Shared Immune and Nutrient Metabolism Pathways Between Autism Spectrum Disorder and Celiac Disease: An In Silico Approach
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Limitations
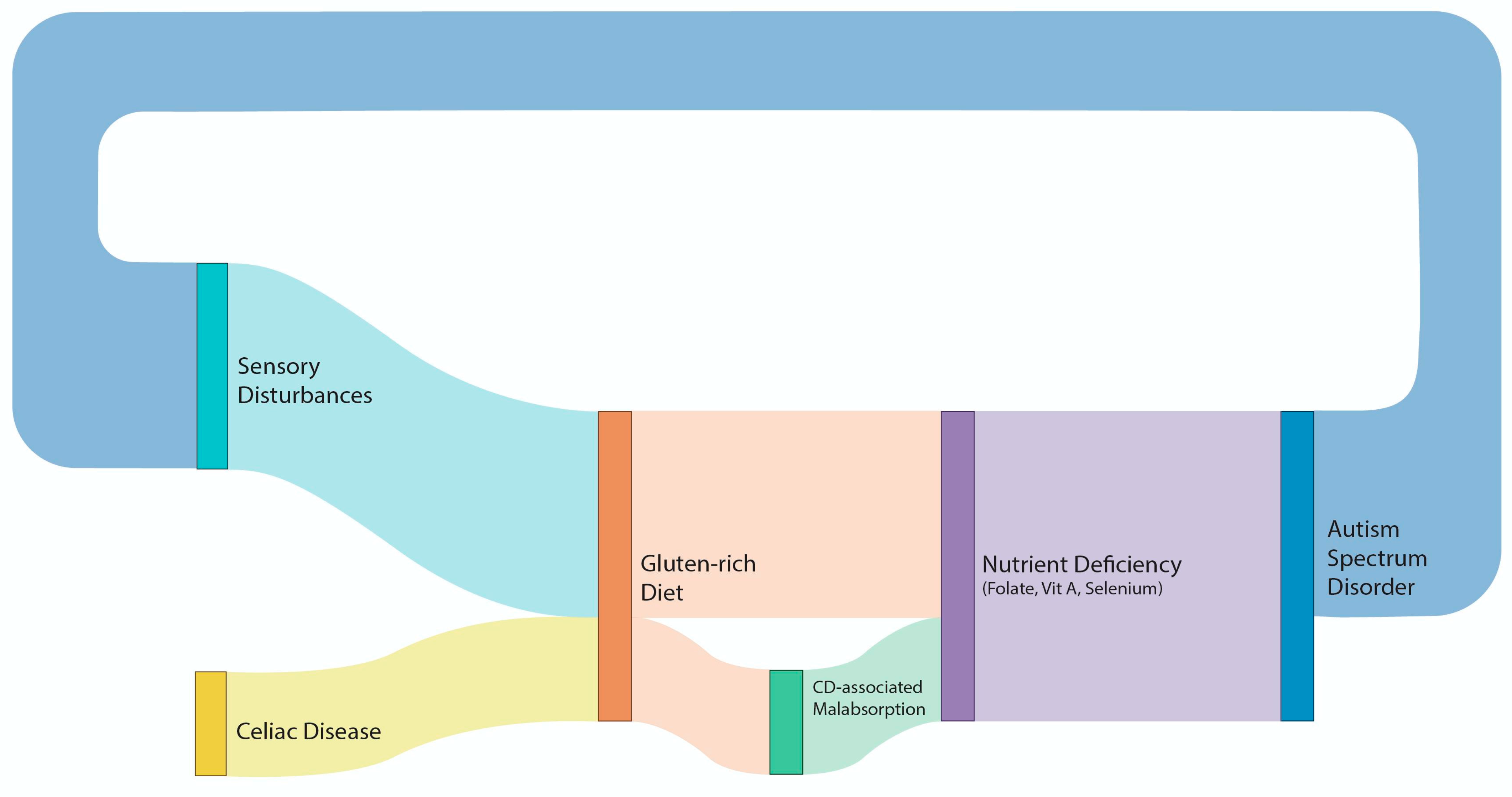
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9 (Suppl. S1), S55–S65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bai, D.; Yip, B.H.K.; Windham, G.C.; Sourander, A.; Francis, R.; Yoffe, R.; Glasson, E.; Mahjani, B.; Suominen, A.; Leonard, H.; et al. Association of Genetic and Environmental Factors with Autism in a 5-Country Cohort. JAMA Psychiatry 2019, 76, 1035–1043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vinci, M.; Treccarichi, S.; Galati Rando, R.; Musumeci, A.; Todaro, V.; Federico, C.; Saccone, S.; Elia, M.; Calì, F. A de novo ARIH2 gene mutation was detected in a patient with autism spectrum disorders and intellectual disability. Sci. Rep. 2024, 14, 15848. [Google Scholar] [CrossRef]
- Rolland, T.; Cliquet, F.; Anney, R.J.L.; Moreau, C.; Traut, N.; Mathieu, A.; Huguet, G.; Duan, J.; Warrier, V.; Portalier, S.; et al. Phenotypic effects of genetic variants associated with autism. Nat. Med. 2023, 29, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Babadi, M.; Namvarhaghighi, H.; Roullet, F.I. Role of environmental factors and epigenetics in autism spectrum disorders. Prog. Mol. Biol. Transl. Sci. 2020, 173, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Emberti Gialloreti, L.; Mazzone, L.; Benvenuto, A.; Fasano, A.; Alcon, A.G.; Kraneveld, A.; Moavero, R.; Raz, R.; Riccio, M.P.; Siracusano, M.; et al. Risk and Protective Environmental Factors Associated with Autism Spectrum Disorder: Evidence-Based Principles and Recommendations. J. Clin. Med. 2019, 8, 217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leader, G.; Abberton, C.; Cunningham, S.; Gilmartin, K.; Grudzien, M.; Higgins, E.; Joshi, L.; Whelan, S.; Mannion, A. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Systematic Review. Nutrients 2022, 14, 1471. [Google Scholar] [CrossRef]
- Baspinar, B.; Yardimci, H. Gluten-Free Casein-Free Diet for Autism Spectrum Disorders: Can It Be Effective in Solving Behavioural and Gastrointestinal Problems? Eurasian J. Med. 2020, 52, 292–297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Croall, I.D.; Hoggard, N.; Hadjivassiliou, M. Gluten and Autism Spectrum Disorder. Nutrients 2021, 13, 572. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elder, J.H.; Shankar, M.; Shuster, J.; Theriaque, D.; Burns, S.; Sherrill, L. The gluten-free, casein-free diet in autism: Results of a preliminary double blind clinical trial. J. Autism Dev. Disord. 2006, 36, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.R.; Cellier, C. Celiac disease. Gastroenterology 2007, 132, 2041–2058. [Google Scholar] [CrossRef]
- Lau, N.M.; Green, P.H.; Taylor, A.K.; Hellberg, D.; Ajamian, M.; Tan, C.Z.; Kosofsky, B.E.; Higgins, J.J.; Rajadhyaksha, A.M.; Alaedini, A. Markers of Celiac Disease and Gluten Sensitivity in Children with Autism. PLoS ONE 2013, 8, e66155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zafirovski, K.; Aleksoska, M.T.; Thomas, J.; Hanna, F. Impact of Gluten-Free and Casein-Free Diet on Behavioural Outcomes and Quality of Life of Autistic Children and Adolescents: A Scoping Review. Children 2024, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Deldar, M.; Sayehmiri, K. The Effects of a Gluten-Free/Casein-Free Diet on Behavioral Indices in Children with Autism Spectrum Disorder: A Systematic Review and Meta-analysis. Iran. J. Pediatr. 2024, 34, e140372. [Google Scholar] [CrossRef]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef]
- Louden, D.N. MedGen: NCBI’s Portal to Information on Medical Conditions with a Genetic Component. Med. Ref. Serv. Q. 2020, 39, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, N.; Kehl, T.; Lenhof, K.; Müller, A.; Mayer, C.; Eckhart, L.; Grammes, N.L.; Diener, C.; Hart, M.; Hahn, O.; et al. GeneTrail 3: Advanced high-throughput enrichment analysis. Nucleic Acids Res. 2020, 48, W515–W520. [Google Scholar] [CrossRef] [PubMed]
- Clappison, E.; Hadjivassiliou, M.; Zis, P. Psychiatric Manifestations of Coeliac Disease, a Systematic Review and Meta-Analysis. Nutrients 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edmiston, E.; Ashwood, P.; Van de Water, J. Autoimmunity, Autoantibodies, and Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 383–390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hadjivassiliou, M.; Sanders, D.S.; Grünewald, R.A.; Woodroofe, N.; Boscolo, S.; Aeschlimann, D. Gluten sensitivity: From gut to brain. Lancet Neurol. 2010, 9, 318–330. [Google Scholar] [CrossRef]
- Zis, P.; Sarrigiannis, P.G.; Rao, D.G.; Hadjivassiliou, M. Gluten neuropathy: Prevalence of neuropathic pain and the role of gluten-free diet. J. Neurol. 2018, 265, 2231–2236. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Davies-Jones, G.A.; Sanders, D.S.; Grünewald, R.A. Dietary treatment of gluten ataxia. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1221–1224. [Google Scholar] [CrossRef]
- Piwowarczyk, A.; Horvath, A.; Pisula, E.; Kawa, R.; Szajewska, H. Gluten-Free Diet in Children with Autism Spectrum Disorders: A Randomized, Controlled, Single-Blinded Trial. J. Autism Dev. Disord. 2020, 50, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Ghalichi, F.; Ghaemmaghami, J.; Malek, A.; Ostadrahimi, A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: A randomized clinical trial. World J. Pediatr. 2016, 12, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, L.; Reale, L.; Spina, M.; Guarnera, M.; Lionetti, E.; Martorana, S.; Mazzone, D. Compliant gluten-free children with celiac disease: An evaluation of psychological distress. BMC Pediatr. 2011, 11, 1–6. [Google Scholar] [CrossRef]
- Bayes, J.; Agrawal, N.; Schloss, J. The Bioavailability of Various Oral Forms of Folate Supplementation in Healthy Populations and Animal Models: A Systematic Review. J. Altern. Complement. Med. 2019, 25, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Ravn-Haren, G. Absorption, distribution, metabolism and excretion (ADME) of oral selenium from organic and inorganic sources: A review. J. Trace Elem. Med. Biol. 2021, 67, 126801. [Google Scholar] [CrossRef] [PubMed]
- von Lintig, J.; Moon, J.; Lee, J.; Ramkumar, S. Carotenoid metabolism at the intestinal barrier. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158580. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balasco, L.; Provenzano, G.; Bozzi, Y. Sensory Abnormalities in Autism Spectrum Disorders: A Focus on the Tactile Domain, From Genetic Mouse Models to the Clinic. Front. Psychiatry 2020, 10, 1016. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hazen, E.P.; Stornelli, J.L.; O’Rourke, J.A.; Koesterer, K.; McDougle, C.J. Sensory symptoms in autism spectrum disorders. Harv. Rev. Psychiatry 2014, 22, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Trott, N.; Hoggard, N.; Sanders, D.S. Sensory Symptoms without Structural Pathology in Patients with Gluten Sensitivity. Nutrients 2024, 16, 1209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marí-Bauset, S.; Zazpe, I.; Mari-Sanchis, A.; Llopis-González, A.; Morales-Suárez-Varela, M. Food selectivity in autism spectrum disorders: A systematic review. J. Child. Neurol. 2014, 29, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Fatoba, A.; Simpson, C. Assessing the causal association between celiac disease and autism spectrum disorder: A two-sample Mendelian randomization approach. Autism Res. 2025, 18, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, S.; Parma, B.; Morabito, V.; Borini, S.; Romaniello, R.; Molteni, M.; Mani, E.; Selicorni, A. Celiac disease in autism spectrum disorder: Data from an Italian child cohort. Ital. J. Pediatr. 2023, 49, 79. [Google Scholar] [CrossRef]

| Gene Ontology | FDRASD | FDRCD |
|---|---|---|
| Response to Stimulus | 1.37 × 10−5 | 3.78 × 10−12 |
| Regulation of Cell Population Proliferation | 6.47 × 10−5 | 3.66 × 10−9 |
| Response to Lipopolysaccharide | 0.0189 | 4.09 × 10−8 |
| Response to Molecules of Bacterial Origin | 0.0324 | 6.69 × 10−8 |
| Response to Lipids | 4.82 × 10−7 | 9.71 × 10−8 |
| Response to Organic Substances | 3.22 × 10−13 | 6.90 × 10−7 |
| Positive Regulation of Cell Population Proliferation | 0.0025 | 4.62 × 10−6 |
| Positive Regulation of Cell Differentiation | 0.0079 | 8.70 × 10−6 |
| Positive Regulation of Transport | 0.0482 | 6.58 × 10−5 |
| Cellular Response to Organic Substances | 1.01 × 10−7 | 1.01 × 10−4 |
| Regulation of Cell Differentiation | 5.51 × 10−4 | 1.02 × 10−4 |
| Chemical Homeostasis | 2.93 × 10−7 | 1.94 × 10−4 |
| Homeostatic Process | 1.98 × 10−8 | 2.75 × 10−4 |
| Positive Regulation of Signal Transduction | 0.0076 | 6.97 × 10−4 |
| Positive Regulation of Cation Transmembrane Transport | 0.0482 | 0.0015 |
| Positive Regulation of Ion Transmembrane Transport | 0.0125 | 0.0021 |
| Cellular Homeostasis | 1.26 × 10−5 | 0.0025 |
| Cellular Process | 7.01 × 10−18 | 0.0026 |
| Cellular Metal ion Homeostasis | 0.0131 | 0.0031 |
| Cellular Chemical Homeostasis | 4.00 × 10−4 | 0.0035 |
| Cell Motility | 1.26 × 10−5 | 0.0042 |
| Retinoid Metabolic Process | 2.35 × 10−5 | 0.0049 |
| Metal Ion Homeostasis | 2.96 × 10−4 | 0.0055 |
| Diterpenoid Metabolic Process | 1.04 × 10−5 | 0.0056 |
| Cellular Cation Homeostasis | 0.0017 | 0.0060 |
| Cellular Ion Homeostasis | 0.0025 | 0.0068 |
| Biological Process | 2.16 × 10−15 | 0.0068 |
| Response to Amyloid-Beta | 0.0083 | 0.0071 |
| Locomotion | 1.04 × 10−5 | 0.0078 |
| Cell Migration | 0.0021 | 0.0081 |
| Response to Oxidative Stress | 0.0303 | 0.0085 |
| Catabolic Process | 9.14 × 10−8 | 0.0089 |
| Response to Inorganic Substances | 0.0011 | 0.0094 |
| Positive Regulation of Metabolic Process | 1.97 × 10−5 | 0.0099 |
| Cation Homeostasis | 1.61 × 10−5 | 0.0104 |
| Isoprenoid Metabolic Process | 2.86 × 10−5 | 0.0105 |
| Positive Regulation of Nitrogen Compound Metabolic Process | 0.0039 | 0.0150 |
| Positive Regulation of Ion Transport | 0.0209 | 0.0164 |
| Ion Homeostasis | 1.03 × 10−4 | 0.0190 |
| Regulation of Signalling | 4.12 × 10−4 | 0.0191 |
| Regulation of Cellular Component Organization | 0.0010 | 0.0214 |
| Positive Regulation of Cellular Metabolic Process | 0.0020 | 0.0220 |
| Response to Nutrient Levels | 3.32 × 10−4 | 0.0229 |
| Cellular Response to Drugs | 0.0067 | 0.0243 |
| Positive Regulation of Cellular Biosynthetic Process | 0.0278 | 0.0249 |
| Response to Vitamins | 2.44 × 10−4 | 0.0255 |
| Positive Regulation of Gene Expression | 0.0010 | 0.0257 |
| Alcohol Metabolic Process | 2.07 × 10−9 | 0.0258 |
| Carboxylic Acid Metabolic Process | 5.93 × 10−18 | 0.0287 |
| Response to Extracellular Stimulus | 2.92 × 10−5 | 0.0287 |
| Cell Communication | 1.94 × 10−7 | 0.0290 |
| Monocarboxylic Acid Metabolic Process | 8.55 × 10−18 | 0.0358 |
| Response to Nutrient | 1.27 × 10−6 | 0.0379 |
| Cellular Lipid Metabolic Process | 4.87 × 10−10 | 0.0416 |
| Gene Ontology | FDRASD | FDRCD |
|---|---|---|
| Folate Metabolism | 0.0074 | 6.37 × 10−4 |
| Selenium Micronutrient Network | 9.64 × 10−4 | 0.0013 |
| Vitamin A and Carotenoid Metabolism | 0.0420 | 0.0024 |
| Nuclear Receptors Meta-Pathway | 9.43 × 10−9 | 0.0121 |
| Aryl Hydrocarbon Receptor Pathway | 3.11 × 10−5 | 0.0393 |
| Genes Involved in Male Infertility | 4.12 × 10−5 | 0.0393 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sykioti, P.; Zis, P.; Hadjikonstanti, D.; Hadjivassiliou, M.; Vavougios, G.D. Shared Immune and Nutrient Metabolism Pathways Between Autism Spectrum Disorder and Celiac Disease: An In Silico Approach. Nutrients 2025, 17, 1439. https://doi.org/10.3390/nu17091439
Sykioti P, Zis P, Hadjikonstanti D, Hadjivassiliou M, Vavougios GD. Shared Immune and Nutrient Metabolism Pathways Between Autism Spectrum Disorder and Celiac Disease: An In Silico Approach. Nutrients. 2025; 17(9):1439. https://doi.org/10.3390/nu17091439
Chicago/Turabian StyleSykioti, Panagiota, Panagiotis Zis, Despina Hadjikonstanti, Marios Hadjivassiliou, and George D. Vavougios. 2025. "Shared Immune and Nutrient Metabolism Pathways Between Autism Spectrum Disorder and Celiac Disease: An In Silico Approach" Nutrients 17, no. 9: 1439. https://doi.org/10.3390/nu17091439
APA StyleSykioti, P., Zis, P., Hadjikonstanti, D., Hadjivassiliou, M., & Vavougios, G. D. (2025). Shared Immune and Nutrient Metabolism Pathways Between Autism Spectrum Disorder and Celiac Disease: An In Silico Approach. Nutrients, 17(9), 1439. https://doi.org/10.3390/nu17091439








