The Combined Effect of High-Intensity Interval Training and Time-Restricted Feeding on the AKT-IGF-1-mTOR Signaling Pathway in the Muscle Tissue of Type 2 Diabetic Rats
Abstract
1. Introduction
2. Materials and Methods
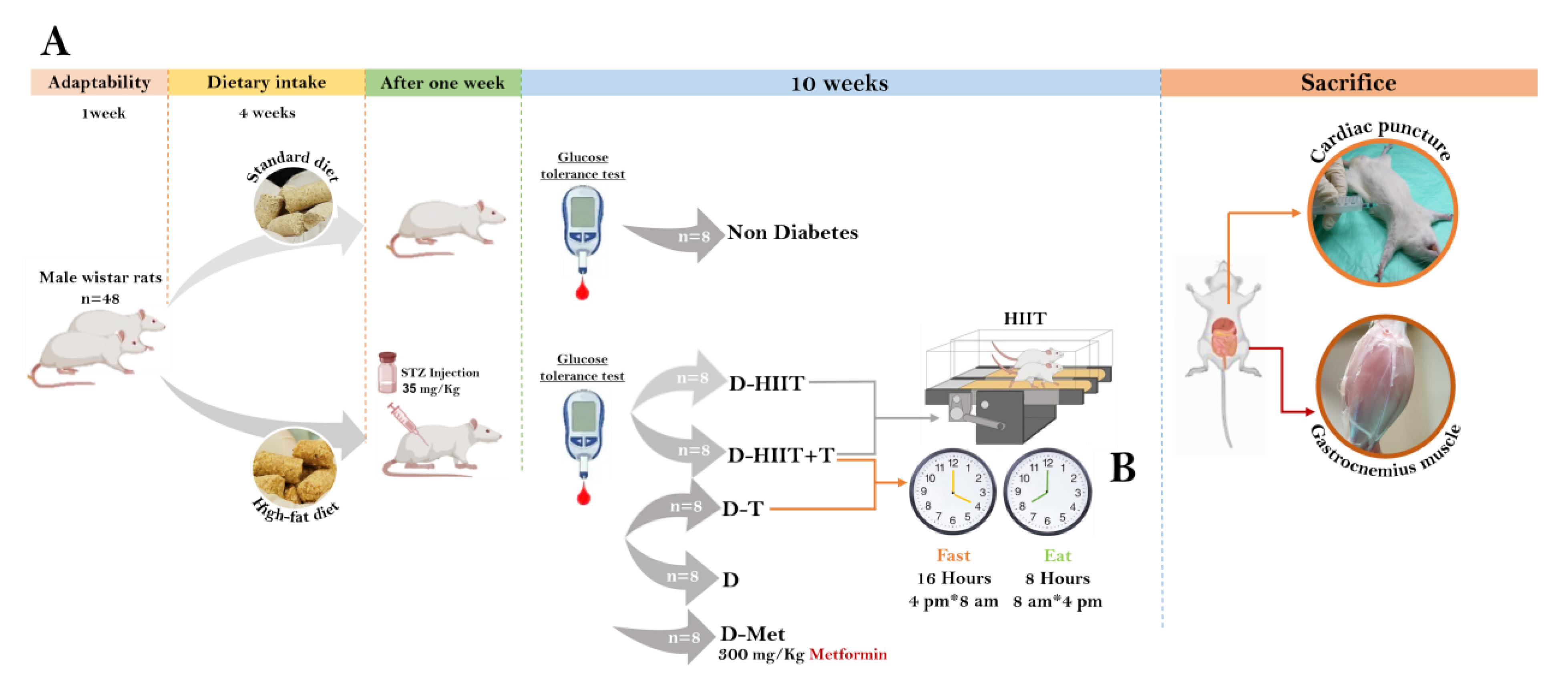
2.1. Animals
2.2. Diabetes Induction
2.3. Processes and Protocols
2.3.1. Adaptation Process
2.3.2. The HIIT Protocol
2.3.3. IF Protocol
2.4. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.5. Muscle Tissue Preparation
2.6. Western Blot
2.7. Tissue Sectioning and Staining
2.8. Frozen Section Preparation and Sudan Black-B Staining
2.9. Biochemical Parameters
2.10. Statistical Analysis
3. Results
3.1. Intraperitoneal Glucose Tolerance Tests (IPGTT) in the Experimental Groups
3.2. Serum Blood Glucose, Insulin, HOMA-IR
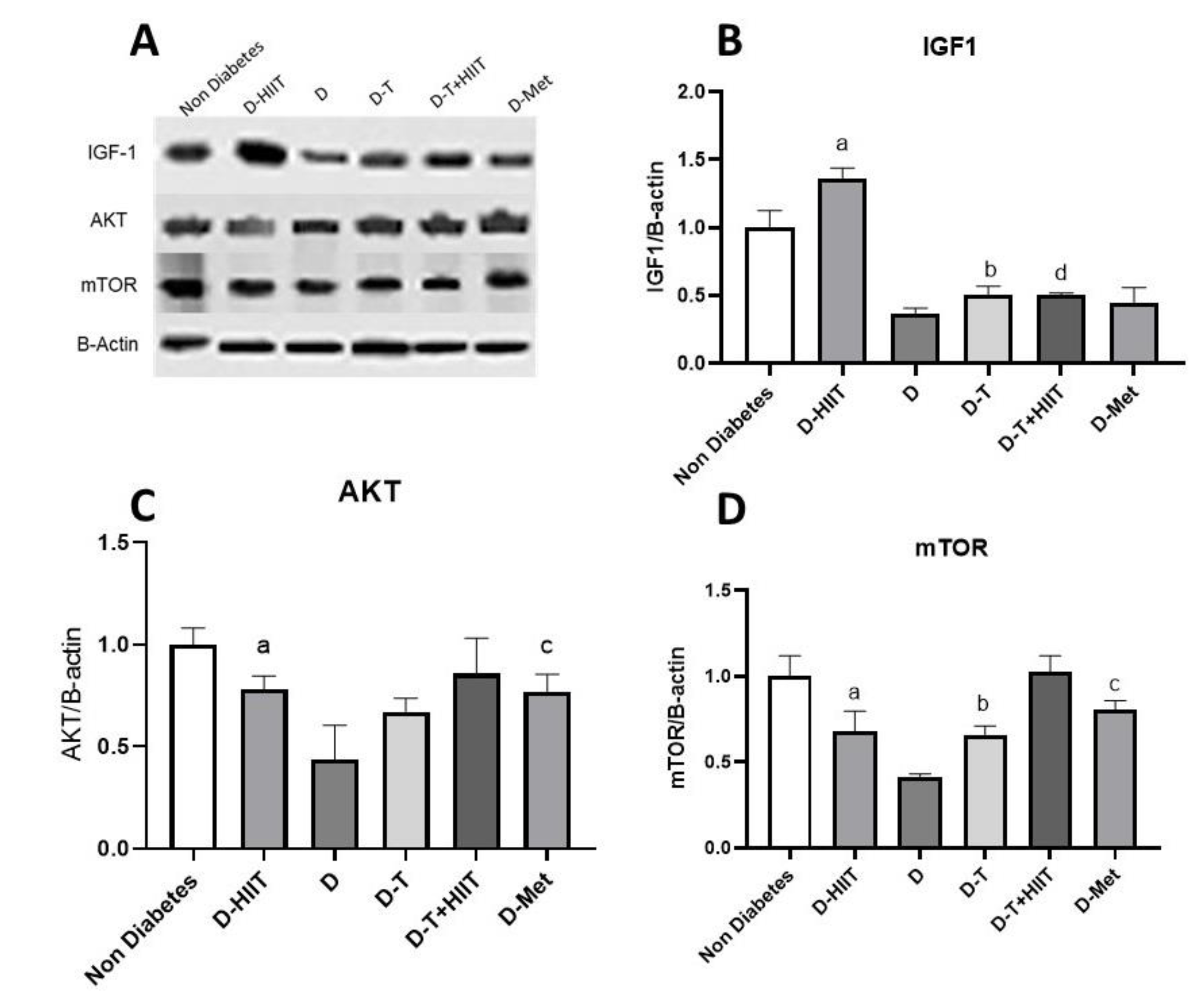
3.3. Expressions of IGF-1, AKT, mTOR
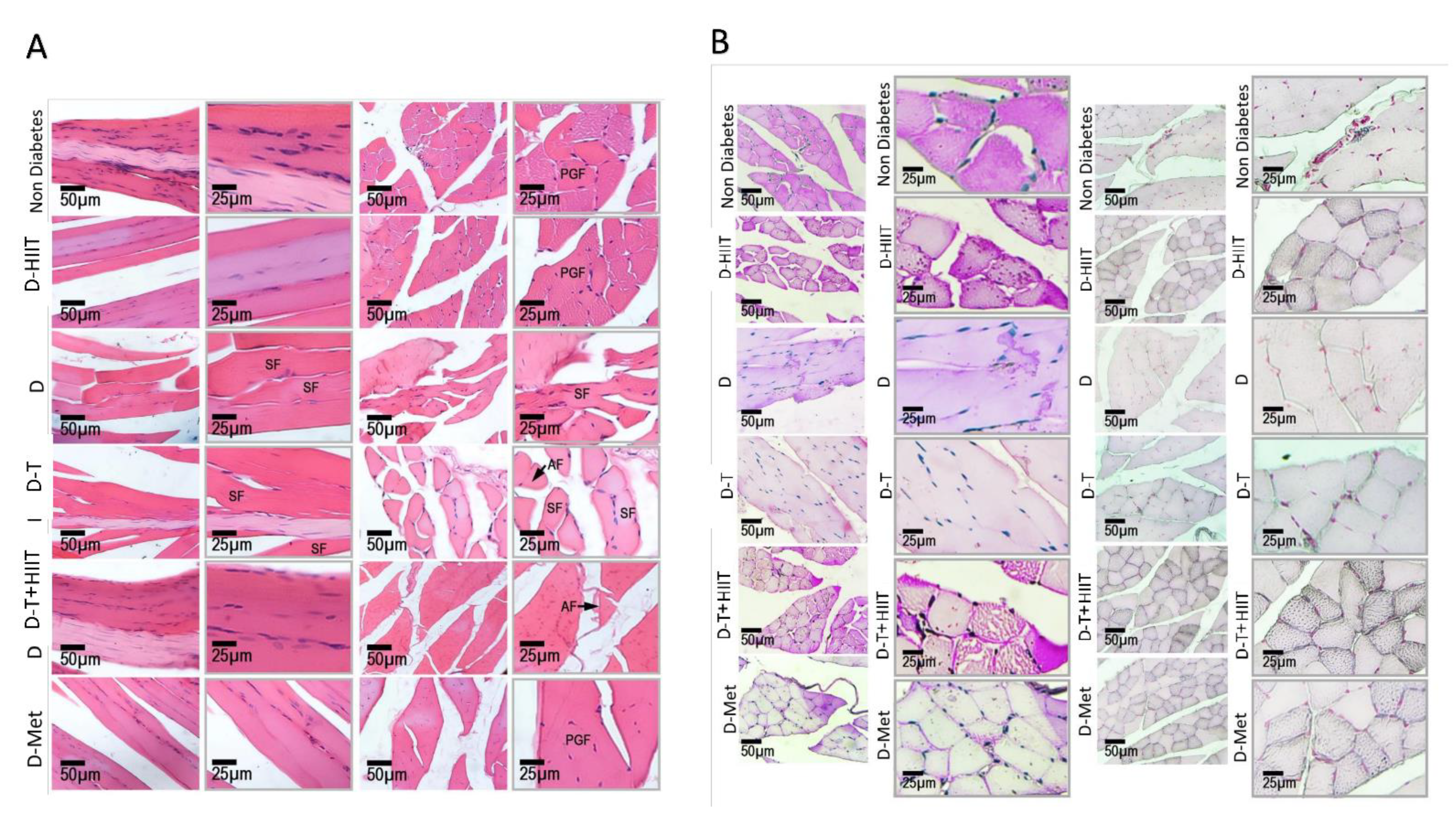
3.4. Histological Changes of the Muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergman, B.C.; Goodpaster, B.H. Exercise and muscle lipid content, composition, and localization: Influence on muscle insulin sensitivity. Diabetes 2020, 69, 848–858. [Google Scholar] [CrossRef] [PubMed]
- De Franco, E. From biology to genes and back again: Gene discovery for monogenic forms of beta-cell dysfunction in diabetes. J. Mol. Biol. 2020, 432, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Salgin, B.; Sleigh, A.J.; Williams, R.M.; Jackson, S.J.; Bluck, L.J.; Murgatroyd, P.R.; Humphreys, S.M.; Harding, S.; Carpenter, T.A.; Dunger, D.B. Intramyocellular lipid levels are associated with peripheral, but not hepatic, insulin sensitivity in normal healthy subjects. Clin. Sci. 2009, 117, 111–118. [Google Scholar] [CrossRef]
- DeFronzo, R.A. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef]
- Mahashabde, M.; Chaudhary, G.; Kanchi, G.; Rohatgi, S.; Rao, P.; Patil, R.; Nallamothu, V. An unusual case of critical illness polyneuromyopathy. Indian J. Crit. Care Med. Peer-Rev. Off. Publ. Indian Soc. Crit. Care Med. 2020, 24, 133. [Google Scholar]
- Wang, W.; Shen, D.; Zhang, L.; Ji, Y.; Xu, L.; Chen, Z.; Shen, Y.; Gong, L.; Zhang, Q.; Shen, M. SKP-SC-EVs mitigate denervated muscle atrophy by inhibiting oxidative stress and inflammation and improving microcirculation. Antioxidants 2021, 11, 66. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Uusitupa, M.; Khan, T.A.; Viguiliouk, E.; Kahleova, H.; Rivellese, A.A.; Hermansen, K.; Pfeiffer, A.; Thanopoulou, A.; Salas-Salvadó, J.; Schwab, U. Prevention of type 2 diabetes by lifestyle changes: A systematic review and meta-analysis. Nutrients 2019, 11, 2611. [Google Scholar] [CrossRef]
- Artinian, N.T.; Fletcher, G.F.; Mozaffarian, D.; Kris-Etherton, P.; Van Horn, L.; Lichtenstein, A.H.; Kumanyika, S.; Kraus, W.E.; Fleg, J.L.; Redeker, N.S. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: A scientific statement from the American Heart Association. Circulation 2010, 122, 406–441. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Clevel. Clin. J. Med. 2017, 84, S15. [Google Scholar] [CrossRef] [PubMed]
- Azemi, A.K.; Siti-Sarah, A.R.; Mokhtar, S.S.; Rasool, A.H.G. Time-Restricted feeding improved vascular endothelial function in a high-fat diet-induced obesity rat model. Vet. Sci. 2022, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; ZhuGe, F.; Sun, L.; Ni, Y.; Fu, O.; Gao, G.; Chen, J.; Kato, H.; Fu, Z. Enhanced effect of daytime restricted feeding on the circadian rhythm of streptozotocin-induced type 2 diabetic rats. Am. J. Physiol.-Endocrinol. Metab. 2012, 302, E1027–E1035. [Google Scholar] [CrossRef][Green Version]
- Rami, M.; Rahdar, S.; Ahmadi Hekmatikar, A.; Awang Daud, D.M. Highlighting the novel effects of high-intensity interval training on some histopathological and molecular indices in the heart of type 2 diabetic rats. Front. Endocrinol. 2023, 14, 1175585. [Google Scholar] [CrossRef]
- Gui, D.; Cui, Z.; Zhang, L.; Yu, C.; Yao, D.; Xu, M.; Chen, M.; Wu, P.; Li, G.; Wang, L. Salidroside attenuates hypoxia-induced pulmonary arterial smooth muscle cell proliferation and apoptosis resistance by upregulating autophagy through the AMPK-mTOR-ULK1 pathway. BMC Pulm. Med. 2017, 17, 191. [Google Scholar] [CrossRef]
- Matos, M.A.d.; Vieira, D.V.; Pinhal, K.C.; Lopes, J.F.; Dias-Peixoto, M.F.; Pauli, J.R.; Castro Magalhães, F.d.; Little, J.P.; Rocha-Vieira, E.; Amorim, F.T. High-intensity interval training improves markers of oxidative metabolism in skeletal muscle of individuals with obesity and insulin resistance. Front. Physiol. 2018, 9, 1451. [Google Scholar] [CrossRef]
- Madsen, S.M.; Thorup, A.C.; Overgaard, K.; Jeppesen, P.B. High intensity interval training improves glycaemic control and pancreatic β cell function of type 2 diabetes patients. PLoS ONE 2015, 10, e0133286. [Google Scholar] [CrossRef]
- Antoni, R.; Johnston, K.L.; Collins, A.L.; Robertson, M.D. The effects of intermittent energy restriction on indices of cardiometabolic health. Res. Endocrinol. 2014, 2014, 459119. [Google Scholar] [CrossRef]
- Wu, N.N.; Tian, H.; Chen, P.; Wang, D.; Ren, J.; Zhang, Y. Physical exercise and selective autophagy: Benefit and risk on cardiovascular health. Cells 2019, 8, 1436. [Google Scholar] [CrossRef]
- Almalki, M.H.; Alshahrani, F. Options for controlling type 2 diabetes during Ramadan. Front. Endocrinol. 2016, 7, 32. [Google Scholar] [CrossRef]
- Liu, P.-j.; Hu, Y.-s.; Wang, M.-j.; Kang, L. Nutrient weight against sarcopenia: Regulation of the IGF-1/PI3K/Akt/FOXO pathway in quinoa metabolites. Curr. Opin. Pharmacol. 2021, 61, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Skovsø, S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J. Diabetes Investig. 2014, 5, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Khalifi, S.; Jedi, S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes. Acta Physiol. Hung. 2014, 101, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Okoduwa, S.I.R.; Umar, I.A.; James, D.B.; Inuwa, H.M. Appropriate insulin level in selecting fortified diet-fed, streptozotocin-treated rat model of type 2 diabetes for anti-diabetic studies. PLoS ONE 2017, 12, e0170971. [Google Scholar] [CrossRef]
- Rad, M.G.; Sharifi, M.; Meamar, R.; Soltani, N. The role of pancreas to improve hyperglycemia in STZ-induced diabetic rats by thiamine disulfide. Nutr. Diabetes 2022, 12, 32. [Google Scholar] [CrossRef]
- Madsen, S.M.; Thorup, A.C.; Overgaard, K.; Bjerre, M.; Jeppesen, P.B. Functional and structural vascular adaptations following 8 weeks of low volume high intensity interval training in lower leg of type 2 diabetes patients and individuals at high risk of metabolic syndrome. Arch. Physiol. Biochem. 2015, 121, 178–186. [Google Scholar] [CrossRef]
- Leandro, C.G.; Levada, A.C.; Hirabara, S.M.; Manhas-De-Castro, R.; De-Castro, C.B.; Curi, R.; Pithon-Curi, T.C. Aprogram of moderate physical training for wistar rats based on maximal oxygen consumption. J. Strength Cond. Res. 2007, 21, 751–756. [Google Scholar] [CrossRef]
- Qin, F.; Dong, Y.; Wang, S.; Xu, M.; Wang, Z.; Qu, C.; Yang, Y.; Zhao, J. Maximum oxygen consumption and quantification of exercise intensity in untrained male Wistar rats. Sci. Rep. 2020, 10, 11520. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Hashim, M.A.; Yam, M.F.; Hor, S.Y.; Lim, C.P.P.; Asmawi, M.Z.; Sadikun, A. Anti-hyperglycaemic activity of swietenia macrophylla king (meliaceae) seed extracts in normoglycaemic rats undergoing glucose tolerance tests. Chin. Med. 2013, 8, 11. [Google Scholar] [CrossRef]
- Depner, H.; Lützkendorf, J.; Babkir, H.A.; Sigrist, S.J.; Holt, M.G. Differential centrifugation–based biochemical fractionation of the Drosophila adult CNS. Nat. Protoc. 2014, 9, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Dimauro, I.; Pearson, T.; Caporossi, D.; Jackson, M.J. A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res. Notes 2012, 5, 513. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Tosun, A.B.; Fine, J.L.; Lee, A.V.; Taylor, D.L.; Chennubhotla, S.C. Spatial statistics for segmenting histological structures in H&E stained tissue images. IEEE Trans. Med. Imaging 2017, 36, 1522–1532. [Google Scholar] [PubMed]
- Kleczek, P.; Jaworek-Korjakowska, J.; Gorgon, M. A novel method for tissue segmentation in high-resolution H&E-stained histopathological whole-slide images. Comput. Med. Imaging Graph. 2020, 79, 101686. [Google Scholar]
- Bhattacharya, A.; Dhar, P.; Mehra, R.D. Preliminary morphological and biochemical changes in rat liver following postnatal exposure to sodium arsenite. Anat. Cell Biol. 2012, 45, 229. [Google Scholar] [CrossRef]
- Mojiminiyi, O.A.; Abdella, N.A. Effect of homeostasis model assessment computational method on the definition and associations of insulin resistance. Clin. Chem. Lab. Med. 2010, 48, 1629–1634. [Google Scholar] [CrossRef]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am. J. Physiol.-Endocrinol. Metab. 2008, 294, E15–E26. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic adipose tissue inflammation linking obesity to insulin resistance and type 2 diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Schleh, M. Integration of Insulin Sensitivity in Adipose Tissue and Skeletal Muscle, and the Role of Exercise on Muscle Lipid Regulation. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2022. [Google Scholar]
- Gilbert, M. Role of skeletal muscle lipids in the pathogenesis of insulin resistance of obesity and type 2 diabetes. J. Diabetes Investig. 2021, 12, 1934–1941. [Google Scholar] [CrossRef]
- Yin, R.; Xue, Y.; Hu, J.; Hu, X.; Shen, Q. The effects of diet and streptozotocin on metabolism and gut microbiota in a type 2 diabetes mellitus mouse model. Food Agric. Immunol. 2020, 31, 723–739. [Google Scholar] [CrossRef]
- Guo, X.-x.; Wang, Y.; Wang, K.; Ji, B.-p.; Zhou, F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J. Zhejiang Univ. Sci. B 2018, 19, 559. [Google Scholar] [CrossRef] [PubMed]
- Janssen, J.A. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int. J. Mol. Sci. 2021, 22, 7797. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.G.; Sharifi, M.; Meamar, R.; Soltani, N. Long term administration of thiamine disulfide improves FOXO1/PEPCK pathway in liver to reduce insulin resistance in type 1 diabetes rat model. Biomed. Pharmacother. 2024, 177, 117053. [Google Scholar] [CrossRef]
- Thonusin, C.; Pantiya, P.; Sumneang, N.; Chunchai, T.; Nawara, W.; Arunsak, B.; Siri-Angkul, N.; Sriwichaiin, S.; Chattipakorn, S.C.; Chattipakorn, N. Effectiveness of high cardiorespiratory fitness in cardiometabolic protection in prediabetic rats. Mol. Med. 2022, 28, 31. [Google Scholar] [CrossRef]
- Carnagarin, R.; Dharmarajan, A.M.; Dass, C.R. Molecular aspects of glucose homeostasis in skeletal muscle–A focus on the molecular mechanisms of insulin resistance. Mol. Cell. Endocrinol. 2015, 417, 52–62. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Pereira, D.F.; Kappel, V.D.; Folador, P.; Figueiredo, M.d.S.R.B.; Pizzolatti, M.G.; Silva, F.R.M.B. Insulin signaling: A potential signaling pathway for the stimulatory effect of kaempferitrin on glucose uptake in skeletal muscle. Eur. J. Pharmacol. 2013, 712, 1–7. [Google Scholar] [CrossRef]
- Mann, G.; Riddell, M.C.; Adegoke, O.A. Effects of acute muscle contraction on the key molecules in insulin and Akt signaling in skeletal muscle in health and in insulin resistant states. Diabetology 2022, 3, 423–446. [Google Scholar] [CrossRef]
- Sirago, G.; Picca, A.; Calvani, R.; Coelho-Júnior, H.J.; Marzetti, E. Mammalian target of rapamycin (mTOR) signaling at the crossroad of muscle fiber fate in sarcopenia. Int. J. Mol. Sci. 2022, 23, 13823. [Google Scholar] [CrossRef]
- Zhang, Y.; Whaley-Connell, A.T.; Sowers, J.R.; Ren, J. Autophagy as an emerging target in cardiorenal metabolic disease: From pathophysiology to management. Pharmacol. Ther. 2018, 191, 1–22. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef]
- Yao, H.; Han, X.; Han, X. The cardioprotection of the insulin-mediated PI3K/Akt/mTOR signaling pathway. Am. J. Cardiovasc. Drugs 2014, 14, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Fuentealba, C.; Contreras-Ferrat, A.E.; Altamirano, F.; Espinosa, A.; Li, Q.; Niu, W.; Lavandero, S.; Klip, A.; Jaimovich, E. Electrical stimuli release ATP to increase GLUT4 translocation and glucose uptake via PI3Kγ-Akt-AS160 in skeletal muscle cells. Diabetes 2013, 62, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Colomiere, M.; Permezel, M.; Lappas, M. Diabetes and obesity during pregnancy alter insulin signalling and glucose transporter expression in maternal skeletal muscle and subcutaneous adipose tissue. J. Mol. Endocrinol. 2010, 44, 213–223. [Google Scholar] [CrossRef]
- Xue, R.; Hao, D.-D.; Sun, J.-P.; Li, W.-W.; Zhao, M.-M.; Li, X.-H.; Chen, Y.; Zhu, J.-H.; Ding, Y.-J.; Liu, J. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid. Redox Signal. 2013, 19, 5–23. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483. [Google Scholar] [CrossRef]
- Zhang, H.-p.; Jiang, R.-y.; Zhu, J.-y.; Sun, K.-n.; Huang, Y.; Zhou, H.-h.; Zheng, Y.-b.; Wang, X.-j. PI3K/AKT/mTOR signaling pathway: An important driver and therapeutic target in triple-negative breast cancer. Breast Cancer 2024, 31, 539–551. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR signaling in growth, metabolism, and disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Rozengurt, E.; Sinnett-Smith, J.; Kisfalvi, K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: A novel target for the antidiabetic drug metformin in pancreatic cancer. Clin. Cancer Res. 2010, 16, 2505–2511. [Google Scholar] [CrossRef]
- Suhara, T.; Baba, Y.; Shimada, B.K.; Higa, J.K.; Matsui, T. The mTOR signaling pathway in myocardial dysfunction in type 2 diabetes mellitus. Curr. Diabetes Rep. 2017, 17, 38. [Google Scholar] [CrossRef]
- Kohler, Z.M.; Trencsenyi, G.; Juhasz, L.; Zvara, A.; Szabo, J.P.; Dux, L.; Puskas, L.G.; Rovo, L.; Keller-Pinter, A. Tilorone increases glucose uptake in vivo and in skeletal muscle cells by enhancing Akt2/AS160 signaling and glucose transporter levels. J. Cell. Physiol. 2023, 238, 1080–1094. [Google Scholar] [CrossRef]
- Atherton, P.J.; Phillips, B.E.; Wilkinson, D.J. Exercise and regulation of protein metabolism. Prog. Mol. Biol. Transl. Sci. 2015, 135, 75–98. [Google Scholar] [PubMed]
- Fogarty, C.E.; Bergmann, A. Killers creating new life: Caspases drive apoptosis-induced proliferation in tissue repair and disease. Cell Death Differ. 2017, 24, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Sen, I.; Bozkurt, O.; Aras, E.; Heise, S.; Brockmann, G.A.; Severcan, F. Lipid profiles of adipose and muscle tissues in mouse models of juvenile onset of obesity without high fat diet induction: A Fourier transform infrared (FT-IR) spectroscopic study. Appl. Spectrosc. 2015, 69, 679–688. [Google Scholar] [CrossRef] [PubMed]
- O’neill, H.M. AMPK and exercise: Glucose uptake and insulin sensitivity. Diabetes Metab. J. 2013, 37, 1–21. [Google Scholar] [CrossRef]
- Bharath, L.P.; Nikolajczyk, B.S. The intersection of metformin and inflammation. Am. J. Physiol.-Cell Physiol. 2021, 320, C873–C879. [Google Scholar] [CrossRef]
- Saisho, Y. Metformin and inflammation: Its potential beyond glucose-lowering effect. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2015, 15, 196–205. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, W.; Xu, G. Fuzhu jiangtang granules combined with metformin reduces insulin resistance in skeletal muscle of diabetic rats via PI3K/Akt signaling. Pharm. Biol. 2019, 57, 660–668. [Google Scholar] [CrossRef]
- Yang, X.; Kord-Varkaneh, H.; Talaei, S.; Clark, C.C.; Zanghelini, F.; Tan, S.C.; Zarezadeh, M.; Mousavi, S.M.; Rahmani, J.; Zhang, Y. The influence of metformin on IGF-1 levels in humans: A systematic review and meta-analysis. Pharmacol. Res. 2020, 151, 104588. [Google Scholar] [CrossRef]
- Jiating, L.; Buyun, J.; Yinchang, Z. Role of metformin on osteoblast differentiation in type 2 diabetes. BioMed Res. Int. 2019, 2019, 9203934. [Google Scholar] [CrossRef]


| High-Fat Diet (60%) | Standard Diet | Parameters |
|---|---|---|
| 24 | 23 | Protein (%) |
| 26 | 50.3 | Carbohydrate (%) |
| 35 | 4.5 | Fat (%) |
| 15 | 22.2 | Others (%) |
| 60 | 10 | Fat (%) |
| 5.2 | 3.1 | Calories (kcal/g) |
| Week | Warm-Up (Min) | Intensity Warm-Up (%Vo2max) | Treadmill Speed Warmup Time (Meters/Min) | Repetition | Ratio of Training Time to Rest (Min) | Training Intensity (%Vo2max) | Treadmill Speed During Exercise (Meters/Min) | Rest Time Intensity (%Vo2max) | Treadmill Speed at Rest (Meters/Min) | Cool Down (Min) | Intensity Cool Down (%Vo2max) | Treadmill Speed Cool Down Time (Meters/Min) | Total Duration (Min) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vmax test evaluation | |||||||||||||
| 1 | 5 | 30–40 | 10 | 15 | 3:2 | 85–90 | 26 | 30–40 | 10 | 5 | 30–40 | 10 | 48 |
| 2 | 5 | 30–40 | 10 | 15 | 3:2 | 85–90 | 26 | 30–40 | 10 | 5 | 30–40 | 10 | 48 |
| 3 | 5 | 30–40 | 10 | 15 | 3:2 | 85–90 | 26 | 30–40 | 10 | 5 | 30–40 | 10 | 48 |
| 4 | 5 | 30–40 | 10 | 15 | 3:2 | 85–90 | 26 | 30–40 | 10 | 5 | 30–40 | 10 | 48 |
| Vmax test evaluation | |||||||||||||
| 5 | 5 | 30–40 | 12 | 15 | 4:2 | 85–90 | 30 | 30–40 | 12 | 5 | 30–40 | 12 | 56 |
| 6 | 5 | 30–40 | 12 | 15 | 4:2 | 85–90 | 30 | 30–40 | 12 | 5 | 30–40 | 12 | 56 |
| 7 | 5 | 30–40 | 12 | 15 | 4:2 | 85–90 | 30 | 30–40 | 12 | 5 | 30–40 | 12 | 56 |
| 8 | 5 | 30–40 | 12 | 15 | 4:2 | 85–90 | 30 | 30–40 | 12 | 5 | 30–40 | 12 | 56 |
| 9 | 5 | 30–40 | 12 | 15 | 4:2 | 85–90 | 30 | 30–40 | 12 | 5 | 30–40 | 12 | 56 |
| 10 | 5 | 30–40 | 12 | 15 | 4:2 | 85–90 | 30 | 30–40 | 12 | 5 | 30–40 | 12 | 56 |
| Two-Way ANOVA for Protein Content of AKT, IGF1, and mTOR | ||||||
|---|---|---|---|---|---|---|
| Main Effect, p-Value, Effect Size | Interaction, p-Value, Effect Size | |||||
| Parameters | HIIT | TRF | TRF × HIIT | |||
| p-Value | Effect Size | p-Value | Effect Size | p-Value | Effect Size | |
| Serum glucose (mg/dL) | <0.001 | 0.66 | <0.001 | 0.61 | 0.34 | 0.075 |
| Serum insulin (microU/L) | <0.001 | 0.67 | <0.001 | 0.68 | 0.74 | 0.009 |
| HOMA-IR | <0.001 | 0.87 | <0.001 | 0.63 | 0.52 | 0.035 |
| IGF-1/β-Actin protein | <0.001 | 0.98 | 0.015 | 0.54 | <0.001 | 0.96 |
| AKT/β-Actin protein | 0.011 | 0.57 | 0.057 | 0.38 | 0.32 | 0.12 |
| mTOR/β-Actin protein | 0.004 | 0.67 | 0.006 | 0.63 | 0.33 | 0.116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohebinejad, M.; Kazeminasab, F.; Ghanbari Rad, M.; Bagheri, R.; Razi, M.; Willoughby, D.; Dutheil, F. The Combined Effect of High-Intensity Interval Training and Time-Restricted Feeding on the AKT-IGF-1-mTOR Signaling Pathway in the Muscle Tissue of Type 2 Diabetic Rats. Nutrients 2025, 17, 1404. https://doi.org/10.3390/nu17091404
Mohebinejad M, Kazeminasab F, Ghanbari Rad M, Bagheri R, Razi M, Willoughby D, Dutheil F. The Combined Effect of High-Intensity Interval Training and Time-Restricted Feeding on the AKT-IGF-1-mTOR Signaling Pathway in the Muscle Tissue of Type 2 Diabetic Rats. Nutrients. 2025; 17(9):1404. https://doi.org/10.3390/nu17091404
Chicago/Turabian StyleMohebinejad, Motahareh, Fatemeh Kazeminasab, Mahtab Ghanbari Rad, Reza Bagheri, Mazdak Razi, Darryn Willoughby, and Fred Dutheil. 2025. "The Combined Effect of High-Intensity Interval Training and Time-Restricted Feeding on the AKT-IGF-1-mTOR Signaling Pathway in the Muscle Tissue of Type 2 Diabetic Rats" Nutrients 17, no. 9: 1404. https://doi.org/10.3390/nu17091404
APA StyleMohebinejad, M., Kazeminasab, F., Ghanbari Rad, M., Bagheri, R., Razi, M., Willoughby, D., & Dutheil, F. (2025). The Combined Effect of High-Intensity Interval Training and Time-Restricted Feeding on the AKT-IGF-1-mTOR Signaling Pathway in the Muscle Tissue of Type 2 Diabetic Rats. Nutrients, 17(9), 1404. https://doi.org/10.3390/nu17091404









