Efficacy and Tolerability of a Chemically Characterized Scutellaria lateriflora L. Extract-Based Food Supplement for Sleep Management: A Single-Center, Controlled, Randomized, Crossover, Double-Blind Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. RP-UHPLC-MS Analysis of S. lateriflora Extract
2.2. Food Supplement and Placebo
2.3. Study Design
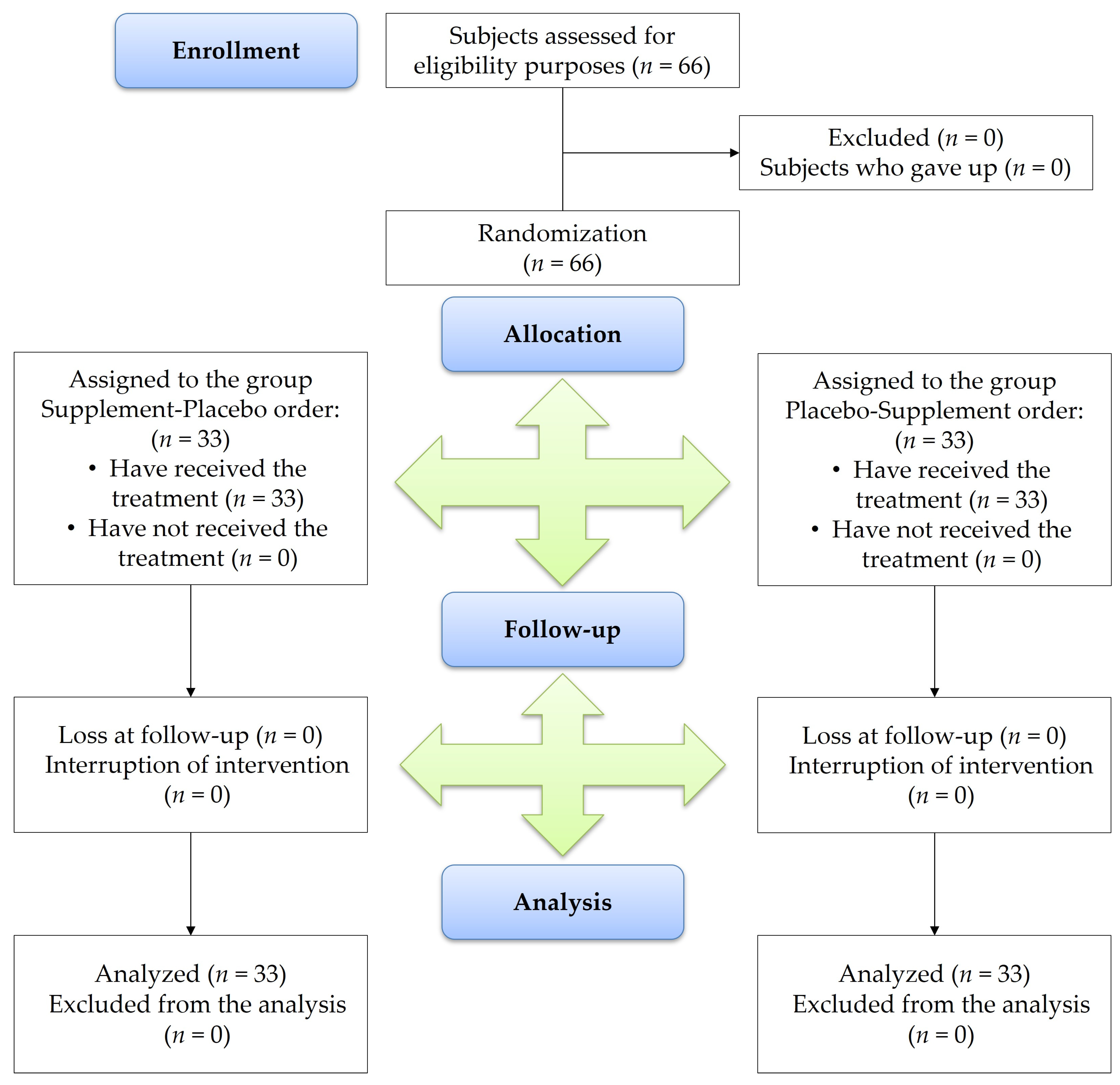
2.4. Participants and Recruiting
2.5. Outcomes of Study
2.6. Data Collection
2.7. Safety and Tolerability
2.8. Statistical Analysis
3. Results
3.1. Chemical Profile of S. lateriflora Extract
3.2. Efficacy and Tolerability of Food Supplement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRF | Case Reporting Form |
| GABA | Gamma-aminobutyric acid |
| GAD-7 | Generalized Anxiety Disorder 7 |
| ISI | Insomnia Severity Index |
| LMM | Linear Mixed Model |
| NR | Number of Awakenings |
| PHQ-9 | Patient Health Questionnaire-9 |
| PSQI | Pittsburgh Sleep Quality Index |
| SEI | Sleep Efficiency Index |
| SOL | Sleep Onset Latency |
| TST | Total Sleep Time |
| TTL | Total Time in Bed |
| VAS | Visual Analog Scale |
| WASO | Wakefulness After Sleep Onset |
References
- Burman, D. Sleep disorders: Insomnia. FP Essent. 2017, 460, 22–28. [Google Scholar] [PubMed]
- Rosenberg, R.S.; Van Hout, S. The American Academy of Sleep Medicine inter-scorer reliability program: Sleep stage scoring. J. Clin. Sleep Med. 2013, 9, 81–87. [Google Scholar] [CrossRef]
- Perlis, M.; Gehrman, P. Types of insomnia. Encycl. Sleep 2013, 1, 199–202. [Google Scholar]
- Riemann, D.; Nissen, C.; Palagini, L.; Otte, A.; Perlis, M.L.; Spiegelhalder, K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015, 14, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Kraus, S.S.; Rabin, L.A. Sleep America: Managing the crisis of adult chronic insomnia and associated conditions. J. Affect. Disord. 2012, 138, 192–212. [Google Scholar] [CrossRef]
- Balbo, M.; Leproult, R.; Van Cauter, E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int. J. Endocrinol. 2010, 2010, 759234. [Google Scholar] [CrossRef]
- Haimov, I. Association between memory impairment and insomnia among older adults. Eur. J. Ageing 2006, 3, 107–115. [Google Scholar] [CrossRef]
- de Almondes, K.M.; Costa, M.V.; Malloy-Diniz, L.F.; Diniz, B.S. Insomnia and risk of dementia in older adults: Systematic review and meta-analysis. J. Psychiatr. Res. 2016, 77, 109–115. [Google Scholar] [CrossRef]
- Okajima, I.; Komada, Y.; Nomura, T.; Nakashima, K.; Inoue, Y. Insomnia as a risk for depression: A longitudinal epidemiologic study on a Japanese rural cohort. J. Clin. Psychiatry 2012, 73, 377–383. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chen, Y.T.; Chen, M.H.; Huang, C.C.; Chiang, C.H.; Huang, P.H.; Chen, J.W.; Chen, T.J.; Lin, S.J.; Leu, H.B.; et al. The association between insomnia and increased future cardiovascular events: A nationwide population-based study. Psychosom. Med. 2015, 77, 743–751. [Google Scholar] [CrossRef]
- Léger, D.; Poursain, B.; Neubauer, D.; Uchiyama, M. An international survey of sleeping problems in the general population. Curr. Med. Res. Opin. 2008, 24, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Kripke, D.F. Hypnotic drug risks of mortality, infection, depression, and cancer: But lack of benefit. F1000Research 2016, 5, 918. [Google Scholar] [CrossRef]
- Buscemi, N.; Vandermeer, B.; Friesen, C.; Bialy, L.; Tubman, M.; Ospina, M.; Klassen, T.P.; Witmans, M. The efficacy and safety of drug treatments for chronic insomnia in adults: A meta-analysis of RCTs. J. Gen. Intern. Med. 2007, 22, 1335–1350. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; Gaulier, B.; Barry, T.; Kowatch, R.A. Patients’ acceptance of psychological and pharmacological therapies for insomnia. Sleep 1992, 15, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Guadagna, S.; Barattini, D.F.; Rosu, S.; Ferini-Strambi, L. Plant extracts for sleep disturbances: A systematic review. Evid. Based Complement. Alternat. Med. 2020, 2020, 3792390. [Google Scholar] [CrossRef]
- Sánchez-Ortuño, M.M.; Bélanger, L.; Ivers, H.; LeBlanc, M.; Morin, C.M. The use of natural products for sleep: A common practice? Sleep Med. 2009, 10, 982–987. [Google Scholar] [CrossRef]
- Maroo, N.; Hazra, A.; Das, T. Efficacy and safety of a polyherbal sedative-hypnotic formulation NSF-3 in primary insomnia in comparison to zolpidem: A randomized controlled trial. Indian J. Pharmacol. 2013, 45, 34–39. [Google Scholar]
- Schulz, V.; Hänsel, R.; Tyler, V.E. Rational Phytotherapy: A Physicians’ Guide to Herbal Medicine, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Wheatley, D. Herbal products, stress, and the mind. In Nutrients, Stress, and Medical Disorders; Yehuda, S., Mostofsky, D.I., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 137–153. [Google Scholar]
- Sherman, S.H.; Joshee, N. Current status of research on medicinal plant Scutellaria lateriflora: A review. J. Med. Aromat. Plants 2022, 11, 22–38. [Google Scholar]
- Upton, R.; Dayu, R.H. Skullcap Scutellaria lateriflora L.: An American nervine. J. Herb. Med. 2012, 2, 76–96. [Google Scholar] [CrossRef]
- Buccato, D.G.; Ullah, H.; De Lellis, L.F.; Piccinocchi, R.; Baldi, A.; Xiao, X.; Arciola, C.R.; Di Minno, A.; Daglia, M. In Vitro Assessment of Cortisol Release Inhibition, Bioaccessibility and Bioavailability of a Chemically Characterized Scutellaria lateriflora L. Hydroethanolic Extract. Mol. 2024, 29, 586. [Google Scholar] [CrossRef]
- Sarris, J.; McIntyre, E.; Camfield, D.A. Plant-based medicines for anxiety disorders, part 2: A review of clinical studies with supporting preclinical evidence. CNS Drugs 2013, 27, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Di Minno, A.; De Filippis, A.; Sommella, E.; Buccato, D.G.; De Lellis, L.F.; El-Seedi, H.R.; Khalifa, S.A.M.; Piccinocchi, R.; Galdiero, M.; et al. In vitro antimicrobial and antibiofilm properties and bioaccessibility after oral digestion of chemically characterized extracts obtained from Cistus × incanus L., Scutellaria lateriflora L., and their combination. Foods, 2023; 12, 1826. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Pasquarella, C.; Odone, A.; Colucci, M.E.; Costanza, A.; Serafini, G.; Aguglia, A.; Murri, M.B.; Brakoulias, V.; Amore, M.; et al. Screening for depression in primary care with Patient Health Questionnaire-9 (PHQ-9): A systematic review. J. Affect. Disord. 2021, 279, 473–483. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Baek, J.; Han, K.; Choi-Kwon, S. Sleep diary-and actigraphy-derived sleep parameters of 8-hour fast-rotating shift work nurses: A prospective descriptive study. Int. J. Nurs. Stud. 2020, 112, 103719. [Google Scholar] [CrossRef]
- Alqurashi, Y.D.; Dawidziuk, A.; Alqarni, A.; Kelly, J.; Moss, J.; Polkey, M.I.; Morrell, M.J. A visual analog scale for the assessment of mild sleepiness in patients with obstructive sleep apnea and healthy participants. Ann. Thorac. Med. 2021, 16, 141–147. [Google Scholar] [CrossRef] [PubMed]
- VigiErbe. Available online: www.vigierbe.it (accessed on 28 August 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2014, 67, 1–48. [Google Scholar]
- The R Core Team. R: A Language and Environment for Statistical Computing. 2022. Available online: http://www.r-project.org/index.html (accessed on 24 December 2024).
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical analysis working group (CAWG) metabolomics standards initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Calvert, M.; Blazeby, J.; Altman, D.G.; Revicki, D.A.; Moher, D.; Brundage, M.D.; Group, C.P. Reporting of patient-reported outcomes in randomized trials: The CONSORT PRO extension. JAMA 2013, 309, 814–822. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Draft guidance on the scientific requirements for health claims related to neurological and psychological functions. EFSA J. 2011, 9, 2170. [Google Scholar]
- Brock, C.; Whitehouse, J.; Tewfik, I.; Towell, T. American skullcap (Scutellaria lateriflora): A randomised, double-blind placebo-controlled crossover study of its effects on mood in healthy volunteers. Phytother. Res. 2014, 28, 692–698. [Google Scholar] [CrossRef]
- Pary, R.; Matuschka, P.R.; Lewis, S.; Caso, W.; Lippmann, S. Generalized anxiety disorder. South. Med. J. 2003, 96, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Brock, C.; Whitehouse, J.; Tewfik, I.; Towell, T. The use of Scutellaria lateriflora: A pilot survey amongst herbal medicine practitioners. J. Herb. Med. 2012, 2, 34–41. [Google Scholar] [CrossRef]
- Awad, R.; Arnason, J.T.; Trudeau, V.; Bergeron, C.; Budzinski, J.W.; Foster, B.C.; Merali, Z. Phytochemical and biological analysis of skullcap (Scutellaria lateriflora L.): A medicinal plant with anxiolytic properties. Phytomedicine 2003, 10, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yan Huen, M.S.; Tsang, S.Y.; Xue, H. Neuroactive flavonoids interacting with GABAA receptor complex. CNS Neurol. Disord. Drug Targets 2005, 4, 575–585. [Google Scholar] [CrossRef]
- Hui, K.M.; Wang, X.H.; Xue, H. Interaction of flavones from the roots of Scutellaria baicalensis with the benzodiazepine site. Planta Med. 2000, 66, 91–93. [Google Scholar] [CrossRef]
- de Carvalho, R.S.M.; Duarte, F.S.; de Lima, T.C.M. Involvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in mice. Behav. Brain Res. 2011, 221, 75–82. [Google Scholar] [CrossRef]
- Ruan, L.; Guan, K.; Wang, Y.; Gu, M.; Chen, Y.; Cai, L.; Ye, R.; Huang, Z.; Guo, A.; Su, Z.; et al. Baicalein exerts anxiolytic and antinociceptive effects in a mouse model of posttraumatic stress disorder: Involvement of the serotonergic system and spinal delta-opioid receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 122, 110689. [Google Scholar] [CrossRef]
- Chang, H.H.; Lu, Y.-P.; Cheng, C.-H.; Lu, C.-Y.; Hsiao, Y.-T.; Tsai, Y.-F.; Li, C.L.; Chang, F.-C. Biphasic effects of baicalin, an active constituent of Scutellaria baicalensis Georgi, in the spontaneous sleep-wake regulation. J. Ethnopharmacol. 2011, 135, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.M.; Huen, M.S.; Wang, H.Y.; Zheng, H.; Sigel, E.; Baur, R.; Ren, H.; Li, Z.W.; Wong, J.; Xue, H. Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem. Pharmacol. 2002, 64, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Donath, F.; Quispe, S.; Diefenbach, K.; Maurer, A.; Fietze, I.; Roots, I. Critical evaluation of the effect of valerian extract on sleep structure and sleep quality. Pharmacopsychiatry 2000, 33, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Chandra Shekhar, H.; Joshua, L.; Thomas, J.V. Standardized extract of Valeriana officinalis improves overall sleep quality in human subjects with sleep complaints: A randomized, double-blind, placebo-controlled, clinical study. Adv. Ther. 2024, 41, 246–261. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y.; Lv, F.; Hu, Y.; Yao, Z.; Wu, Y.; Lian, F.; Yang, J.; Xu, X. Valerian for insomnia on subjective and objective sleep parameters: A meta-analysis of randomized controlled trials. Curr. Sleep Med. Rep. 2023, 9, 211–224. [Google Scholar] [CrossRef]
- Stevinson, C.; Ernst, E. Valerian for insomnia: A systematic review of randomized clinical trials. Sleep Med. 2000, 1, 91–99. [Google Scholar] [CrossRef]
- Bent, S.; Padula, A.; Moore, D.; Patterson, M.; Mehling, W. Valerian for sleep: A systematic review and meta-analysis. Am. J. Med. 2006, 119, 1005–1012. [Google Scholar] [CrossRef]
- O‘G‘Li, F.J.S. Chamomile: A herbal medicine of the past with bright future. Eur. Int. J. Multidiscip. Res. Manag. Stud. 2022, 2, 251–254. [Google Scholar]
- Kazemi, A.; Shojaei-Zarghani, S.; Eskandarzadeh, P.; Hashempur, M.H. Effects of chamomile (Matricaria chamomilla L.) on sleep: A systematic review and meta-analysis of clinical trials. Complement. Ther. Med. 2024, 84, 103071. [Google Scholar] [CrossRef]
- Poza, J.J.; Pujol, M.; Ortega-Albás, J.J.; Romero, O. Melatonin in sleep disorders. Neurología 2022, 37, 575–585. [Google Scholar] [CrossRef]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-analysis: Melatonin for the treatment of primary sleep disorders. PLoS ONE 2013, 8, e63773. [Google Scholar] [CrossRef] [PubMed]

| Characteristics of Enrolled Subjects | Group Treatment-Control Order (n = 33) | Group Control-Treatment Order (n = 33) |
|---|---|---|
| Mean age (years) | 44.2 ± 15.5 | 44.1 ± 15.0 |
| Men | 39.2 ± 13.9 | 46.8 ± 15.5 |
| Women | 50.3 ± 15.6 | 40.3 ± 14.2 |
| Gender | ||
| Men | 18 | 19 |
| Women | 15 | 14 |
| Body mass index | ||
| Men | 20.8 ± 2.1 | 21.3 ± 1.2 |
| Women | 22.4 ± 2.4 | 21.0 ± 2.6 |
| Abdominal circumference (cm) | ||
| Men | 90.8 ± 3.1 | 89.5 ± 2.3 |
| Women | 76.2 ± 1.4 | 75.7 ± 1.8 |
| Ethnicity: Caucasian | 33 | 33 |
| Placebo | Supplement | |||||
|---|---|---|---|---|---|---|
| t0 | t1 | t2 | t0 | t1 | t2 | |
| PSQI | 11.5 ± 3.0 | 12.1 ± 2.7 | 13.3 ± 3.0 | 13 ± 2.9 | 11.2 ± 2.7 | 9.5 ± 3.5 |
| (6–18) | (8–19) | (9–20) | (9–19) | (6–17) | (5–19) | |
| Sleep Onset Latency | 1.9 ± 0.8 | 2.2 ± 0.6 | 2.3 ± 0.7 | 2.3 ± 0.6 | 1.8 ± 0.7 | 1.5 ± 0.8 |
| (0–3) | (1–3) | (1–3) | (1–3) | (1–3) | (0–3) | |
| Sleep Effectiveness | 75.7 ± 10 | 76 ± 10.5 | 73.8 ± 9.6 | 71.3 ± 10.1 | 74.4 ± 10.8 | 80.3 ± 8.6 |
| (50–88) | (50–100) | (57–100) | (50–100) | (50–100) | (57–100) | |
| Total Sleep Time | 5.4 ± 1.0 | 5.2 ± 0.9 | 5.0 ± 1.0 | 5.0 ± 1.0 | 5.5 ± 1 | 6.2 ± 1.2 |
| (2–7) | (2–8) | (2–7) | (1–7) | (2–7) | (2–8) | |
| VAS | 2.1 ± 2.5 | 2.1 ± 2.7 | 3.0 ± 3.1 | 2.8 ± 3.1 | 1.7 ± 2.4 | 1.7 ± 2.2 |
| (0–8) | (0–7) | (0–8) | (0–8) | (0–7) | (0–9) | |
| Variable | F | Df | p-Value |
|---|---|---|---|
| PSQI | |||
| Measurement | 9.473 | 2.320 | <0.001 |
| Treatment | 36.26 | 1.320 | <0.001 |
| Order | 0.251 | 1.62 | 0.62 |
| Sex | 0.003 | 1.62 | 0.96 |
| Age | 38.40 | 1.62 | <0.001 |
| Measurement × Treatment | 74.53 | 2.320 | <0.001 |
| Measurement × Order | 0.665 | 2.320 | 0.51 |
| Order × Treatment | 0.099 | 1.320 | 0.75 |
| Measurement × Treatment × Order | 5.572 | 2.320 | <0.001 |
| Sleep Onset Latency | |||
| Measurement | 5.944 | 2.320 | <0.001 |
| Treatment | 20.614 | 1.320 | <0.001 |
| Order | 0.370 | 1.62 | 0.55 |
| Sex | 2.845 | 1.62 | 0.10 |
| Age | 4.411 | 1.62 | 0.040 |
| Measurement × Treatment | 44.521 | 2.320 | <0.001 |
| Measurement × Order | 0.905 | 2.320 | 0.41 |
| Order × Treatment | 0.756 | 1.320 | 0.39 |
| Measurement × Treatment × Order | 6.747 | 2.320 | <0.001 |
| Sleep Efficiency | |||
| Measurement | 5.959 | 2.320 | <0.001 |
| Treatment | 0.034 | 1.320 | 0.85 |
| Order | 0.246 | 1.62 | 0.62 |
| Sex | 0.029 | 1.62 | 0.87 |
| Age | 1.829 | 1.62 | 0.18 |
| Measurement × Treatment | 15.10 | 2.320 | <0.001 |
| Measurement × Order | 2.812 | 2.320 | 0.062 |
| Order × Treatment | 0.018 | 1.320 | 0.89 |
| Measurement × Treatment × Order | 2.038 | 2.320 | 0.13 |
| Total Sleep Time | |||
| Measurement | 10.87 | 2.320 | <0.001 |
| Treatment | 28.44 | 1.320 | <0.001 |
| Order | 5.109 | 1.62 | 0.027 |
| Sex | 0.200 | 1.62 | 0.66 |
| Age | 10.53 | 1.62 | <0.001 |
| Measurement × Treatment | 46.82 | 2.320 | <0.001 |
| Measurement × Order | 1.158 | 2.320 | 0.32 |
| Order × Treatment | 0.048 | 1.320 | 0.83 |
| Measurement × Treatment × Order | 2.033 | 2.320 | 0.13 |
| Variable | X2 | Df | P |
|---|---|---|---|
| VAS | |||
| Measurement | 17.96 | 2 | <0.001 |
| Treatment | 16.29 | 1 | <0.001 |
| Order | 0.003 | 1 | 0.98 |
| Sex | 2.409 | 1 | 0.12 |
| Age | 54.05 | 1 | <0.001 |
| Measurement × Treatment | 52.12 | 2 | <0.001 |
| Measurement × Order | 5.145 | 2 | 0.076 |
| Order × Treatment | 0.236 | 1 | 0.63 |
| Measurement × Treatment × Order | 2.590 | 2 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Minno, A.; Morone, M.V.; Buccato, D.G.; De Lellis, L.F.; Ullah, H.; Piccinocchi, R.; Cordara, M.; Larsen, D.S.; Di Guglielmo, A.; Baldi, A.; et al. Efficacy and Tolerability of a Chemically Characterized Scutellaria lateriflora L. Extract-Based Food Supplement for Sleep Management: A Single-Center, Controlled, Randomized, Crossover, Double-Blind Clinical Trial. Nutrients 2025, 17, 1491. https://doi.org/10.3390/nu17091491
Di Minno A, Morone MV, Buccato DG, De Lellis LF, Ullah H, Piccinocchi R, Cordara M, Larsen DS, Di Guglielmo A, Baldi A, et al. Efficacy and Tolerability of a Chemically Characterized Scutellaria lateriflora L. Extract-Based Food Supplement for Sleep Management: A Single-Center, Controlled, Randomized, Crossover, Double-Blind Clinical Trial. Nutrients. 2025; 17(9):1491. https://doi.org/10.3390/nu17091491
Chicago/Turabian StyleDi Minno, Alessandro, Maria Vittoria Morone, Daniele Giuseppe Buccato, Lorenza Francesca De Lellis, Hammad Ullah, Roberto Piccinocchi, Marcello Cordara, Danaé S. Larsen, Antonietta Di Guglielmo, Alessandra Baldi, and et al. 2025. "Efficacy and Tolerability of a Chemically Characterized Scutellaria lateriflora L. Extract-Based Food Supplement for Sleep Management: A Single-Center, Controlled, Randomized, Crossover, Double-Blind Clinical Trial" Nutrients 17, no. 9: 1491. https://doi.org/10.3390/nu17091491
APA StyleDi Minno, A., Morone, M. V., Buccato, D. G., De Lellis, L. F., Ullah, H., Piccinocchi, R., Cordara, M., Larsen, D. S., Di Guglielmo, A., Baldi, A., Piccinocchi, G., Xiao, X., Sacchi, R., & Daglia, M. (2025). Efficacy and Tolerability of a Chemically Characterized Scutellaria lateriflora L. Extract-Based Food Supplement for Sleep Management: A Single-Center, Controlled, Randomized, Crossover, Double-Blind Clinical Trial. Nutrients, 17(9), 1491. https://doi.org/10.3390/nu17091491







