Curcumin Modulation of the Gut–Brain Axis for Neuroinflammation and Metabolic Disorders Prevention and Treatment
Abstract
:1. Introduction
1.1. Polyphenols and Curcumin: Structure and Function
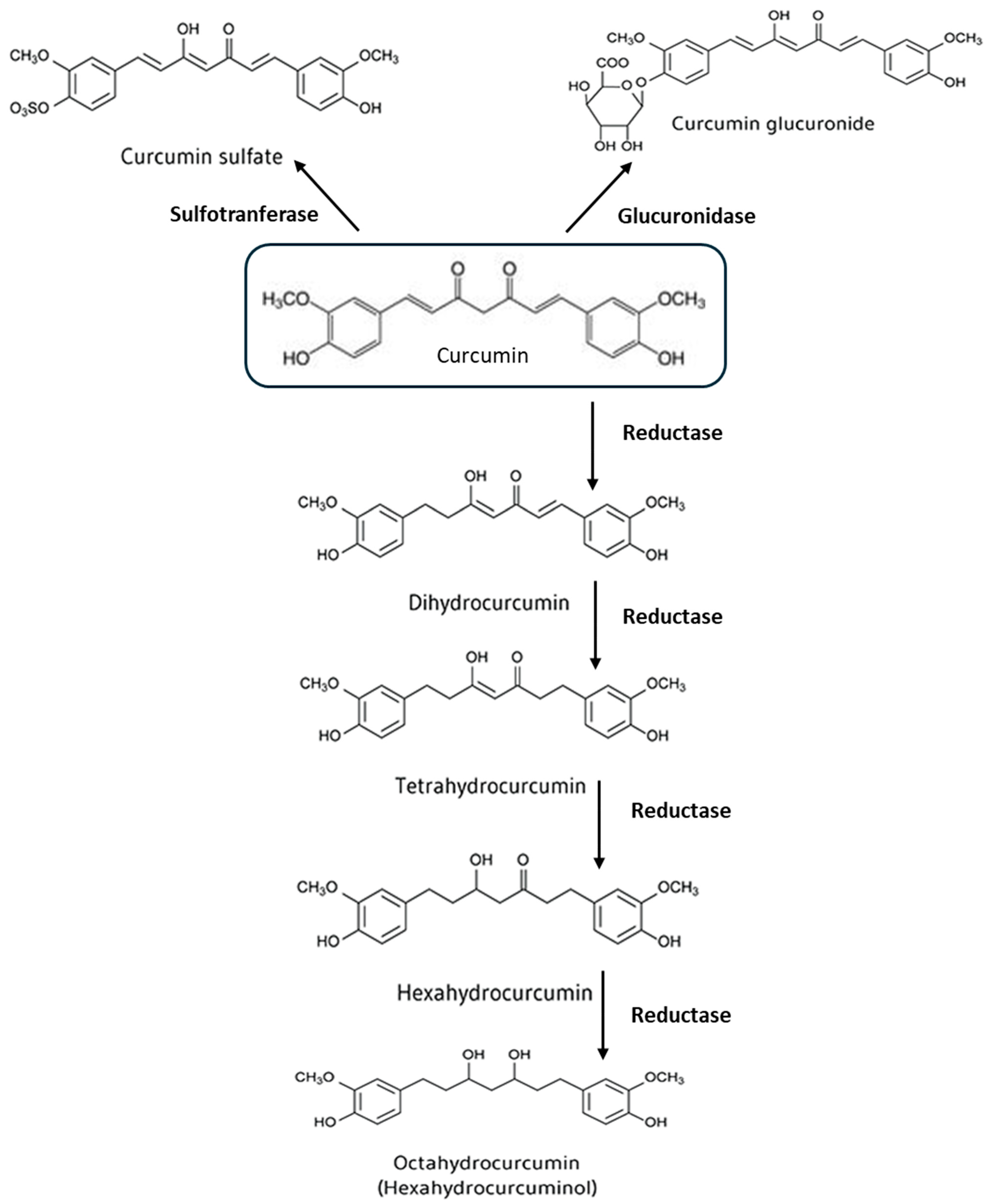
1.2. Metabolism of Curcumin
1.3. Curcumin Metabolites and Their Effects
1.4. Curcumin’s Health Benefits and Applications
2. Effects of Curcumin on Body Composition and Obesity: Efficacy, Challenges, and Strategies to Improve Bioavailability
3. Adipocyte Remodeling, Systemic Inflammation, and the Role of Hypoxia in Obesity: Mechanisms and Implications
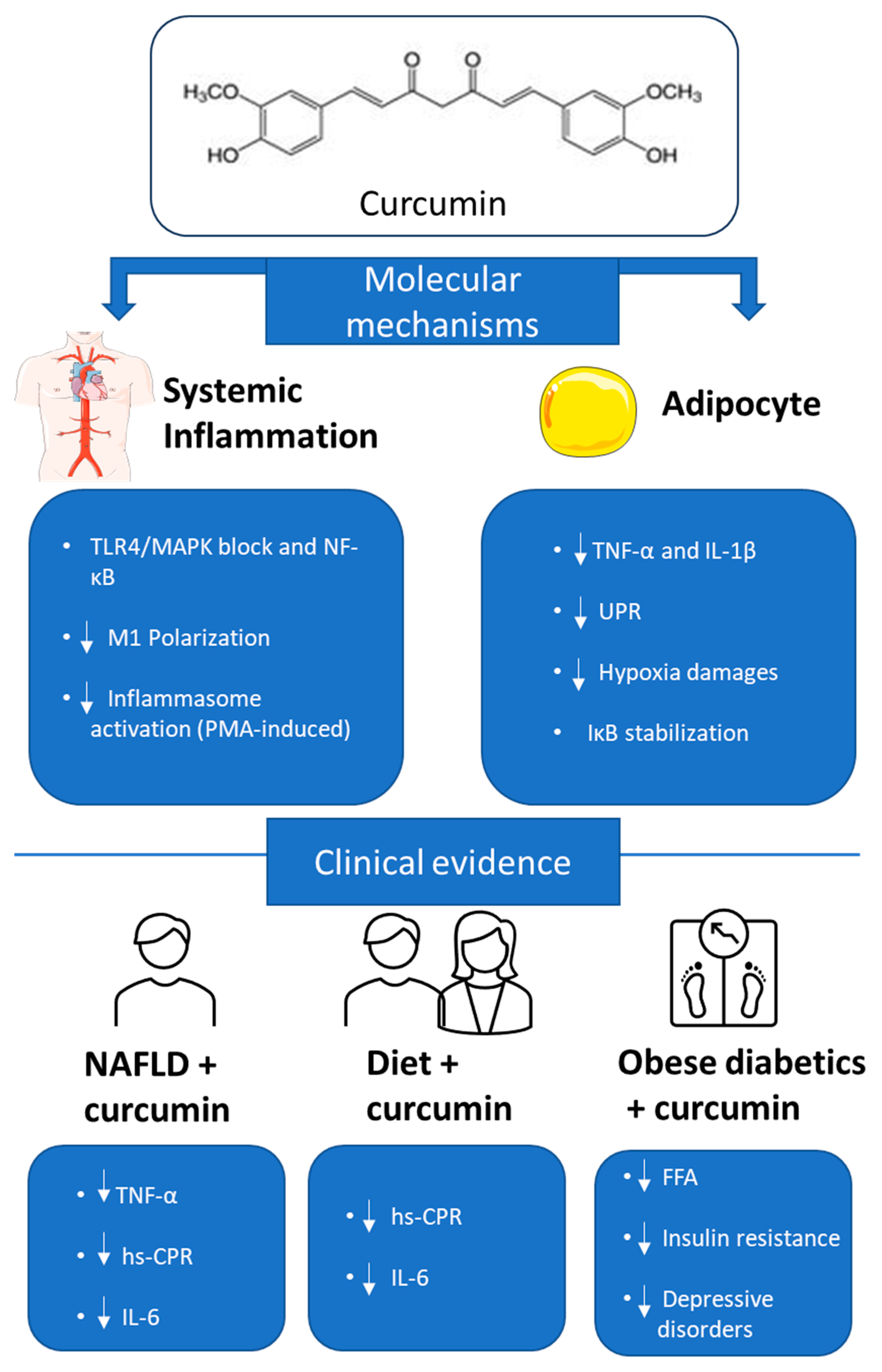
4. Curcumin Anti-Inflammatory Effects in Obesity: Evidence from In Vitro, In Vivo, and Clinical Studies
5. Curcumin Role in Modulating Oxidative Stress and the Keap1-NRF2 Pathway in Obesity-Related Inflammation
6. The Role of Curcumin in Preventing Metabolic Diseases in Adolescence and Early Childhood
7. Effects of Curcumin Treatment on Brain Inflammation Associated with Obesity
8. Impact of Curcumin on Gut Microbiota and Metabolism: Implications for Obesity and Health
9. Curcumin Promotes the Well-Being of the Intestinal Barrier
10. Curcumin and Gut Health: Potential Neuroprotective Effects Through the Gut–Brain Axis in Neurodegenerative Diseases and Obesity
11. Conclusions
Funding
Conflicts of Interest
Abbreviations
| ACC | acetyl CoA carboxylase |
| AMPK | AMP-activated kinase |
| BAT | brown adipose tissue |
| CLSs | crown-like structures |
| CPT-1 | carnitine palmitoyltransferase-1 |
| DCA | deoxycholic acid |
| FFA | free fatty acids |
| FMT | Fecal microbiota transplantation |
| GPAT-1 | glycerol-3-phosphate acyltransferase-1 |
| HFD | high-fat diet |
| HIF | Hypoxia-inducible factor |
| HO-1 | hemoxygenase 1 |
| hs-CRP | high-sensitivity C-reactive protein |
| IPA | intestinal alkaline phosphatase |
| LCA | lithocholic acid |
| MCE | mitotic clonal expansion |
| MLCK | myosin light chain kinase |
| NAFLD | nonalcoholic fatty liver disease |
| PDE3 | phosphodiesterase 3 |
| PKA | protein kinase A |
| PMA | phorbol myristate acetate |
| PPARs | peroxisome proliferator-activated receptors |
| Rb | Retinoblastoma protein |
| SFA | saturated fatty acids |
| SCFA | short-chain fatty acids |
| UPR | protein unfolding response |
| WAT | white adipose tissue |
| ZO-1 | zonula occludens 1 |
References
- Mengoni, B.; Armeli, F.; Schifano, E.; Prencipe, S.A.; Pompa, L.; Sciubba, F.; Brasili, E.; Giampaoli, O.; Mura, F.; Reverberi, M.; et al. In Vitro and In Vivo Antioxidant and Immune Stimulation Activity of Wheat Product Extracts. Nutrients 2025, 17, 302. [Google Scholar] [CrossRef] [PubMed]
- Armeli, F.; Mengoni, B.; Schifano, E.; Lenz, T.; Archer, T.; Uccelletti, D.; Businaro, R. The Probiotic Yeast, Milmed, Promotes Autophagy and Antioxidant Pathways in BV-2 Microglia Cells and C. Elegans. Antioxidants 2025, 14, 393. [Google Scholar] [CrossRef]
- Armeli, F.; Bonucci, A.; Maggi, E.; Pinto, A.; Businaro, R. Mediterranean Diet and Neurodegenerative Diseases: The Neglected Role of Nutrition in the Modulation of the Endocannabinoid System. Biomolecules 2021, 11, 790. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Gancarz, M.; Kondracka, A.; Rusinek, R.; Oniszczuk, A. Curcumin and Weight Loss: Does It Work? Int. J. Mol. Sci. 2022, 23, 639. [Google Scholar] [CrossRef]
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of Ultra-Performance Liquid Chromatography Coupled with Quadrupole Time-of-Flight Mass Spectrometry to Identify Curcumin Metabolites Produced by Human Intestinal Bacteria. J. Chromatogr. B 2015, 985, 38–47. [Google Scholar] [CrossRef]
- Pinkaew, D.; Changtam, C.; Tocharus, C.; Govitrapong, P.; Jumnongprakhon, P.; Suksamrarn, A.; Tocharus, J. Association of Neuroprotective Effect of Di-O-Demethylcurcumin on Aβ25–35-Induced Neurotoxicity with Suppression of NF-κB and Activation of Nrf2. Neurotox. Res. 2016, 29, 80–91. [Google Scholar] [CrossRef]
- Alsharif, F.J.; Almuhtadi, Y.A. The Effect of Curcumin Supplementation on Anthropometric Measures among Overweight or Obese Adults. Nutrients 2021, 13, 680. [Google Scholar] [CrossRef]
- Chandrasekaran, P.R.; Madanagopalan, V.G. Role of Curcumin in Retinal Diseases—A Review. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 1457–1473. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. Protective Effects of Curcumin in Cardiovascular Diseases—Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-Y.; Sun, Y.-Y.; Chang, P.; Huang, H.-C. Curcumin Inhibits Cell Damage and Apoptosis Caused by Thapsigargin-Induced Endoplasmic Reticulum Stress Involving the Recovery of Mitochondrial Function Mediated by Mitofusin-2. Neurotox. Res. 2022, 40, 449–460. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P. Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Mirzaei, H.; Shakeri, A.; Rashidi, B.; Jalili, A.; Banikazemi, Z.; Sahebkar, A. Phytosomal Curcumin: A Review of Pharmacokinetic, Experimental and Clinical Studies. Biomed. Pharmacother. 2017, 85, 102–112. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Chen, C.-W.; Chen, L.-K.; Chou, H.-Y.; Chang, C.-L.; Juan, C.-C. Curcumin Attenuates Adipogenesis by Inducing Preadipocyte Apoptosis and Inhibiting Adipocyte Differentiation. Nutrients 2019, 11, 2307. [Google Scholar] [CrossRef]
- Fajas, L. Adipogenesis: A Cross-Talk between Cell Proliferation and Cell Differentiation. Ann. Med. 2003, 35, 79–85. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Curcumin-Induced Suppression of Adipogenic Differentiation Is Accompanied by Activation of Wnt/β-Catenin Signaling. Am. J. Physiol. Cell Physiol. 2010, 298, C1510–C1516. [Google Scholar] [CrossRef]
- Bennett, C.N.; Ross, S.E.; Longo, K.A.; Bajnok, L.; Hemati, N.; Johnson, K.W.; Harrison, S.D.; MacDougald, O.A. Regulation of Wnt Signaling during Adipogenesis. J. Biol. Chem. 2002, 277, 30998–31004. [Google Scholar] [CrossRef]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin Inhibits Adipogenesis in 3T3-L1 Adipocytes and Angiogenesis and Obesity in C57/BL Mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin Induces Brown Fat-like Phenotype in 3T3-L1 and Primary White Adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Mele, L.; Bidault, G.; Mena, P.; Crozier, A.; Brighenti, F.; Vidal-Puig, A.; Del Rio, D. Dietary (Poly)Phenols, Brown Adipose Tissue Activation, and Energy Expenditure: A Narrative Review. Adv. Nutr. 2017, 8, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of Fat Cell Turnover in Humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Kawasaki, N.; Asada, R.; Saito, A.; Kanemoto, S.; Imaizumi, K. Obesity-Induced Endoplasmic Reticulum Stress Causes Chronic Inflammation in Adipose Tissue. Sci. Rep. 2012, 2, 799. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Huang, F.; Liu, B.; Xie, Y. Curcumin Inhibits Lipolysis via Suppression of ER Stress in Adipose Tissue and Prevents Hepatic Insulin Resistance. J. Lipid Res. 2016, 57, 1243–1255. [Google Scholar] [CrossRef]
- Giordano, A.; Murano, I.; Mondini, E.; Perugini, J.; Smorlesi, A.; Severi, I.; Barazzoni, R.; Scherer, P.E.; Cinti, S. Obese Adipocytes Show Ultrastructural Features of Stressed Cells and Die of Pyroptosis. J. Lipid Res. 2013, 54, 2423–2436. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte Death Defines Macrophage Localization and Function in Adipose Tissue of Obese Mice and Humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- Fujisaka, S.; Usui, I.; Ikutani, M.; Aminuddin, A.; Takikawa, A.; Tsuneyama, K.; Mahmood, A.; Goda, N.; Nagai, Y.; Takatsu, K.; et al. Adipose Tissue Hypoxia Induces Inflammatory M1 Polarity of Macrophages in an HIF-1α-Dependent and HIF-1α-Independent Manner in Obese Mice. Diabetologia 2013, 56, 1403–1412. [Google Scholar] [CrossRef]
- Priyanka, A.; Anusree, S.S.; Nisha, V.M.; Raghu, K.G. Curcumin Improves Hypoxia Induced Dysfunctions in 3T3-L1 Adipocytes by Protecting Mitochondria and down Regulating Inflammation. BioFactors 2014, 40, 513–523. [Google Scholar] [CrossRef]
- Varì, R.; Scazzocchio, B.; Silenzi, A.; Giovannini, C.; Masella, R. Obesity-Associated Inflammation: Does Curcumin Exert a Beneficial Role? Nutrients 2021, 13, 1021. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Giraud, J.; Davis, R.J.; White, M.F. C-Jun N-Terminal Kinase (JNK) Mediates Feedback Inhibition of the Insulin Signaling Cascade. J. Biol. Chem. 2003, 278, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Olefsky, J.M. Inflammation and Insulin Resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, T.; Wang, X.; Wei, X.; Chen, Y.; Guo, L.; Zhang, J.; Wang, C. Curcumin Modulates Macrophage Polarization Through the Inhibition of the Toll-Like Receptor 4 Expression and Its Signaling Pathways. Cell Physiol. Biochem. 2015, 36, 631–641. [Google Scholar] [CrossRef]
- Kong, F.; Ye, B.; Cao, J.; Cai, X.; Lin, L.; Huang, S.; Huang, W.; Huang, Z. Curcumin Represses NLRP3 Inflammasome Activation via TLR4/MyD88/NF-κB and P2X7R Signaling in PMA-Induced Macrophages. Front. Pharmacol. 2016, 7, 369. [Google Scholar] [CrossRef]
- Gonzales, A.M.; Orlando, R.A. Curcumin and Resveratrol Inhibit Nuclear Factor-kappaB-Mediated Cytokine Expression in Adipocytes. Nutr. Metab. 2008, 5, 17. [Google Scholar] [CrossRef]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.-J. Nano-Curcumin Improves Glucose Indices, Lipids, Inflammation, and Nesfatin in Overweight and Obese Patients with Non-Alcoholic Fatty Liver Disease (NAFLD): A Double-Blind Randomized Placebo-Controlled Clinical Trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of Curcumin Supplementation on Markers of Inflammation and Oxidative Stress among Healthy Overweight and Obese Girl Adolescents: A Randomized Placebo-controlled Clinical Trial. Phytother. Res. 2019, 33, 2015–2022. [Google Scholar] [CrossRef]
- Na, L.; Li, Y.; Pan, H.; Zhou, X.; Sun, D.; Meng, M.; Li, X.; Sun, C. Curcuminoids Exert Glucose-lowering Effect in Type 2 Diabetes by Decreasing Serum Free Fatty Acids: A Double-blind, Placebo-controlled Trial. Mol. Nutr. Food Res. 2013, 57, 1569–1577. [Google Scholar] [CrossRef]
- Yaikwawong, M.; Jansarikit, L.; Jirawatnotai, S.; Chuengsamarn, S. Curcumin Reduces Depression in Obese Patients with Type 2 Diabetes: A Randomized Controlled Trial. Nutrients 2024, 16, 2414. [Google Scholar] [CrossRef]
- Ebrahimzadeh, A.; Mohseni, S.; Safargar, M.; Mohtashamian, A.; Niknam, S.; Bakhoda, M.; Afshari, S.; Jafari, A.; Ebrahimzadeh, A.; Fooladshekan, S.; et al. Curcumin Effects on Glycaemic Indices, Lipid Profile, Blood Pressure, Inflammatory Markers and Anthropometric Measurements of Non-Alcoholic Fatty Liver Disease Patients: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Complement. Ther. Med. 2024, 80, 103025. [Google Scholar] [CrossRef] [PubMed]
- Armeli, F.; Mengoni, B.; Laskin, D.L.; Businaro, R. Interplay among Oxidative Stress, Autophagy, and the Endocannabinoid System in Neurodegenerative Diseases: Role of the Nrf2- P62/SQSTM1 Pathway and Nutraceutical Activation. Curr. Issues Mol. Biol. 2024, 46, 6868–6884. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent Advances of Natural Polyphenols Activators for Keap1-Nrf2 Signaling Pathway. Chem. Biodivers. 2019, 16, e1900400. [Google Scholar] [CrossRef] [PubMed]
- Sikalidis, A.K.; Mazor, K.M.; Lee, J.-I.; Roman, H.B.; Hirschberger, L.L.; Stipanuk, M.H. Upregulation of Capacity for Glutathione Synthesis in Response to Amino Acid Deprivation: Regulation of Glutamate–Cysteine Ligase Subunits. Amino Acids 2014, 46, 1285–1296. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin Attenuates Oxidative Stress in RAW264.7 Cells by Increasing the Activity of Antioxidant Enzymes and Activating the Nrf2-Keap1 Pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin Activates the Haem Oxygenase-1 Gene via Regulation of Nrf2 and the Antioxidant-Responsive Element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef]
- Shin, J.W.; Chun, K.-S.; Kim, D.-H.; Kim, S.-J.; Kim, S.H.; Cho, N.-C.; Na, H.-K.; Surh, Y.-J. Curcumin Induces Stabilization of Nrf2 Protein through Keap1 Cysteine Modification. Biochem. Pharmacol. 2020, 173, 113820. [Google Scholar] [CrossRef]
- Mameli, C.; Mazzantini, S.; Zuccotti, G. Nutrition in the First 1000 Days: The Origin of Childhood Obesity. Int. J. Environ. Res. Public Health 2016, 13, 838. [Google Scholar] [CrossRef]
- Du, S.; Zhou, N.; Zheng, W.; Zhu, X.; Ling, R.; Zhou, W.; Li, X. Prepuberty Is a Window Period for Curcumin to Prevent Obesity in Postnatal Overfed Rats. Pediatr. Res. 2024, 96, 104–114. [Google Scholar] [CrossRef]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity Is Associated with Hypothalamic Injury in Rodents and Humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia Dictate the Impact of Saturated Fat Consumption on Hypothalamic Inflammation and Neuronal Function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin Inhibits LPS-Induced Neuroinflammation by Promoting Microglial M2 Polarization via TREM2/TLR4/NF-κB Pathways in BV2 Cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-X.; Yu, R.; Shao, L.-F.; Zhang, Y.-X.; Ge, C.-X.; Liu, X.-M.; Wu, W.-Y.; Li, J.-M.; Kong, L.-D. Up-Regulated Fractalkine (FKN) and Its Receptor CX3CR1 Are Involved in Fructose-Induced Neuroinflammation: Suppression by Curcumin. Brain Behav. Immun. 2016, 58, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Armeli, F.; Mengoni, B.; Maggi, E.; Mazzoni, C.; Preziosi, A.; Mancini, P.; Businaro, R.; Lenz, T.; Archer, T. Milmed Yeast Alters the LPS-Induced M1 Microglia Cells to Form M2 Anti-Inflammatory Phenotype. Biomedicines 2022, 10, 3116. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Ji, H.-F. Regulative Effects of Curcumin Spice Administration on Gut Microbiota and Its Pharmacological Implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef]
- Li, S.; You, J.; Wang, Z.; Liu, Y.; Wang, B.; Du, M.; Zou, T. Curcumin Alleviates High-Fat Diet-Induced Hepatic Steatosis and Obesity in Association with Modulation of Gut Microbiota in Mice. Food Res. Int. 2021, 143, 110270. [Google Scholar] [CrossRef]
- Li, R.; Yao, Y.; Gao, P.; Bu, S. The Therapeutic Efficacy of Curcumin vs. Metformin in Modulating the Gut Microbiota in NAFLD Rats: A Comparative Study. Front. Microbiol. 2021, 11, 555293. [Google Scholar] [CrossRef]
- Duarte, L.; Gasaly, N.; Poblete-Aro, C.; Uribe, D.; Echeverria, F.; Gotteland, M.; Garcia-Diaz, D.F. Polyphenols and Their Anti-Obesity Role Mediated by the Gut Microbiota: A Comprehensive Review. Rev. Endocr. Metab. Disord. 2021, 22, 367–388. [Google Scholar] [CrossRef]
- Han, Z.; Yao, L.; Zhong, Y.; Xiao, Y.; Gao, J.; Zheng, Z.; Fan, S.; Zhang, Z.; Gong, S.; Chang, S.; et al. Gut Microbiota Mediates the Effects of Curcumin on Enhancing Ucp1-Dependent Thermogenesis and Improving High-Fat Diet-Induced Obesity. Food Funct. 2021, 12, 6558–6575. [Google Scholar] [CrossRef]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin Improves Intestinal Barrier Function: Modulation of Intracellular Signaling, and Organization of Tight Junctions. Am. J. Physiol. Cell Physiol. 2017, 312, C438–C445. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral Supplementation with Non-Absorbable Antibiotics or Curcumin Attenuates Western Diet-Induced Atherosclerosis and Glucose Intolerance in LDLR−/−Mice—Role of Intestinal Permeability and Macrophage Activation. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Wei, W.; Li, Y.; Wu, J.; Zhao, Y.; Zhang, L.; Wang, T.; Zhong, X. Curcumin and Resveratrol Regulate Intestinal Bacteria and Alleviate Intestinal Inflammation in Weaned Piglets. Molecules 2019, 24, 1220. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guan, B.; Lin, L.; Wang, Y. Improvement of Intestinal Barrier Function, Gut Microbiota, and Metabolic Endotoxemia in Type 2 Diabetes Rats by Curcumin. Bioengineered 2021, 12, 11947–11958. [Google Scholar] [CrossRef]
- D’Antongiovanni, V.; Fornai, M.; Benvenuti, L.; Di Salvo, C.; Pellegrini, C.; Cappelli, F.; Masi, S.; Antonioli, L. Dietary Supplement, Containing the Dry Extract of Curcumin, Emblica and Cassia, Counteracts Intestinal Inflammation and Enteric Dysmotility Associated with Obesity. Metabolites 2023, 13, 410. [Google Scholar] [CrossRef]
- Zhong, L.; Cai, B.; Wang, Q.; Li, X.; Xu, W.; Chen, T. Exploring the Neuroprotective Mechanism of Curcumin Inhibition of Intestinal Inflammation against Parkinson’s Disease Based on the Gut-Brain Axis. Pharmaceuticals 2022, 16, 39. [Google Scholar] [CrossRef]
- Cai, B.; Zhong, L.; Wang, Q.; Xu, W.; Li, X.; Chen, T. Curcumin Alleviates 1-Methyl- 4-Phenyl- 1,2,3,6-Tetrahydropyridine- Induced Parkinson’s Disease in Mice via Modulating Gut Microbiota and Short-Chain Fatty Acids. Front. Pharmacol. 2023, 14, 1198335. [Google Scholar] [CrossRef]
- Cui, C.; Han, Y.; Li, H.; Yu, H.; Zhang, B.; Li, G. Curcumin-Driven Reprogramming of the Gut Microbiota and Metabolome Ameliorates Motor Deficits and Neuroinflammation in a Mouse Model of Parkinson’s Disease. Front. Cell. Infect. Microbiol. 2022, 12, 887407. [Google Scholar] [CrossRef]
- Lamichhane, G.; Liu, J.; Lee, S.-J.; Lee, D.-Y.; Zhang, G.; Kim, Y. Curcumin Mitigates the High-Fat High-Sugar Diet-Induced Impairment of Spatial Memory, Hepatic Metabolism, and the Alteration of the Gut Microbiome in Alzheimer’s Disease-Induced (3xTg-AD) Mice. Nutrients 2024, 16, 240. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Salbaum, J.M.; Luo, M.; Blanchard, E.; Taylor, C.M.; Welsh, D.A.; Berthoud, H.-R. Obese-Type Gut Microbiota Induce Neurobehavioral Changes in the Absence of Obesity. Biol. Psychiatry 2015, 77, 607–615. [Google Scholar] [CrossRef]
- Pereira, L.T.G.; Vilela, W.R.; Bellozi, P.M.Q.; Engel, D.F.; De Paula, G.C.; De Andrade, R.R.; Mortari, M.R.; De Melo Teixeira, M.; Coleine, C.; Figueiredo, C.P.; et al. Fecal Microbiota Transplantation Ameliorates High-fat Diet-induced Memory Impairment in Mice. J. Neurochem. 2024, 168, 2893–2907. [Google Scholar] [CrossRef]
- Syeda, T.; Sánchez-Tapia, M.; Orta, I.; Granados-Portillo, O.; Pérez-Jimenez, L.; Rodríguez-Callejas, J.-D.; Toribio, S.; Silva-Lucero, M.-C.; Rivera, A.-L.; Tovar, A.; et al. Bioactive Foods Decrease Liver and Brain Alterations Induced by a High-Fat-Sucrose Diet through Restoration of Gut Microbiota and Antioxidant Enzymes. Nutrients 2021, 14, 22. [Google Scholar] [CrossRef]



| Patients | Inclusion Criteria | Experimental Group Composition | Treatment | Main Effects | Type of Study | Reference |
|---|---|---|---|---|---|---|
| 84 overweight/obese patients suffering from NAFLD, male and female | 25–50 years old, NAFLD diagnosed using ultrasonography and 25 ≤ BMI < 35 kg/m2. | 23 male+ 19 females in each group | Nano-curcumin capsules, twice daily, 80 mg/day, for 3 months | -decreased fatty liver degree -improvement in glycaemic, lipid and insulin resistance parameters (fasting glucose, HbA1c, HOMA-IR and others) -reduction of inflammatory response (TNFα, hs-CRP, IL-6 reduction) | double-blind, randomized, placebo-controlled clinical trial | [37] |
| 60 overweight or obese adolescent girls | 13–18 years old, having a menstruation cycle more than 6 months. overweight and obesity were defined as body mass index (BMI) percentile for age between 85th and 95th and BMI percentile for age more than 95th, respectively. | 30 subjects in each group | -slight weight loss diet -500-mg curcumin capsule a day for 10 weeks. | -reduction of inflammatory markers (hs-CRP, IL-6) -increased antioxidant response | double-blind. randomized placebo-controlled clinical trial | [38] |
| 100 patients with T2DM, male and female (50/experimental group palcebo/curcumin) | 18–65 years old. BMI ≥ 24.0; fasting blood glucose ≥ 7.0 mmol/L or postprandial blood glucose ≥ 11.1 mmol/L) | 25 male+ 25 females in placebo group 24 male+ 26 females in treated group | -150 mg curcuminoids capsule twice daily, 300 mg daily, for 3 months | -improvement of diabetic condition and reduction of fasting glucose, HbA1c, and HOMA-IR -decreased concentration of serum total FFAs | randomized, double-blind, placebo- controlled trial | [39] |
| 227 patients with T2DM and depression, male and female | >35 years old. BMI ≥ 23 kg/m2 and well-controlled blood glucose (glycated hemoglobin [HbA1c] < 6.5% and fasting plasma glucose [FPG] < 110 mg/dL). | 54 male+ 80 females in placebo group 62 male+ 73 females in treated group | -curcuminoids 3 capsules, twice daily, (1500 mg daily) for one year | -improved depression severity and plasmatic serotonin levels -improvement of glycaemic and insulin resistance indices (HbA1c, HOMA-IR) -reduction of inflammatory markers (TNFα, IL-1β, IL 6) -increased antioxidant defence (GPX and SOD) | randomized, double-blind, placebo- controlled trial | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerullo, M.; Armeli, F.; Mengoni, B.; Menin, M.; Crudeli, M.L.; Businaro, R. Curcumin Modulation of the Gut–Brain Axis for Neuroinflammation and Metabolic Disorders Prevention and Treatment. Nutrients 2025, 17, 1430. https://doi.org/10.3390/nu17091430
Cerullo M, Armeli F, Mengoni B, Menin M, Crudeli ML, Businaro R. Curcumin Modulation of the Gut–Brain Axis for Neuroinflammation and Metabolic Disorders Prevention and Treatment. Nutrients. 2025; 17(9):1430. https://doi.org/10.3390/nu17091430
Chicago/Turabian StyleCerullo, Miriam, Federica Armeli, Beatrice Mengoni, Martina Menin, Maria Luisa Crudeli, and Rita Businaro. 2025. "Curcumin Modulation of the Gut–Brain Axis for Neuroinflammation and Metabolic Disorders Prevention and Treatment" Nutrients 17, no. 9: 1430. https://doi.org/10.3390/nu17091430
APA StyleCerullo, M., Armeli, F., Mengoni, B., Menin, M., Crudeli, M. L., & Businaro, R. (2025). Curcumin Modulation of the Gut–Brain Axis for Neuroinflammation and Metabolic Disorders Prevention and Treatment. Nutrients, 17(9), 1430. https://doi.org/10.3390/nu17091430






