., & Suzuki, Y. (2025). The Impact of Macronutrient Intake on Sleep Quality in Female Endurance Athletes: A Pilot Observational Cross-Sectional Study. Nutrients, 17(8), 1368. https://doi.org/10.3390/nu17081368









