Abnormal Plasma/Serum Magnesium, Copper, and Zinc Concentrations Associate with the Future Development of Cardiovascular Diseases
Highlights
- Abnormal plasma levels of magnesium, copper, and zinc were associated with an increased risk of major adverse cardiovascular events (MACEs) in a cohort of the Scottish population.
- 2High copper levels and low magnesium and zinc levels were linked to an increased prevalence of circulatory system diseases.
- Electronic health records and ICD-10 coding enabled the retrospective identification of MACEs and disease subtypes.
- Logistic regression analysis revealed specific metal ion profiles associated with distinct circulatory disease groups.
- These findings underscore the value of monitoring trace metal status in cardiovascular risk assessment and prevention strategies.
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Overall Populations and MACE Patient Identification
2.3. Analysis of Plasma/Serum Magnesium, Copper, and Zinc Concentration Data in MACE Patients
2.4. Analysis of Plasma/Serum Magnesium, Copper, and Zinc Concentration Data in MACE Patients
3. Results
3.1. Magnesium, Copper, and Zinc Status in MACE Patients and Control Groups
3.2. Logistic Regression Analysis of Magnesium, Copper, and Zinc Status Prior to the Development of Circulatory System Diseases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHI | Community health index |
| CI | Confidence interval |
| CVD | Cardiovascular disease |
| EHRs | Electronic health records |
| glm | Generalised linear model |
| HIC | Health Informatics Centre |
| ICD-10 | International Classification of Diseases, 10th Revision |
| MACEs | Major adverse cardiovascular events |
| MI | Myocardial infarction |
| NHS | National Health Service |
| OR | Odds ratio |
| PROCHI | Pseudo-CHI |
| SMRs | Scottish morbidity records |
| WHO | World Health Organisation |
References
- WHO. Cardiovascular Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 7 March 2025).
- GBD. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Hong, X.; Tian, G.; Zhu, Y.; Ren, T. Exogeneous metal ions as therapeutic agents in cardiovascular disease and their delivery strategies. Regen. Biomater. 2023, 11, rbad103. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Liu, J.; O’Keefe, J.H. Magnesium for the prevention and treatment of cardiovascular disease. Open Heart 2018, 5, e000775. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, R.; Davies, A.; Veenhuizen, M.; Campbell, M.; Pitt, S.J.; Ajjan, R.A.; Stewart, A.J. Magnesium deficiency and cardiometabolic disease. Nutrients 2023, 15, 2355. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. The role of dietary magnesium in cardiovascular disease. Nutrients 2024, 16, 4223. [Google Scholar] [CrossRef]
- Klevay, L.M. Cardiovascular disease from copper deficiency—A history. J. Nutr. 2000, 130, 489S–492S. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Q.; Liang, R.; Zhang, D.; Liu, X.; Zhang, M.; Xiong, Y.; Xu, M.; Liu, Q.; Li, P.; et al. Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death Dis. 2023, 14, 105. [Google Scholar] [CrossRef]
- Vu, T.; Fredenburgh, J.; Weitz, J. Zinc: An important cofactor in haemostasis and thrombosis. Thromb. Haemost. 2013, 109, 421–430. [Google Scholar] [CrossRef]
- Guo, D.; Du, Y.; Wu, Q.; Jiang, W.; Bi, H. Disrupted calcium homeostasis is involved in elevated zinc ion-induced photoreceptor cell death. Arch. Biochem. Biophys. 2014, 560, 44–51. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pagidipati, N.J.; Califf, R.M.; McGuire, D.K.; Green, J.B.; Demets, D.; George, J.T.; Gerstein, H.C.; Hobbs, T.; Holman, R.R.; et al. Impact of regulatory guidance on evaluating cardiovascular risk of new Glucose-Lowering therapies to treat Type 2 diabetes mellitus. Circulation 2020, 141, 843–862. [Google Scholar] [CrossRef]
- Marx, N.; McGuire, D.K.; Perkovic, V.; Woerle, H.-J.; Broedl, U.C.; Von Eynatten, M.; George, J.T.; Rosenstock, J. Composite primary end points in cardiovascular outcomes trials involving Type 2 diabetes patients: Should unstable angina be included in the primary end point? Diabetes Care 2017, 40, 1144–1151. [Google Scholar] [CrossRef]
- Bosco, E.; Hsueh, L.; McConeghy, K.W.; Gravenstein, S.; Saade, E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: A systematic review. BMC Med. Res. Methodol. 2021, 21, 241. [Google Scholar] [CrossRef]
- HIC. Data service at HIC. University of Dundee, UK. Available online: https://www.dundee.ac.uk/hic/data-service (accessed on 7 March 2025).
- McQueenie, R.; Nicholl, B.I.; Jani, B.D.; Canning, J.; Macdonald, S.; McCowan, C.; Neary, J.; Browne, S.; Mair, F.S.; Siebert, S. Patterns of multimorbidity and their effects on adverse outcomes in rheumatoid arthritis: A study of 5658 UK Biobank participants. BMJ Open 2020, 10, e038829. [Google Scholar] [CrossRef]
- WHO. International Statistical Classification of Diseases and Related Health Problems, 10th Revision, 2019 Edition. Available online: https://icd.who.int/browse10/2019/en (accessed on 7 March 2025).
- Public Health England. National Diet and Nutrition Survey: Results from Years 9 to 11 (2016/2017 to 2018/2019); Department of Health and Social Care: London, UK, 2020. Available online: https://www.gov.uk/government/statistics/ndns-results-from-years-9-to-11-2016-to-2017-and-2018-to-2019 (accessed on 9 April 2025).
- Goff, J.P. Invited review: Mineral absorption mechanisms, mineral interactions that affect acid–base and antioxidant status, and diet considerations to improve mineral status. J. Dairy Sci. 2018, 101, 2763–2813. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.-C.; Wang, W.-F.; Li, Z.-M.; Duan, Y.-J.; Chen, M.; Wu, Y.-N.; Hu, Y.-M. The Relationship between Dietary Patterns and Blood Mineral Concentration among Children in Hunan Province of China. BMC Public Health 2023, 23, 1518. [Google Scholar] [CrossRef]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.R.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome Connections with Host Metabolism and Habitual Diet from 1,098 Deeply Phenotyped Individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chen, B.; Zhang, F.; Zhang, B.; Guo, Y.; Pang, M.; Huang, L.; Wang, T. Toxic and Essential Metals: Metabolic Interactions with the Gut Microbiota and Health Implications. Front. Nutr. 2024, 11, 1448388. [Google Scholar] [CrossRef]
- Pajarillo, E.A.B.; Lee, E.; Kang, D.-K. Trace Metals and Animal Health: Interplay of the Gut Microbiota with Iron, Manganese, Zinc, and Copper. Anim. Nutr. 2021, 7, 750–761. [Google Scholar] [CrossRef]
- Del Chierico, F.; Trapani, V.; Petito, V.; Reddel, S.; Pietropaolo, G.; Graziani, C.; Masi, L.; Gasbarrini, A.; Putignani, L.; Scaldaferri, F.; et al. Dietary Magnesium Alleviates Experimental Murine Colitis through Modulation of Gut Microbiota. Nutrients 2021, 13, 4188. [Google Scholar] [CrossRef]
- Kumar, R.; Grover, S.; Batish, V.K. Nutraceuticals and Gut Microbiota: Understanding the Connection for Improved Health. Trends Food Sci. Technol. 2020, 102, 555–566. [Google Scholar] [CrossRef]
- Tan, M.-Y.; Mo, C.-Y.; Zhao, Q. The association between magnesium depletion score and hypertension in US adults: Evidence from the national Health and nutrition examination survey (2007–2018). Biol. Trace Elem. Res. 2023, 202, 4418–4430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, L.; Dong, Y.; Xu, J.; Wei, Y.; Yu, D.; Xu, J.; Zhang, W. The effect of magnesium intake on stroke incidence: A systematic review and meta-analysis with trial sequential analysis. Front. Neurol. 2019, 10, 852. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; O’Keefe, J.H.; Wilson, W. Subclinical magnesium deficiency: A principal driver of cardiovascular disease and a public health crisis. Open Heart 2018, 5, e000668. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Dietrich, H.H.; Horiuchi, T.; Hongo, K.; Dacey, R.G. Mechanisms of magnesium-induced vasodilation in cerebral penetrating arterioles. Neurosci. Res. 2015, 107, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Xu, H.; Wang, C.; Qin, H.; An, Z. Magnesium enhances the chondrogenic differentiation of mesenchymal stem cells by inhibiting activated macrophage-induced inflammation. Sci. Rep. 2018, 8, 3406. [Google Scholar] [CrossRef]
- Song, B.; Jiang, M.; Zhang, Y.; Xu, Y.; Wu, C.; Wu, D.; Zhou, C.; Li, M.; Ji, X. Research hotpots and frontier trends of neuroprotective effects of magnesium from 1999 to 2023: A bibliometric analysis. CNS Neurosci. Ther. 2024, 30, e14597. [Google Scholar] [CrossRef]
- Zhao, H.; Mei, K.; Hu, Q.; Wu, Y.; Xu, Y.; Qinling, N.; Yu, P.; Deng, Y.; Zhu, W.; Yan, Z.; et al. Circulating copper levels and the risk of cardio-cerebrovascular diseases and cardiovascular and all-cause mortality: A systematic review and meta-analysis of longitudinal studies. Environ. Pollut. 2023, 340, 122711. [Google Scholar] [CrossRef]
- Schuschke, D.A. Dietary copper in the physiology of the microcirculation. J. Nutr. 1997, 127, 2274–2281. [Google Scholar] [CrossRef]
- Cheng, F.; Peng, G.; Lu, Y.; Wang, K.; Ju, Q.; Ju, Y.; Ouyang, M. Relationship between copper and immunity: The potential role of copper in tumor immunity. Front. Oncol. 2022, 12, 1019153. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.I.; Lee, C.C.; Norvell, J.E. Effect of copper deficiency on the lymphatic absorption of cholesterol, plasma chylomicron clearance, and postheparin lipase activities. Exp. Biol. Med. 1988, 188, 410–419. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Mangan, D.; O’Keefe, J.H. Copper deficiency may be a leading cause of ischaemic heart disease. Open Heart 2018, 5, e000784. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Su, Y.; Zheng, Y.; Fu, B.; Tang, L.; Qin, Y.-X. Zinc regulates vascular endothelial cell activity through zinc-sensing receptor ZnR/GPR39. Am. J. Physiol. Cell Physiol. 2017, 314, C404–C414. [Google Scholar] [CrossRef]
- Betrie, A.H.; Brock, J.A.; Harraz, O.F.; Bush, A.I.; He, G.-W.; Nelson, M.T.; Angus, J.A.; Wright, C.E.; Ayton, S. Zinc drives vasorelaxation by acting in sensory nerves, endothelium and smooth muscle. Nat. Commun. 2021, 12, 3296. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Huang, L.; Zhao, J.; Wang, Z.; Yao, W.; Wu, X.; Huang, J.; Bian, B. The relationship between serum zinc level and heart failure: A meta-analysis. BioMed. Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Tanita, A.; Namiuchi, S.; Onodera, K.; Sunamura, S.; Ogata, T.; Noda, K.; Takii, T. Serum zinc concentration in patients with myocardial infarction: A retrospective study. BMC Cardiovasc. Disord. 2024, 24, 107. [Google Scholar] [CrossRef]
- Munk, D.E.; Laursen, T.L.; Kirk, F.T.; Vilstrup, H.; Ala, A.; Gormsen, L.C.; Ott, P.; Sandahl, T.D. Effect of oral zinc regimens on human hepatic copper content: A randomized intervention study. Sci. Rep. 2022, 12, 14714. [Google Scholar] [CrossRef]
- Lee, S.R. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid. Med. Cell Longev. 2018, 2018, 11. [Google Scholar] [CrossRef]
- Tubek, S. Correlations between serum zinc concentrations and oxygen balance parameters in patients with primary arterial hypertension. Biol. Trace Elem. Res. 2007, 115, 213–222. [Google Scholar] [CrossRef]
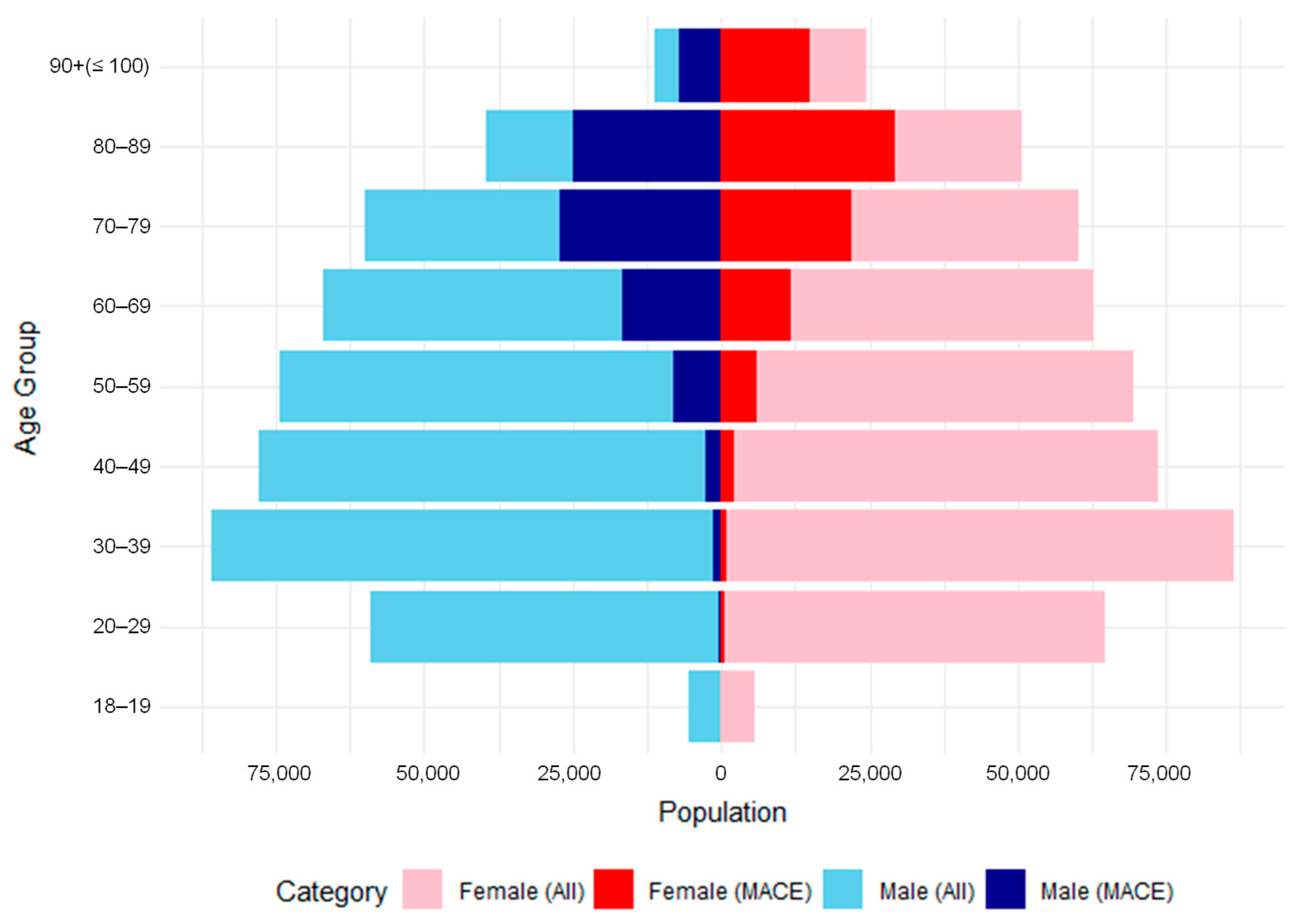


| ICD-10 Code | Disease Category |
|---|---|
| I00–I02 | Acute rheumatic fever |
| I05–I09 | Chronic rheumatic heart diseases |
| I10–I15 | Hypertensive diseases |
| I20–I25 | Ischaemic heart diseases |
| I26–I28 | Pulmonary heart disease and diseases of pulmonary circulation |
| I30–I52 | Other forms of heart disease |
| I60–I69 | Cerebrovascular diseases |
| I70–I78 | Diseases of arteries, arterioles, and capillaries |
| G45.0 | Vertebro-basilar artery syndrome |
| G45.1 | Carotid artery syndrome (hemispheric) |
| G45.2 | Multiple and bilateral precerebral artery syndromes |
| G45.3 | Amaurosis fugax |
| G45.4 | Transient global amnesia |
| G45.8 | Other transient cerebral ischaemic attacks and related syndromes |
| G45.9 | Transient cerebral ischaemic attack, unspecified |
| G46.0 | Middle cerebral artery syndrome |
| G46.1 | Anterior cerebral artery syndrome |
| G46.2 | Posterior cerebral artery syndrome |
| G46.3 | Brain stem stroke syndrome |
| G46.4 | Cerebellar stroke syndrome |
| Magnesium | ||||||
|---|---|---|---|---|---|---|
| Status | High | Normal | Low | |||
| Total number | 4164 | 104,818 | 15,704 | |||
| Group | MACE patients | Control | MACE patients | Control | MACE patients | Control |
| Number | 1223 | 2941 | 113,886 | 410,181 | 5455 | 10,249 |
| Percentages | 29.4% | 70.6% | 27.9% | 72.1% | 34.7% | 65.3% |
| p-value | High vs. Normal; p < 0.0001 **** | Normal vs. Low; p < 0.0001 **** | ||||
| Copper | ||||||
| Status | High | Normal | Low | |||
| Total number | 568 | 3361 | 101 | |||
| Group | MACE patients | Control | MACE patients | Control | MACE patients | Control |
| Number | 103 | 465 | 492 | 2869 | 10 | 91 |
| Percentages | 18.1% | 81.9% | 14.6% | 85.4% | 9.9% | 90.1% |
| p-value | High vs. Normal; p = 0.0316 * | Normal vs. Low; p = 0.1828 | ||||
| Zinc | ||||||
| Status | High | Normal | Low | |||
| Total number | 64 | 1893 | 404 | |||
| Group | MACE patients | Control | MACE patients | Control | MACE patients | Control |
| Number | 6 | 58 | 164 | 1729 | 73 | 331 |
| Percentages | 9.4% | 90.6% | 8.7% | 91.3% | 18.1% | 81.9% |
| p-value | High vs. Normal; p = 0.8424 | Normal vs. Low; p < 0.0001 **** | ||||
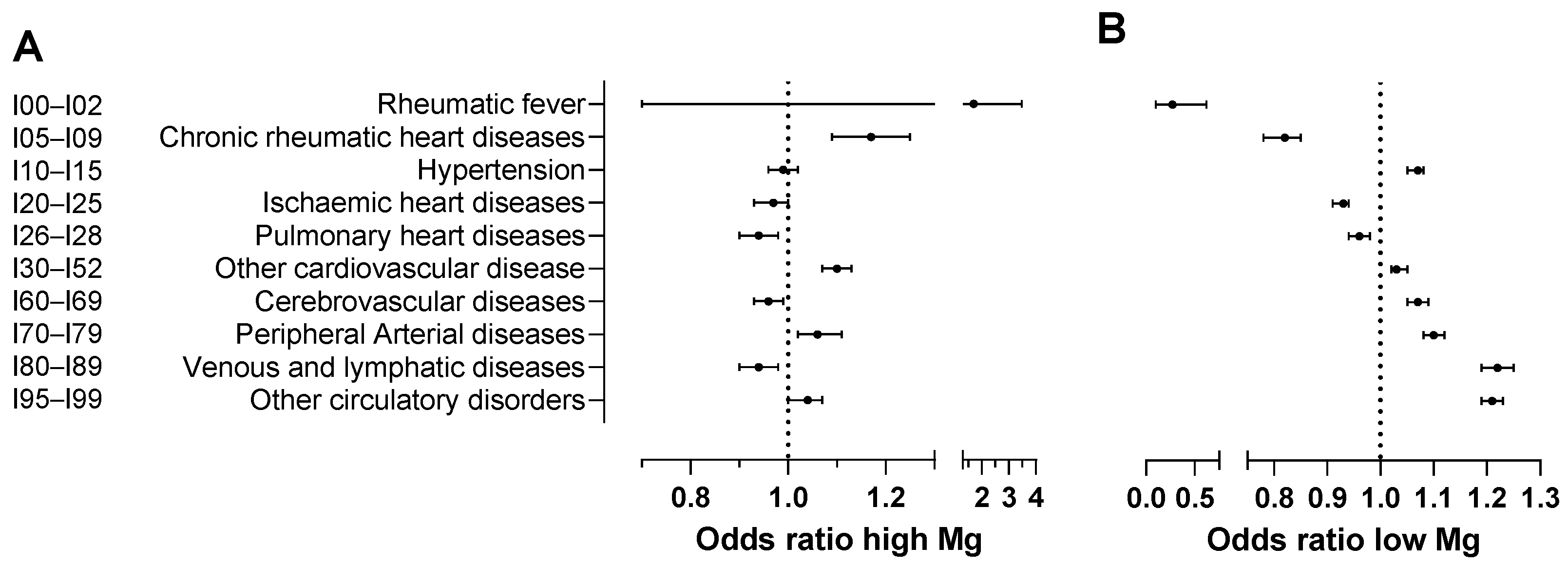
| ICD-10 Codes | Diseases of the Circulatory System | OR for High Magnesium | 95%CI for High Magnesium | OR for Low Magnesium | 95%CI for Low Magnesium |
|---|---|---|---|---|---|
| I00–I02 | Acute rheumatic fever | 1.70 | [0.77, 3.73] | 0.27 ** | [0.11, 0.68] |
| I05–I09 | Chronic rheumatic heart diseases | 1.17 *** | [1.09, 1.26] | 0.82 *** | [0.78, 0.85] |
| I10–I15 | Hypertensive diseases | 0.99 | [0.96, 1.02] | 1.07 *** | [1.05, 1.08] |
| I20–I25 | Ischaemic heart diseases | 0.97 * | [0.93, 1.00] | 0.93 *** | [0.91, 0.94] |
| I26–I28 | Pulmonary heart disease and diseases of pulmonary circulation | 0.94 ** | [0.90, 0.98] | 0.96 *** | [0.94, 0.98] |
| I30–I52 | Other forms of heart disease | 1.10 *** | [1.07, 1.13] | 1.03 *** | [1.02, 1.05] |
| I60–I69 | Cerebrovascular diseases | 0.96 * | [0.93, 0.99] | 1.07 *** | [1.05, 1.09] |
| I70–I79 | Diseases of arteries, arterioles and capillaries | 1.06 ** | [1.02, 1.11] | 1.10 *** | [1.08, 1.12] |
| I80–I89 | Diseases of veins, lymphatic vessels and lymph nodes, not elsewhere classified | 0.94 | [0.90, 0.98] | 1.22 *** | [1.19, 1.25] |
| I95–I99 | Other unspecified disorders of the circulatory system | 1.04 * | [1.00, 1.07] | 1.21 *** | [1.19, 1.23] |
| ICD-10 Codes | Diseases of the Circulatory System | OR for High Copper | 95%CI for High Copper | OR for Low Copper | 95%CI for Low Copper |
|---|---|---|---|---|---|
| I00–I02 | Acute rheumatic fever | No data available | |||
| I05–I09 | Chronic rheumatic heart diseases | 2.26 * | [1.07, 4.76] | 1.03 | [0.14, 7.99] |
| I10–I15 | Hypertensive diseases | 1.78 *** | [1.40, 2.26] | 0.51 | [0.24, 1.10] |
| I20–I25 | Ischaemic heart diseases | 1.64 ** | [1.19, 2.25] | 0.26 | [0.06, 1.06] |
| I26–I28 | Pulmonary heart disease and diseases of pulmonary circulation | 1.52 | [0.96, 2.41] | 0.58 | [0.14, 2.36] |
| I30–I52 | Other forms of heart disease | 1.37 | [1.07, 1.76] | 1.21 | [0.73, 2.00] |
| I60–I69 | Cerebrovascular diseases | 1.62 ** | [1.17, 2.23] | 0.28 | [0.07, 1.13] |
| I70–I79 | Diseases of arteries, arterioles and capillaries | 1.48 * | [1.01, 2.16] | 0.75 | [0.27, 2.04] |
| I80–I89 | Diseases of veins, lymphatic vessels and lymph nodes, not elsewhere classified | 1.37 | [0.98, 1.91] | 2.64 *** | [1.59, 4.38] |
| I95–I99 | Other unspecified disorders of the circulatory system | 1.20 | [0.90, 1.59] | 1.19 | [0.67, 2.11] |
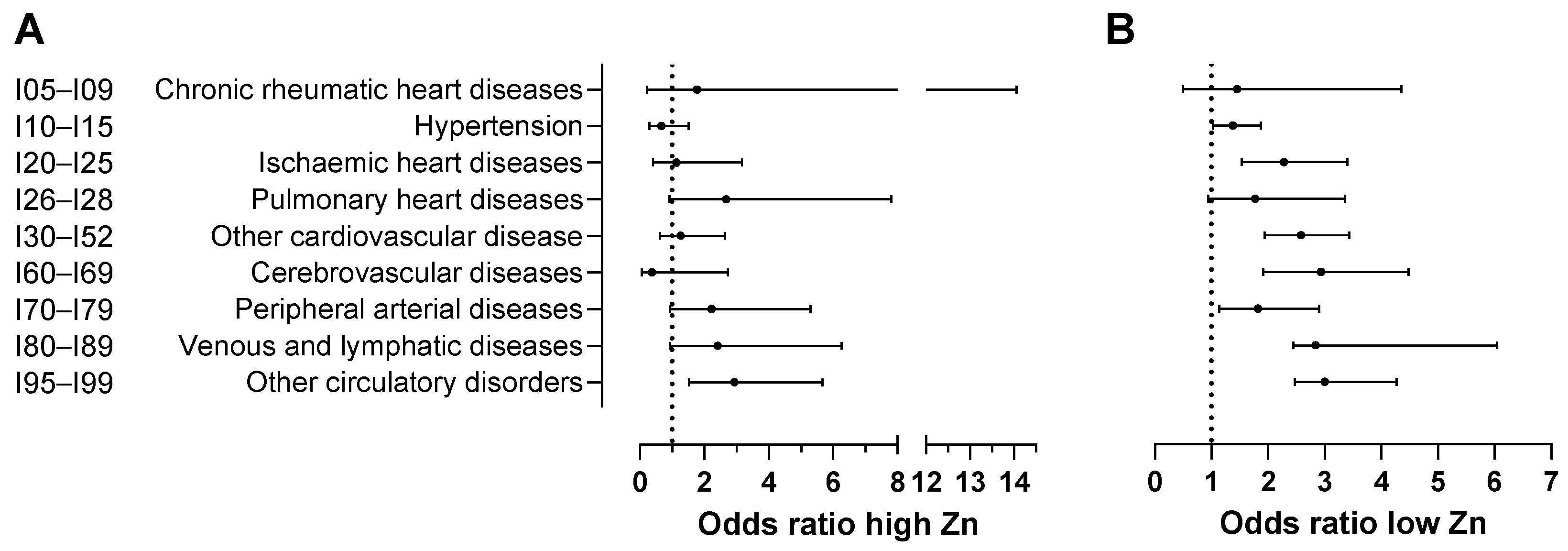
| ICD-10 Codes | Diseases of the Circulatory System | OR for High Zinc | 95%CI for High Zinc | OR for Low Zinc | 95%CI for Low Zinc |
|---|---|---|---|---|---|
| I00–I02 | Acute rheumatic fever | No data available | |||
| I05–I09 | Chronic rheumatic heart diseases | 1.77 | [0.22, 14.05] | 1.45 | [0.49, 4.35] |
| I10–I15 | Hypertensive diseases | 0.66 | [0.29, 1.51] | 1.38 * | [1.02, 1.87] |
| I20–I25 | Ischaemic heart diseases | 1.14 | [0.41, 3.17] | 2.28 *** | [1.53, 3.40] |
| I26–I28 | Pulmonary heart disease and diseases of pulmonary circulation | 2.68 | [0.92, 7.81] | 1.77 | [0.94, 3.35] |
| I30–I52 | Other forms of heart disease | 1.26 | [0.61, 2.64] | 2.58 *** | [1.94, 3.43] |
| I60–I69 | Cerebrovascular diseases | 0.37 | [0.05, 2.73] | 2.93 *** | [1.91, 4.48] |
| I70–I79 | Diseases of arteries, arterioles and capillaries | 2.23 | [0.94, 5.29] | 1.82 * | [1.14, 2.90] |
| I80–I89 | Diseases of veins, lymphatic vessels and lymph nodes, not elsewhere classified | 2.41 | [0.93, 6.26] | 3.84 *** | [2.44, 6.04] |
| I95–I99 | Other unspecified disorders of the circulatory system | 2.93 ** | [1.52, 5.67] | 3.00 *** | [2.11, 4.27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, B.; Alexander, R.; Fritzen, R.; Mills, S.; Stewart, A.J.; McCowan, C. Abnormal Plasma/Serum Magnesium, Copper, and Zinc Concentrations Associate with the Future Development of Cardiovascular Diseases. Nutrients 2025, 17, 1447. https://doi.org/10.3390/nu17091447
Lin B, Alexander R, Fritzen R, Mills S, Stewart AJ, McCowan C. Abnormal Plasma/Serum Magnesium, Copper, and Zinc Concentrations Associate with the Future Development of Cardiovascular Diseases. Nutrients. 2025; 17(9):1447. https://doi.org/10.3390/nu17091447
Chicago/Turabian StyleLin, Boyang, Robin Alexander, Remi Fritzen, Sarah Mills, Alan J. Stewart, and Colin McCowan. 2025. "Abnormal Plasma/Serum Magnesium, Copper, and Zinc Concentrations Associate with the Future Development of Cardiovascular Diseases" Nutrients 17, no. 9: 1447. https://doi.org/10.3390/nu17091447
APA StyleLin, B., Alexander, R., Fritzen, R., Mills, S., Stewart, A. J., & McCowan, C. (2025). Abnormal Plasma/Serum Magnesium, Copper, and Zinc Concentrations Associate with the Future Development of Cardiovascular Diseases. Nutrients, 17(9), 1447. https://doi.org/10.3390/nu17091447






