Selective Influence of Hemp Fiber Ingestion on Post-Exercise Gut Permeability: A Metabolomics-Based Analysis
Highlights
- Hemp hull fiber derived from the outer seed coat of hemp is rich in bioactives and insoluble fibers.
- Untargeted metabolomics revealed that the combination of consuming nutrient-rich hemp fiber bars and intensive exercise increased levels of beneficial metabolites, including those derived from the gut, in healthy cyclists.
- Intake of hemp hull fiber did not influence modest changes in gut permeability following 2.25 h of vigorous cycling.
- Taken together, these data indicate that the combination of consuming hemp hull fiber for two weeks with an acute 2.25 hour cycling had a selective effect on gut permeability and a significant influence on lipid-, bile acid-, and amino acid-related metabolic pathways.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
- 7:00 a.m.: Participants turned in the 3 d food record. A 30 mL blood sample was collected. Participants provided DOMS and POMS ratings and completed a 2-week retrospective symptom survey (with ratings of gastrointestinal symptoms, mental health, respiratory illness, sleep quality, pain symptoms, and overall well-being).
- 7:10 a.m.: Participants ingested one supplement bar with one cup of water.
- 7:30 a.m.: After a warm-up, participants cycled for 2.25 h at approximately 70% VO2max on their own bicycles fitted to Saris H3 direct drive smart trainers (Madison, WI, USA) with monitoring by the Zwift online training platform (Long Beach, CA, USA) and the Cosmed CPET metabolic cart (Rome, Italy). Heart rate, cycling speed, cadence, distance, and power were measured and recorded continuously during the 2.25 h bout. Metabolic parameters such as breathing rate, ventilation, and oxygen intake were measured after 15 min and then every 30 min during the cycling session. To ensure performance consistency between trials, performance data from the first trial was used to ensure a similar power and metabolic output during the second and third trials. Participants consumed 3 mL/kg of water every 15 min. No other beverage or food containing energy or nutrients was allowed during the 2.25 h cycling sessions.
- 3 h post-exercise period: Participants ingested 450 mL of SS within the 1st minute of getting off the bicycle, and urine was collected for the next 5 h. Blood samples were collected immediately after completing the cycling session, and then 1.5 h and 3.0 h post-exercise. Participants were allowed to shower and change their clothes. The DOMS and POMS questionnaires were administered each time blood samples were collected. No food or beverage other than water (7 mL/kg) was ingested during the first 1.5 h post-exercise. After the 1.5 h post-exercise blood draw, participants ingested a fortified nutrient beverage (Boost, Nestlé S.A., Vevey, Switzerland). Another blood sample was collected 3 h post-exercise. Afterwards, participants were allowed to stay in the lab to complete the 5 h urine collection or leave the lab and return later in the day to turn in the 5 h urine container.
2.3. Sample Analysis
2.3.1. Urine Sugar Analysis
2.3.2. Plasma Untargeted Metabolomics Analysis and Statistical Procedures
2.4. Additional Statistical Procedures
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, F.M.; Petriz, B.; Marques, G.; Kamilla, L.H.; Franco, O.L. Is there an exercise-intensity threshold capable of avoiding the leaky gut? Front. Nutr. 2021, 8, 627289. [Google Scholar] [CrossRef]
- Keirns, B.H.; Koemel, N.A.; Sciarrillo, C.M.; Anderson, K.L.; Emerson, S.R. Exercise and intestinal permeability: Another form of exercise-induced hormesis? Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G512–G518. [Google Scholar] [CrossRef]
- Chantler, S.; Griffiths, A.; Matu, J.; Davison, G.; Holliday, A.; Jones, B. A systematic review: Role of dietary supplements on markers of exercise-associated gut damage and permeability. PLoS ONE 2022, 17, e0266379. [Google Scholar] [CrossRef]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk-Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. A systematic review of the influence of bovine colostrum supplementation on leaky gut syndrome in athletes: Diagnostic biomarkers and future directions. Nutrients 2022, 14, 2512. [Google Scholar] [CrossRef]
- Tataka, Y.; Haramura, M.; Hamada, Y.; Ono, M.; Toyoda, S.; Yamada, T.; Hiratsu, A.; Suzuki, K.; Miyashita, M. Effects of oral cystine and glutamine on exercise-induced changes in gastrointestinal permeability and damage markers in young men. Eur. J. Nutr. 2022, 61, 2331–2339. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Chen, G.Y.; Zhang, Q.; Sha, W.; Kay, C.D.; Chandra, P.; Kay, K.L.; Lila, M.A. Blueberry and/or banana consumption mitigate arachidonic, cytochrome p450 oxylipin generation during recovery from 75-Km cycling: A randomized trial. Front. Nutr. 2020, 7, 121. [Google Scholar] [CrossRef]
- Nieman, D.C.; Kay, C.D.; Rathore, A.S.; Grace, M.H.; Strauch, R.C.; Stephan, E.H.; Sakaguchi, C.A.; Lila, M.A. Increased plasma levels of gut-derived phenolics linked to walking and running following two weeks of flavonoid supplementation. Nutrients 2018, 10, 1718. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Knab, A.M.; Shanely, R.A.; Pappan, K.L.; Jin, F.; Lila, M.A. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: A randomized trial using a metabolomics approach. PLoS ONE 2013, 8, e72215. [Google Scholar] [CrossRef]
- Flores Martinez, K.E.; Bloszies, C.S.; Bolino, M.J.; Henrick, B.M.; Frese, S.A. Hemp hull fiber and two constituent compounds, N-trans-caffeoyltyramine and N-trans-feruloyltyramine, shape the human gut microbiome in vitro. Food Chem. X 2024, 23, 101611. [Google Scholar] [CrossRef]
- van Klinken, B.J.; Stewart, M.L.; Kalgaonkar, S.; Chae, L. Health-promoting opportunities of hemp hull: The potential of bioactive compounds. J. Diet. Suppl. 2024, 21, 543–557. [Google Scholar] [CrossRef]
- Bolster, D.; Chae, L.; van Klinken, J.W.; Kalgaonkar, S. Impact of selected novel plant bioactives on improvement of impaired gut barrier function using human primary cell intestinal epithelium. J. Food Bioact. 2022, 20, 11–16. [Google Scholar] [CrossRef]
- Lee, S.H.; Veeriah, V.; Levine, F. A potent HNF4α agonist reveals that HNF4α controls genes important in inflammatory bowel disease and Paneth cells. PLoS ONE 2022, 17, e0266066. [Google Scholar] [CrossRef]
- Smith, L.L.; Brunetz, M.H.; Chenier, T.C.; McCammon, M.R.; Houmard, J.A.; Franklin, M.E.; Israel, R.G. The effects of static and ballistic stretching on delayed onset muscle soreness and creatine kinase. Res. Q. Exerc. Sport. 1993, 64, 103–107. [Google Scholar] [CrossRef]
- Curran, S.L.; Andrykowski, M.A.; Studts, J.L. Short form of the Profile of Mood States (POMS-SF): Psychometric information. Psychol. Assess. 1995, 7, 80–83. [Google Scholar] [CrossRef]
- Khoshbin, K.; Khanna, L.; Maselli, D.; Atieh, J.; Breen-Lyles, M.; Arndt, K.; Rhoten, D.; Dyer, R.B.; Singh, R.J.; Nayar, S.; et al. Development and validation of test for “leaky gut” small intestinal and colonic permeability using sugars in healthy adults. Gastroenterology 2021, 161, 463–475.e13. [Google Scholar] [CrossRef]
- Larkey, N.E.; Fatica, E.M.; Singh, R.J. Detection of 13C-mannitol and other saccharides using tandem mass spectrometry for evaluation of intestinal permeability or leaky gut. Methods Mol. Biol. 2022, 2546, 285–294. [Google Scholar] [CrossRef]
- Pathmasiri, W.; Rushing, B.R.; McRitchie, S.; Choudhari, M.; Du, X.; Smirnov, A.; Pelleigrini, M.; Thompson, M.J.; Sakaguchi, C.A.; Nieman, D.C.; et al. Untargeted metabolomics reveal signatures of a healthy lifestyle. Sci. Rep. 2024, 14, 13630. [Google Scholar] [CrossRef]
- Li, Y.Y.; Ghanbari, R.; Pathmasiri, W.; McRitchie, S.; Poustchi, H.; Shayanrad, A.; Roshandel, G.; Etemadi, A.; Pollock, J.D.; Malekzadeh, R.; et al. Untargeted metabolomics: Biochemical perturbations in golestan cohort study opium users inform intervention strategies. Front. Nutr. 2020, 7, 584585. [Google Scholar] [CrossRef]
- Ghanbari, R.; Li, Y.; Pathmasiri, W.; McRitchie, S.; Etemadi, A.; Pollock, J.D.; Poustchi, H.; Rahimi-Movaghar, A.; Amin-Esmaeili, M.; Roshandel, G.; et al. Metabolomics reveals biomarkers of opioid use disorder. Transl. Psychiatry 2021, 11, 103. [Google Scholar] [CrossRef]
- Lynch, D.H.; Rushing, B.R.; Pathmasiri, W.; McRitchie, S.; Batchek, D.J.; Petersen, C.L.; Gross, D.C.; Sumner, S.C.J.; Batsis, J.A. Baseline serum biomarkers predict response to a weight loss intervention in older adults with obesity: A pilot study. Metabolites 2023, 13, 853. [Google Scholar] [CrossRef]
- Sun, J.; Xia, Y. Pretreating and normalizing metabolomics data for statistical analysis. Genes Dis. 2023, 11, 100979. [Google Scholar] [CrossRef]
- Välikangas, T.; Suomi, T.; Elo, L.L. A systematic evaluation of normalization methods in quantitative label-free proteomics. Brief. Bioinform. 2018, 19, 1–11. [Google Scholar] [CrossRef]
- Smirnov, A.; Liao, Y.; Fahy, E.; Subramaniam, S.; Du, X. ADAP-KDB: A spectral knowledgebase for tracking and prioritizing unknown GC-MS spectra in the NIH’s Metabolomics Data Repository. Anal. Chem. 2021, 93, 12213–12220. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Wu, G.; Wang, X.; Zhang, Y.; Jiang, N. Structure-based identification of HNF4α agonists: Rosmarinic acid as a promising candidate for NAFLD treatment. Comput. Struct. Biotechnol. J. 2024, 27, 171–183. [Google Scholar] [CrossRef]
- Deehan, E.C.; Mocanu, V.; Madsen, K.L. Effects of dietary fibre on metabolic health and obesity. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 301–318. [Google Scholar] [CrossRef]
- Mocanu, V.; Madsen, K.L. Dietary fibre and metabolic health: A clinical primer. Clin. Transl. Med. 2024, 14, e70018. [Google Scholar] [CrossRef]
- Wang, Z.; Peters, B.A.; Yu, B.; Grove, M.L.; Wang, T.; Xue, X.; Thyagarajan, B.; Daviglus, M.L.; Boerwinkle, E.; Hu, G.; et al. Gut microbiota and blood metabolites related to fiber intake and type 2 diabetes. Circ. Res. 2024, 134, 842–854. [Google Scholar] [CrossRef]
- Myhrstad, M.C.W.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary fiber, gut microbiota, and metabolic regulation-current status in human randomized trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- Mahalak, K.K.; Liu, L.; Bobokalonov, J.; Narrowe, A.B.; Firrman, J.; Bittinger, K.; Hu, W.; Jones, S.M.; Moustafa, A.M. Supplementation with soluble or insoluble rice-bran fibers increases short-chain fatty acid producing bacteria in the gut microbiota in vitro. Front. Nutr. 2024, 11, 1304045. [Google Scholar] [CrossRef]
- Vinelli, V.; Biscotti, P.; Martini, D.; Del Bo’, C.; Marino, M.; Meroño, T.; Nikoloudaki, O.; Calabrese, F.M.; Turroni, S.; Taverniti, V.; et al. Effects of dietary fibers on short-chain fatty acids and gut microbiota composition in healthy adults: A systematic review. Nutrients 2022, 14, 2559. [Google Scholar] [CrossRef]
- Fernández, M.A.; García, M.D.; Sáenz, M.T. Antibacterial activity of the phenolic acids fractions of Scrophularia frutescens and Scrophularia sambucifolia. J. Ethnopharmacol. 1996, 53, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Sommella, E.; Salviati, E.; Campiglia, P.; Ganguli, K.; Djebali, K.; Zhu, W.; Walker, W.A. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr. Res. 2020, 88, 209–217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alonso-Esteban, J.I.; Pinela, J.; Ćirić, A.; Calhelha, R.C.; Soković, M.; Ferreira, I.C.F.R.; Barros, L.; Torija-Isasa, E.; Sánchez-Mata, M.C. Chemical composition and biological activities of whole and dehulled hemp (Cannabis sativa L.) seeds. Food Chem. 2022, 374, 131754. [Google Scholar] [CrossRef]
- van Wijck, K.; Lenaerts, K.; van Loon, L.J.; Peters, W.H.; Buurman, W.A.; Dejong, C.H. Exercise-induced splanchnic hypoperfusion results in gut dysfunction in healthy men. PLoS ONE 2011, 6, e22366. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Shu, L.Z.; Ding, Y.D.; Xue, Q.M.; Cai, W.; Deng, H. Direct and indirect effects of pathogenic bacteria on the integrity of intestinal barrier. Ther. Adv. Gastroenterol. 2023, 16, 17562848231176427. [Google Scholar] [CrossRef]
- Peters, H.P.; Bos, M.; Seebregts, L.; Akkermans, L.M.; van Berge Henegouwen, G.P.; Bol, E.; Mosterd, W.L.; de Vries, W.R. Gastrointestinal symptoms in long-distance runners, cyclists, and triathletes: Prevalence, medication, and etiology. Am. J. Gastroenterol. 1999, 94, 1570–1581. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Dumke, C.L.; Oley, K.; McAnulty, S.R.; Davis, J.M.; Murphy, E.A.; Utter, A.C.; Lind, R.H.; McAnulty, L.S.; et al. Ibuprofen use, endotoxemia, inflammation, and plasma cytokines during ultramarathon competition. Brain Behav. Immun. 2006, 20, 578–584. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Quaresma, M.V.L.D.S.; Mancin, L.; Paoli, A.; Mota, J.F. The interplay between gut microbiome and physical exercise in athletes. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 428–433. [Google Scholar] [CrossRef]
- Nolte, S.; Krüger, K.; Lenz, C.; Zentgraf, K. Optimizing the Gut Microbiota for Individualized Performance Development in Elite Athletes. Biology 2023, 12, 1491. [Google Scholar] [CrossRef]
- Han, M.; Yang, K.; Yang, P.; Zhong, C.; Chen, C.; Wang, S.; Lu, Q.; Ning, K. Stratification of athletes’ gut microbiota: The multifaceted hubs associated with dietary factors, physical characteristics and performance. Gut Microbes 2020, 12, 1–18. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols-gut microbiota interrelationship: A transition to a new generation of prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]


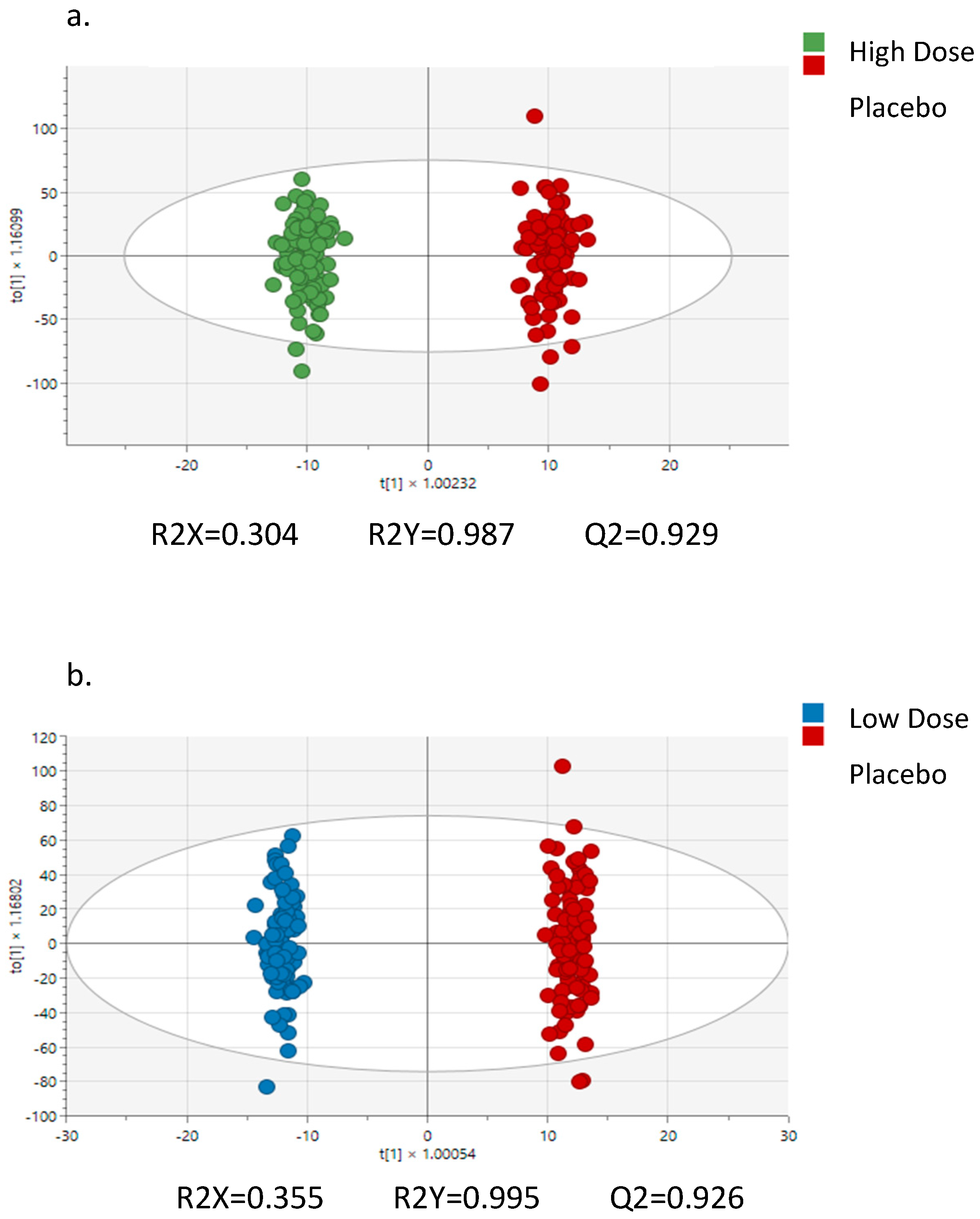
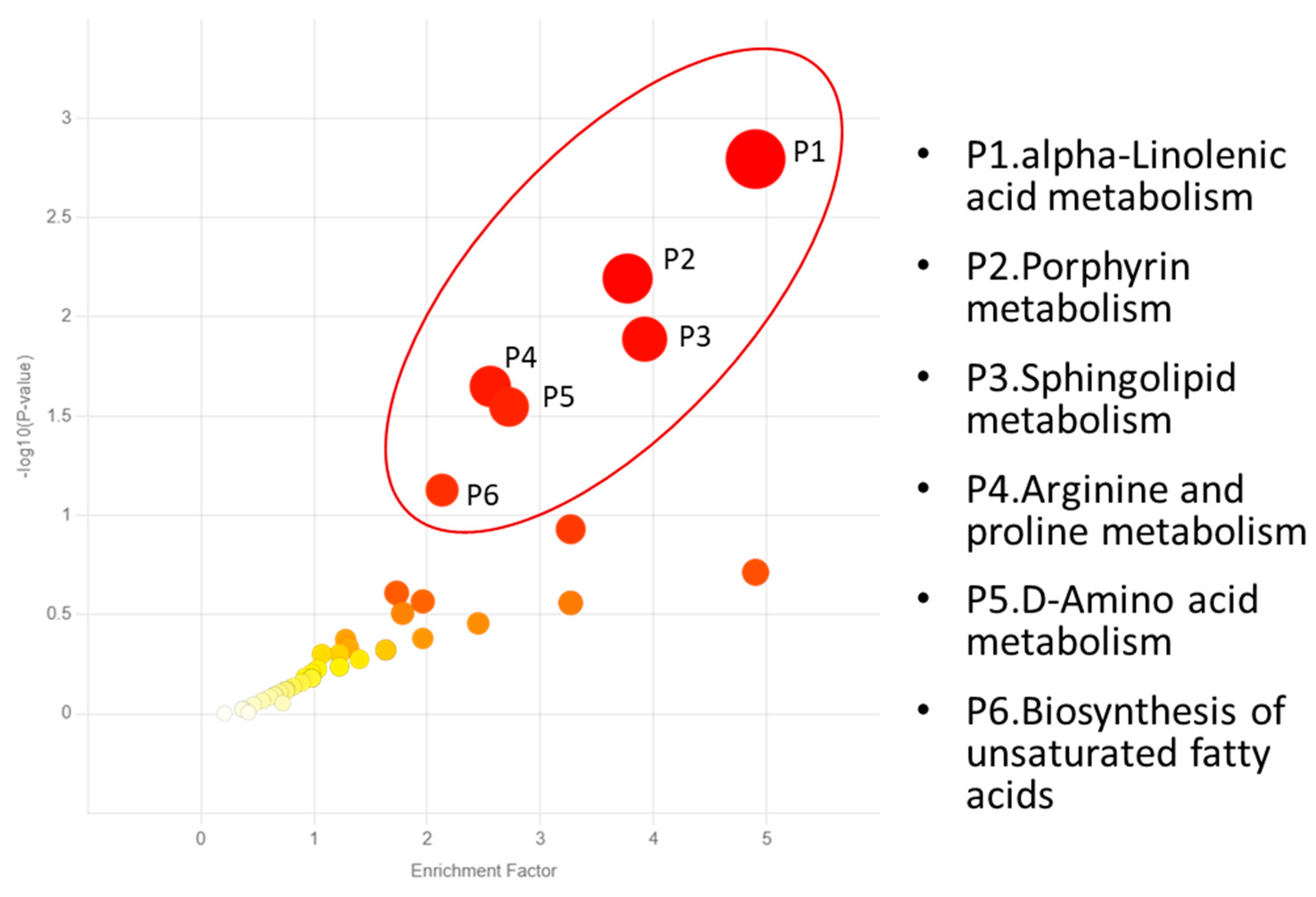
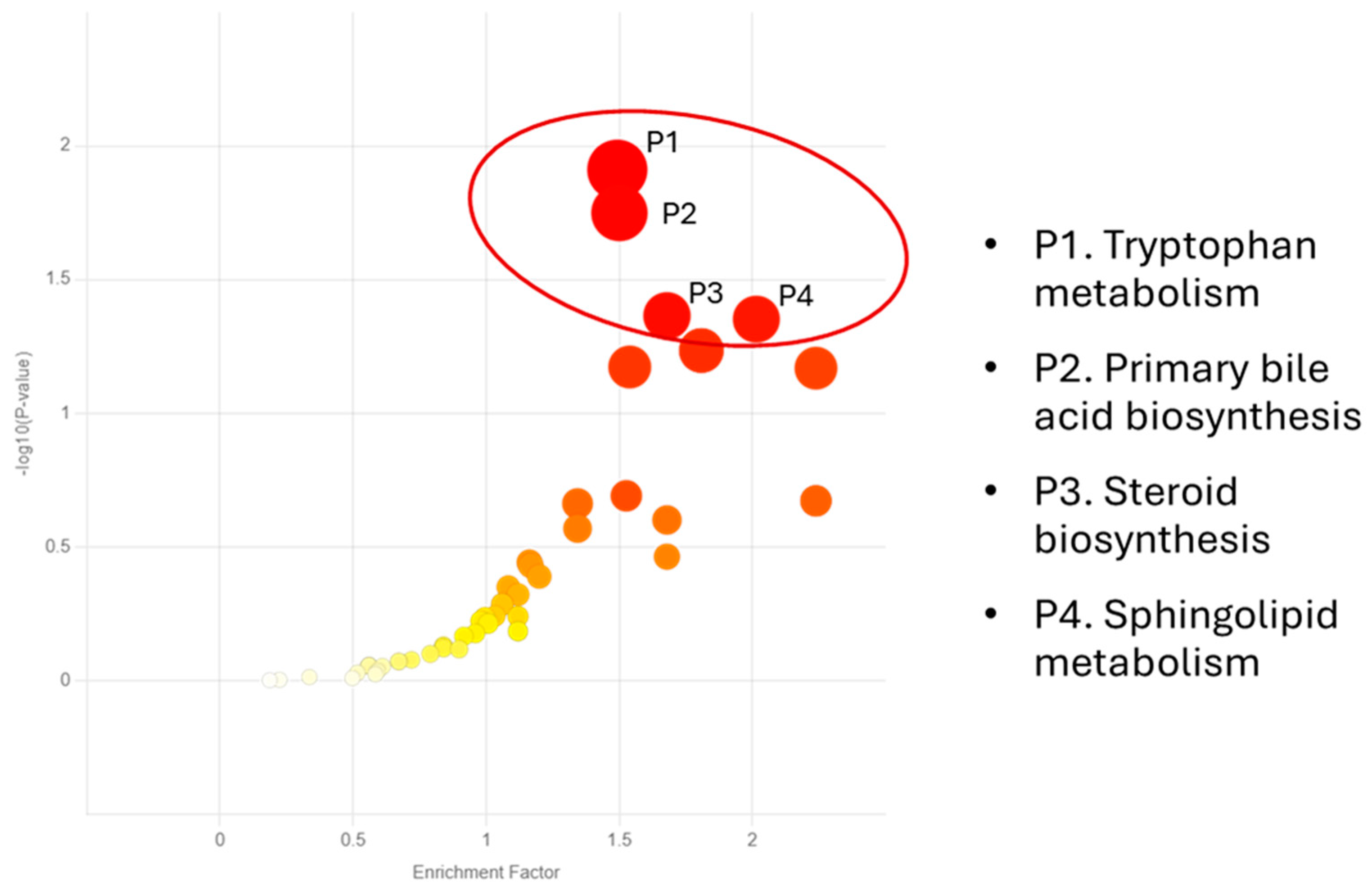
| Ingredient Name | High-Dose Weight (g) | Low-Dose Weight (g) | Placebo Weight (g) |
|---|---|---|---|
| Slurry Binder | 27.45 | 27.45 | 0 |
| Hemp Hull Powder | 10.00 | 2.50 | 0 |
| Colorant | 0.05 | 0.10 | 0.30 |
| Sunflower oil | 1.00 | 1.00 | 1.00 |
| Plain Rice Crisps | 11.50 | 11.50 | 11.50 |
| Milk powder blend | 0 | 7.45 | 9.75 |
| Tapioca and rice flour binder | 0 | 0 | 27.45 |
| Total weight (g) | 50.00 | 50.00 | 50.00 |
| Kilocalories | 182 | 192 | 189 |
| % carbohydrate | 84 | 77 | 75 |
| % fat | 10 | 16 | 18 |
| % protein | 5 | 7 | 7 |
| Sex | Mean ± SE | |
|---|---|---|
| Age (yrs) | M | 45.5 ± 2.2 |
| F | 46.5 ± 4.1 | |
| Weight (kg) | M | 79.2 ± 2.1 * |
| F | 61.7 ± 2.9 | |
| Height (cm) | M | 181 ± 1.4 * |
| F | 165 ± 0.6 | |
| BMI (kg/m2) | M | 24.2 ± 0.5 |
| F | 22.6 ± 1.1 | |
| Body fat (%) | M | 19.4 ± 1.5 * |
| F | 28.0 ± 2.0 | |
| V02max (ml.kg−1min−1) | M | 43.5 ± 1.7 * |
| F | 34.5 ± 3.0 | |
| Max watts | M | 265 ± 10.9 * |
| F | 167 ± 19.0 | |
| Max heart rate (beats/min) | M | 172 ± 2.7 |
| F | 171 ± 5.5 | |
| Max ventilation (L/min) | M | 128 ± 6.0 * |
| F | 79.0 ± 7.5 | |
| Max respiratory rate (breaths/min) | M | 47.1 ± 2.0 |
| F | 40.0 ± 1.5 |
| Performance Measurement Supplement | Mean ± SE | |
|---|---|---|
| Cycling power (watts, % maximum) | High-dose hemp | 138 ± 9.3 (57.2 ± 1.8% max) |
| Low-dose hemp | 138 ± 8.6 (57.6 ± 1.4% max) | |
| Placebo | 137 ± 8.6 (57.1 ± 1.3% max) | |
| Heart rate (beats/min, % maximum) | High-dose hemp | 134 ± 3.4 (78.4 ± 1.5% max) |
| Low-dose hemp | 133 ± 3.1 (77.5 ± 1.4% max) | |
| Placebo | 133 ± 3.6 (77.5 ± 1.8% max) | |
| Oxygen consumption (VO2) (mL·kg−1min−1, % maximum) | High-dose hemp | 30.1 ± 1.1 (73.8 ± 1.5% max) |
| Low-dose hemp | 29.9 ± 1.2 (73.1 ± 1.5% max) | |
| Placebo | 29.5 ± 1.2 (72.2 ± 1.5% max) | |
| Distance cycled (km) | High-dose hemp | 66.1 ± 2.1 |
| Low-dose hemp | 66.2 ± 1.6 | |
| Placebo | 65.8 ± 1.8 | |
| Speed (km/h) | High-dose hemp | 29.0 ± 0.9 |
| Low-dose hemp | 29.3 ± 0.7 | |
| Placebo | 28.6 ± 0.8 | |
| High-Dose Hemp | Low-Dose Hemp | Placebo | Time and Interaction Effects, p-Values | ||||
|---|---|---|---|---|---|---|---|
| Pre-Suppl. | Post-Exerc. | Pre-Suppl. | Post-Exerc. | Pre-Suppl. | Post-Exerc. | ||
| L:C13M | 0.518 ± 0.051 | 0.780 ± 0.230 | 0.450 ± 0.028 | 0.613 ± 0.077 | 0.498 ± 0.039 | 0.490 ± 0.028 | 0.108; 0.195 |
| L:C12M | 0.031 ± 0.003 | 0.053 ± 0.002 | 0.027 ± 0.002 | 0.037 ± 0.004 | 0.033 ± 0.003 | 0.031 ± 0.002 | 0.070; 0.172 |
| VIP | Metabolites | Description |
|---|---|---|
| 2.3 | Uridine | A pyrimidine nucleoside involved with many biological processes, including RNA, glycogen, and biomembrane synthesis. |
| 2.0 | Linoleic acid | An essential polyunsaturated fatty acid (18:2ω6). |
| 1.9 | Uric acid | Chemical created when purines are metabolized. Uric acid is a significant antioxidant in the human body. |
| 1.9 | Valyl-Serine | Dipeptide formed from L-valine and L-serine residues. Incomplete breakdown product of protein digestion or protein catabolism. |
| 1.9 | O-Cresol | A derivative of phenol and an isomer of p-cresol and m-cresol. Phenol is primarily used to synthesize plastics and related materials. |
| 1.9 | 3-hydroxy-4-methoxybenzoic acid | A plant metabolite (isovanillic acid) with antibacterial properties. |
| 1.8 | Stearidonic acid | A plant-based omega-3 fatty acid (18:3 n-3) that increases the levels of long-chain omega-3 PUFAs such as EPA. |
| 1.8 | Glycerophosphocholine | A choline derivative involved in multiple brain functions. |
| 1.8 | Creatinine | An endogenous product of muscle metabolism. |
| 1.8 | 2-Aminoheptanoate | An alpha amino acid. May enhance the effect of ketones as fuel to the Krebs cycle in the brain. |
| 1.8 | Quinaldic acid | A kynurenine metabolite. |
| 1.7 | Phenylacetylglutamine | A novel metabolite derived from gut microbial metabolism of dietary proteins, specifically phenylalanine, which may be linked to risks of adverse cardiovascular events. |
| 1.7 | Indolelactic acid | Formed primarily from gut bacterial metabolism of tryptophan. Functions as an anti-inflammatory molecule. |
| 1.5 | Serotonin | Made from tryptophan, an essential amino acid. A chemical messenger that affects mood, sleep, and digestion. |
| 1.5 | N-Butyrylglycine | An acyl glycine that is a minor metabolite of fatty acids. |
| 1.5 | Cytidine | A pyrimidine nucleoside that serves as a precursor for uridine and is involved in RNA synthesis. |
| 1.5 | S-Allylcysteine | An organosulfur compound that exhibits antioxidant, anti-inflammatory, and redox modulatory activities |
| 1.5 | Adipoyl-L-carnitine | An acylcarnitine. |
| 1.5 | 12,13-DiHOME | An inflammatory oxylipin. |
| 1.5 | N-Acetylmethionine | A derivative of methionine. |
| 1.5 | Calcifediol | The precursor for calcitriol, the active form of vitamin D. |
| 1.4 | 5-Hydroxytryptophan | Metabolite of tryptophan and the immediate precursor of the neurotransmitter serotonin. |
| 1.4 | 4-Methoxycinnamic acid | A methyl derivative of ferulic acid that has been found in hemp fibers. |
| 1.4 | Proline | An amino acid important in protein synthesis, nutrition metabolism, wound healing and immunity, and antioxidative reactions. |
| 1.4 | 5-Aminolevulinic acid | An amino acid that is the first compound in the porphyrin synthesis pathway leading to heme. |
| 1.4 | Procyanidin B1 | A flavonoid group of condensed flavan-3-ols that can be found in many plants. |
| Pathway. | Kegg ID and Metabolites |
|---|---|
| P1. Alpha-linolenic acid metabolism | C06427 Alpha-linolenic acid C16300 Stearidonic acid |
| P2. Porphyrin metabolism | C00931 Porphobilinogen C00430 5 Aminolevulinic acid C00486 Bilirubin |
| P3. Sphingolipid metabolism | C06124 Sphingosine 1-phosphate C00836 Sphinganine C00319 Sphingosine |
| P4. Arginine and proline metabolism | C00555 4-Aminobutyraldehyde C05147 Trans-3-hydroxy-L-proline C00763 D-Proline C01157 4-Hydroxyproline C01165 L-Glutamic gamma-semialdehyde C00077 Ornithine C03564 1-Pyrroline-2-carboxylic acid C00022 Pyruvic acid C03912 1-Pyrroline-5-carboxylic acid |
| P5. D-Amino acid metabolism | C00819 D-Glutamine C00515 D-Ornithine C00763 D-Proline C03440 cis-4-Hydroxy-D-proline C01110 5-Amino-2-oxopentanoic acid C03564 1-Pyrroline-2-carboxylic acid |
| P6. Biosynthesis of unsaturated fatty acids | C00712 Oleic acid C01595 Linoleic acid C00219 Arachidonic acid C06426 gamma-Linolenic acid C06428 Eicosapentaenoic acid C06427 alpha-Linolenic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieman, D.C.; Sakaguchi, C.A.; Williams, J.C.; Pathmasiri, W.; Rushing, B.R.; McRitchie, S.; Sumner, S.J. Selective Influence of Hemp Fiber Ingestion on Post-Exercise Gut Permeability: A Metabolomics-Based Analysis. Nutrients 2025, 17, 1384. https://doi.org/10.3390/nu17081384
Nieman DC, Sakaguchi CA, Williams JC, Pathmasiri W, Rushing BR, McRitchie S, Sumner SJ. Selective Influence of Hemp Fiber Ingestion on Post-Exercise Gut Permeability: A Metabolomics-Based Analysis. Nutrients. 2025; 17(8):1384. https://doi.org/10.3390/nu17081384
Chicago/Turabian StyleNieman, David C., Camila A. Sakaguchi, James C. Williams, Wimal Pathmasiri, Blake R. Rushing, Susan McRitchie, and Susan J. Sumner. 2025. "Selective Influence of Hemp Fiber Ingestion on Post-Exercise Gut Permeability: A Metabolomics-Based Analysis" Nutrients 17, no. 8: 1384. https://doi.org/10.3390/nu17081384
APA StyleNieman, D. C., Sakaguchi, C. A., Williams, J. C., Pathmasiri, W., Rushing, B. R., McRitchie, S., & Sumner, S. J. (2025). Selective Influence of Hemp Fiber Ingestion on Post-Exercise Gut Permeability: A Metabolomics-Based Analysis. Nutrients, 17(8), 1384. https://doi.org/10.3390/nu17081384










