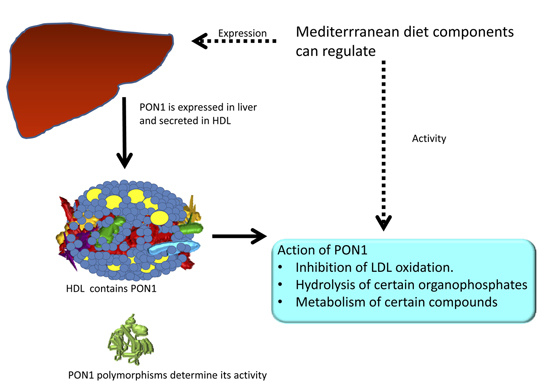
PON1 and Mediterranean Diet
Abstract
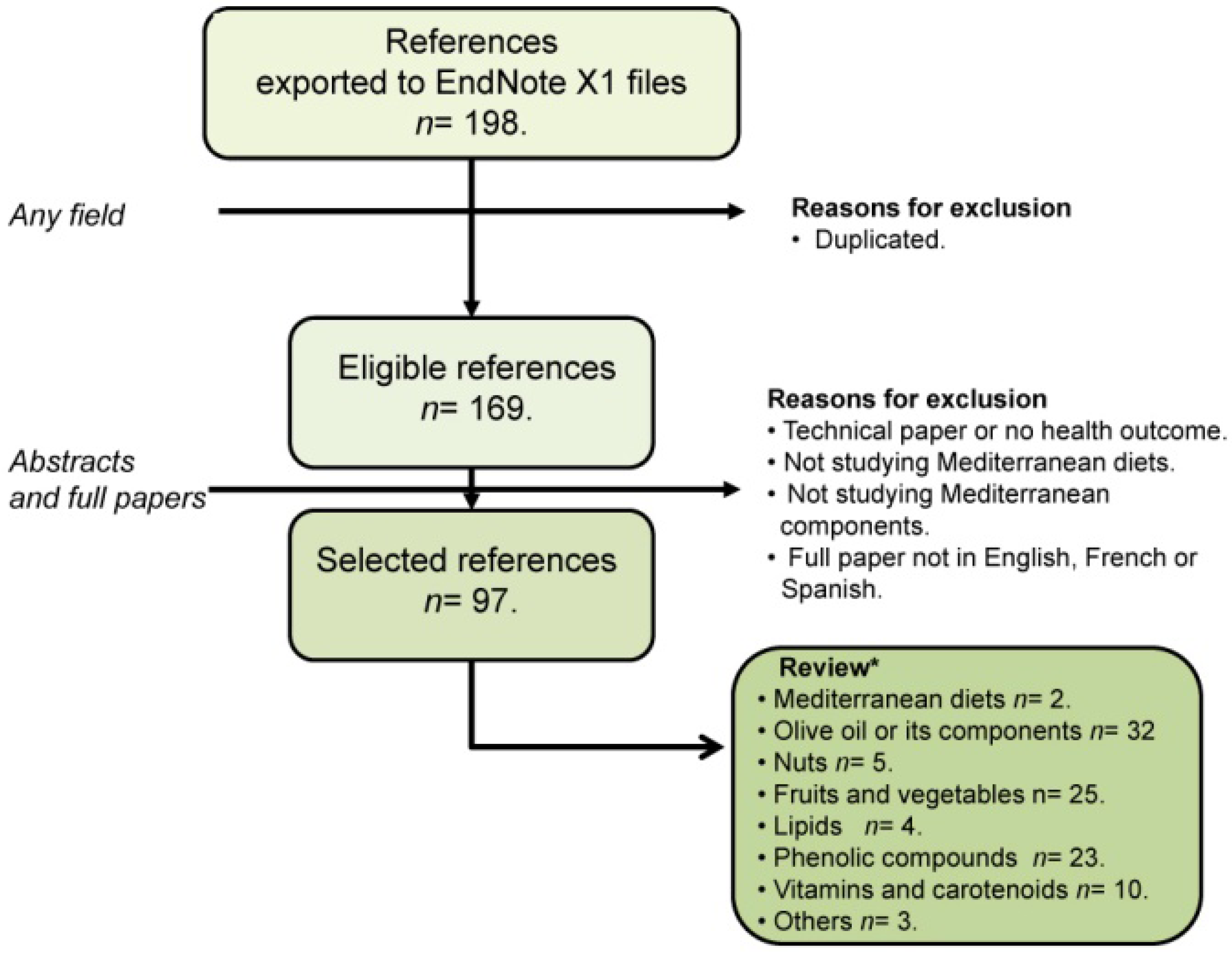
:1. Introduction


2. PON1 and Mediterranean Diet
3. PON1 and Olive Oil or Its Components
| Component | Content (g%) |
|---|---|
| Fatty Acids of Triglycerides | |
| Myristic (14:0) | 0.0–0.05 |
| Palmitic (16:0) | 7.5–20 |
| Palmitoleic (16:1n7) | 0.3–3.5 |
| Margaric (17:0) | 0–0.3 |
| Heptadecenoic (17:1) | 0.0–0.3 |
| Stearic (18:0) | 0.5–5.0 |
| Oleic (18:1n9) | 55–83 |
| Linoleic (18:2n6) | 3.5–21 |
| α-linolenic (18:3n3) | 0.0–0.9 |
| Arachidic (20:0) | 0.0–0.6 |
| Eicosenoic (20:1n9) | 0.0–0.4 |
| Behenic (22:0) | 0.0–0.2 |
| Lignoceric (24:0) | 0.0–0.2 |
| Minor Components | |
| Terpene compounds | 0.1–0.3 |
| Phytosterols | 0.1–0.2 |
| Hydrocarbons | |
| Squalene | 0.1–0.8 |
| Carotenes | 0.05–0.1 |
| Phenolic compounds | 0.05–0.1 |
4. PON1 and Nuts
5. PON1 and Other Constituents of Mediterranean Diet
Fruits and Vegetables
6. PON1 and Chemical Compounds Present in Mediterranean Diet and Their Potential as Nutraceuticals
6.1. Lipids
6.2. Phenolic Compounds
6.3. Vitamins and Carotenoids
6.4. Coenzyme Q10
6.5. Taurine
6.6. Trace Elements
7. Conclusions
| Characteristics of Studies | Findings | References | |
|---|---|---|---|
| Mediterranean diets | Greek tradition vs. Anglo-Celtics | Paraoxonase activity correlated with carotenoid concentrations | [21] |
| Mediterranean-like meal was compared to a Western-like meal | Increase in PON1 activity and carotenoid concentrations | [22] | |
| Olive oil or its components | Virgin olive oil in humans | Increased PON1 levels | [9,36,37] |
| Olive oil in animal models | Favors PON1 | [39,40,45] | |
| Oleic acid intake in humans | The beneficial effect on PON1 activity was dependent on polymorphisms | [26] | |
| Olive oil and green tea phenolics in animals | Increased PON1 activity | [47,48] | |
| Squalene | Variable effects depending on matrix vehicle | [50,51] | |
| Nuts | Human and animal studies | Effect on PON1 may vary according to different nuts and their constituents | [62,63,64,65,66] |
| Fruits and vegetables | Increased consumption in humans | Augmented PON1 with some fruits and phenotypes | [15,70,71,72,73,74,76,89,90,91,92] |
| Lipids | Human and animal studies | Differential effects depending on different fatty acids | [45,93,94,95] |
| Phenolic compounds | Quercetin in mice | PON1 differentially regulated depending on APOE genotype | [96] |
| Anthocyanin in humans | Increased HDL-PON1 | [100] | |
| Flavonoids and isoflavones | Discrepant results in function of experimental approach | [101,102,103,104,108,109,110,111,112,113] | |
| Curcumin | In vivo effect is influenced by the animal model and dietary fat content | [114,115] | |
| Resveratrol | Animal model and dietary regimen modify the outcome | [116,117,118,119,120,121,122] | |
| Vitamins and carotenoids | Vitamin A, C and E supplementation | Positive action on PON1 | [123,124,125,126,127] |
| β-carotene, astaxanthin, lycopene | Increased PON1 activity | [91,128,129,130,131] | |
| Coenzyme Q10 | Humans consuming olive oil enriched with this compound | Increase in PON1 activity | [136] |
| Taurine | Rats with hypothyroidism | Increase in serum paraoxonase | [138] |
| Trace elements | Selenium supplementation to rats | Increase in serum paraoxonase | [139] |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deakin, S.; Leviev, I.; Gomaraschi, M.; Calabresi, L.; Franceschini, G.; James, R.W. Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanism. J. Biol. Chem. 2002, 277, 4301–4308. [Google Scholar] [CrossRef] [PubMed]
- Durrington, P.N.; Mackness, B.; Mackness, M.I. Paraoxonase and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, O.; Rosenblat, M.; Coleman, R.; Shih, D.M.; Aviram, M. Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: Studies in PON1-knockout mice. Free Radic. Biol. Med. 2003, 34, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T. Effect of dietary lipids on paraoxonase-1 activity and gene expression. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Loued, S.; Isabelle, M.; Berrougui, H.; Khalil, A. The anti-inflammatory effect of paraoxonase 1 against oxidized lipids depends on its association with high density lipoproteins. Life Sci. 2012, 90, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.A.; Mackness, M.I.; Kumar, S.; Boulton, A.J.; Durrington, P.N. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1812–1818. [Google Scholar] [CrossRef] [PubMed]
- Seres, I.; Paragh, G.; Deschene, E.; Fulop, T., Jr.; Khalil, A. Study of factors influencing the decreased HDL associated PON1 activity with aging. Exp. Gerontol. 2004, 39, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Deakin, S.P.; James, R.W. Genetic and environmental factors modulating serum concentrations and activities of the antioxidant enzyme paraoxonase-1. Clin. Sci. (Lond.) 2004, 107, 435–447. [Google Scholar] [CrossRef]
- Loued, S.; Berrougui, H.; Componova, P.; Ikhlef, S.; Helal, O.; Khalil, A. Extra-virgin olive oil consumption reduces the age-related decrease in HDL and paraoxonase 1 anti-inflammatory activities. Br. J. Nutr. 2013, 110, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Bub, A.; Barth, S.; Watzl, B.; Briviba, K.; Herbert, B.M.; Luhrmann, P.M.; Neuhauser-Berthold, M.; Rechkemmer, G. Paraoxonase 1 Q192R (PON1–192) polymorphism is associated with reduced lipid peroxidation in R-allele-carrier but not in QQ homozygous elderly subjects on a tomato-rich diet. Eur. J. Nutr. 2002, 41, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Koubaa, N.; Nakbi, A.; Hammami, S.; Attia, N.; Mehri, S.; Ben Hamda, K.; Ben Farhat, M.; Miled, A.; Hammami, M. Association of homocysteine thiolactonase activity and PON1 polymorphisms with the severity of acute coronary syndrome. Clin. Biochem. 2009, 42, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Vitalone, A.; Cole, T.B.; Furlong, C.E. Modulation of paraoxonase (PON1) activity. Biochem. Pharmacol. 2005, 69, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, M.G.; Rumora, L.; Barisic, K. The paraoxonase 1, 2 and 3 in humans. Biochem. Med. (Zagreb) 2011, 21, 122–130. [Google Scholar] [CrossRef]
- Da Costa, L.A.; Badawi, A.; El-Sohemy, A. Nutrigenetics and modulation of oxidative stress. Ann. Nutr. Metab. 2012, 60 (Suppl. 3), 27–36. [Google Scholar] [CrossRef] [PubMed]
- Durrington, P.N.; Mackness, B.; Mackness, M.I. The hunt for nutritional and pharmacological modulators of paraoxonase. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Keys, A. Mediterranean diet and public health: Personal reflections. Am. J. Clin. Nutr. 1995, 61 (Suppl. 6), 1321S–1323S. [Google Scholar] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Wahrburg, U.; Kratz, M.; Cullen, P. Mediterranean diet, olive oil and health. Eur. J. Lipid Sci. Technol. 2002, 104, 698–705. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Pubmed Website. Available online: http://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 11 March 2015).
- Lee, C.T.; Rowley, K.; Jenkins, A.J.; O’Dea, K.; Itsiopoulos, C.; Stoney, R.M.; Su, Q.; Giles, G.G.; Best, J.D. Paraoxonase activity in Greek migrants and Anglo-Celtic persons in the Melbourne Collaborative Cohort Study: Relationship to dietary markers. Eur. J. Nutr. 2005, 44, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Blum, S.; Aviram, M.; Ben-Amotz, A.; Levy, Y. Effect of a Mediterranean meal on postprandial carotenoids, paraoxonase activity and C-reactive protein levels. Ann. Nutr. Metab. 2006, 50, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Kudchodkar, B.J.; Lacko, A.G.; Dory, L.; Fungwe, T.V. Dietary fat modulates serum paraoxonase 1 activity in rats. J. Nutr. 2000, 130, 2427–2433. [Google Scholar] [PubMed]
- Thomas-Moya, E.; Gianotti, M.; Proenza, A.M.; Llado, I. Paraoxonase 1 response to a high-fat diet: Gender differences in the factors involved. Mol. Med. 2007, 13, 203–209. [Google Scholar] [PubMed]
- Hoefel, A.L.; Hansen, F.; Rosa, P.D.; Assis, A.M.; Silveira, S.L.; Denardin, C.C.; Pettenuzzo, L.; Augusti, P.R.; Somacal, S.; Emanuelli, T.; et al. The effects of hypercaloric diets on glucose homeostasis in the rat: Influence of saturated and monounsaturated dietary lipids. Cell Biochem. Funct. 2011, 29, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Tomas, M.; Senti, M.; Elosua, R.; Vila, J.; Sala, J.; Masia, R.; Marrugat, J. Interaction between the Gln-Arg 192 variants of the paraoxonase gene and oleic acid intake as a determinant of high-density lipoprotein cholesterol and paraoxonase activity. Eur. J. Pharmacol. 2001, 432, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, W.H.; Walker, R.J.; de Jong, S.A.; van Rij, A.M.; Phillips, V.; Walker, H.L. Reduced postprandial serum paraoxonase activity after a meal rich in used cooking fat. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Lou-Bonafonte, J.M.; Arnal, C.; Navarro, M.A.; Osada, J. Efficacy of bioactive compounds from extra virgin olive oil to modulate atherosclerosis development. Mol. Nutr. Food Res. 2012, 56, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, F.; Alvarez de Cienfuegos, G.; Badimon, L.; Barja, G.; Battino, M.; Blanco, A.; Bonanome, A.; Colomer, R.; Corella-Piquer, D.; Covas, I.; et al. International conference on the healthy effect of virgin olive oil. Eur. J. Clin. Investig. 2005, 35, 421–424. [Google Scholar] [CrossRef]
- Mata, P.; Alvarez-Sala, L.A.; Rubio, M.J.; Nuno, J.; de Oya, M. Effects of long-term monounsaturated- vs. polyunsaturated-enriched diets on lipoproteins in healthy men and women. Am. J. Clin. Nutr. 1992, 55, 846–850. [Google Scholar] [PubMed]
- Stock, J. Importance of HDL functionality to cardiovascular risk. Atherosclerosis 2011, 218, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Lou-Bonafonte, J.M.; Fito, M.; Covas, M.I.; Farras, M.; Osada, J. HDL-related mechanisms of olive oil protection in cardiovascular disease. Curr. Vasc. Pharmacol. 2012, 10, 392–409. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.W.; Rye, K.A.; Gamble, J.R.; Vadas, M.A.; Barter, P.J. Ability of reconstituted high density lipoproteins to inhibit cytokine-induced expression of vascular cell adhesion molecule-1 in human umbilical vein endothelial cells. J. Lipid Res. 1999, 40, 345–353. [Google Scholar] [PubMed]
- Recalde, D.; Ostos, M.A.; Badell, E.; Garcia-Otin, A.L.; Pidoux, J.; Castro, G.; Zakin, M.M.; Scott-Algara, D. Human apolipoprotein A-IV reduces secretion of proinflammatory cytokines and atherosclerotic effects of a chronic infection mimicked by lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Shih, D.M.; Gu, L.; Xia, Y.R.; Navab, M.; Li, W.F.; Hama, S.; Castellani, L.W.; Furlong, C.E.; Costa, L.G.; Fogelman, A.M.; et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature 1998, 394, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Cherki, M.; Derouiche, A.; Drissi, A.; El Messal, M.; Bamou, Y.; Idrissi-Ouadghiri, A.; Khalil, A.; Adlouni, A. Consumption of argan oil may have an antiatherogenic effect by improving paraoxonase activities and antioxidant status: Intervention study in healthy men. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Manning, P.J.; Jong, S.A.; Ryalls, A.R.; Sutherland, W.H. Paraoxonase 1 activity in chylomicrons and VLDL: The effect of type 2 diabetes and meals rich in saturated fat and oleic acid. Lipids 2012, 47, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.J.; Sutherland, W.H.; Mann, J.I.; Williams, S.M. The effect of meals rich in thermally stressed olive and safflower oils on postprandial serum paraoxonase activity in patients with diabetes. Eur. J. Clin. Nutr. 2001, 55, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Volkova, N.; Aviram, M. Postprandial serum triacylglycerols and oxidative stress in mice after consumption of fish oil, soy oil or olive oil: Possible role for paraoxonase-1 triacylglycerol lipase-like activity. Nutrition 2006, 22, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Acin, S.; Navarro, M.A.; Carnicer, R.; Arbones-Mainar, J.M.; Guzman, M.A.; Arnal, C.; Beltran, G.; Uceda, M.; Maeda, N.; Osada, J. Dietary cholesterol suppresses the ability of olive oil to delay the development of atherosclerotic lesions in apolipoprotein E knockout mice. Atherosclerosis 2005, 182, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Arbones-Mainar, J.M.; Navarro, M.A.; Carnicer, R.; Guillen, N.; Surra, J.C.; Acin, S.; Guzman, M.A.; Sarria, A.J.; Arnal, C.; Aguilera, M.P.; et al. Accelerated atherosclerosis in apolipoprotein E-deficient mice fed Western diets containing palm oil compared with extra virgin olive oils: A role for small, dense high-density lipoproteins. Atherosclerosis 2007, 194, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.; Hassall, D.G.; Rafique, S.; Owen, J.S. Evidence for a paraoxonase-independent inhibition of low-density lipoprotein oxidation by high-density lipoprotein. Atherosclerosis 1997, 135, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Valabhji, J.; McColl, A.J.; Schachter, M.; Dhanjil, S.; Richmond, W.; Elkeles, R.S. High-density lipoprotein composition and paraoxonase activity in Type I diabetes. Clin. Sci. (Lond.) 2001, 101, 659–670. [Google Scholar] [CrossRef]
- Kontush, A.; Chantepie, S.; Chapman, M.J. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Efrat, M.; Rosenblat, M.; Mahmood, S.; Vaya, J.; Aviram, M. Di-oleoyl phosphatidylcholine (PC-18:1) stimulates paraoxonase 1 (PON1) enzymatic and biological activities: In vitro and in vivo studies. Atherosclerosis 2009, 202, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Hussein, O.; Grosovski, M.; Lasri, E.; Svalb, S.; Ravid, U.; Assy, N. Monounsaturated fat decreases hepatic lipid content in non-alcoholic fatty liver disease in rats. World J. Gastroenterol. 2007, 13, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Bayram, B.; Ozcelik, B.; Grimm, S.; Roeder, T.; Schrader, C.; Ernst, I.M.; Wagner, A.E.; Grune, T.; Frank, J.; Rimbach, G. A diet rich in olive oil phenolics reduces oxidative stress in the heart of SAMP8 mice by induction of Nrf2-dependent gene expression. Rejuvenation Res. 2012, 15, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Volkova, N.; Coleman, R.; Almagor, Y.; Aviram, M. Antiatherogenicity of extra virgin olive oil and its enrichment with green tea polyphenols in the atherosclerotic apolipoprotein-E-deficient mice: Enhanced macrophage cholesterol efflux. J. Nutr. Biochem. 2008, 19, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Acin, S.; Navarro, M.A.; Arbones-Mainar, J.M.; Guillen, N.; Sarria, A.J.; Carnicer, R.; Surra, J.C.; Orman, I.; Segovia, J.C.; Torre, R.; et al. Hydroxytyrosol administration enhances atherosclerotic lesion development in apo E deficient mice. J. Biochem. 2006, 140, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Guillen, N.; Acin, S.; Navarro, M.A.; Perona, J.S.; Arbones-Mainar, J.M.; Arnal, C.; Sarria, A.J.; Surra, J.C.; Carnicer, R.; Orman, I.; et al. Squalene in a sex-dependent manner modulates atherosclerotic lesion which correlates with hepatic fat content in apoE-knockout male mice. Atherosclerosis 2008, 197, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Gabas-Rivera, C.; Barranquero, C.; Martinez-Beamonte, R.; Navarro, M.A.; Surra, J.C.; Osada, J. Dietary squalene increases high density lipoprotein-cholesterol and paraoxonase 1 and decreases oxidative stress in mice. PLoS ONE 2014, 9, e104224. [Google Scholar] [CrossRef] [PubMed]
- Arunima, S.; Rajamohan, T. Effect of virgin coconut oil enriched diet on the antioxidant status and paraoxonase 1 activity in ameliorating the oxidative stress in rats–A comparative study. Food Funct. 2013, 4, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D. Olive Oil, Chemistry and Technology; AOCS Press: Champaign, IL, USA, 1996. [Google Scholar]
- Civantos, L.; Contreras, R.; Grana, R. Obtención del Aceite de Oliva Virgen, 2nd ed.; Agrícola Española, S.A., Ed.; Editorial Agrícola Española: Madrid, Spain, 1999. [Google Scholar]
- Harwood, J.; Aparicio, R. Handbook of Olive Oil: Analysis and Properties; Kluwer Academic: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Jiménez, J.; Rondón, D.; Martínez, L.; Mataix, J. Composición química de los aceites de oliva. In Aceite de Oliva Virgen: Nuestro patrimonio alimentario; Mataix, J., Ed.; Universidad de Granada, Puleva Food: Granada, Spain, 2001; pp. 115–136. [Google Scholar]
- Montedoro, G.F.; Taticchi, A.; Esposto, S.; Selvaggini, R.; Urbani, S.; Servilli, M. Antioxidants in virgin olive oil. Olea 2007, 26, 5–13. [Google Scholar]
- Sabate, J.; Wien, M. Nuts, blood lipids and cardiovascular disease. Asia Pac. J. Clin. Nutr. 2010, 19, 131–136. [Google Scholar] [PubMed]
- Fito, M.; Guxens, M.; Corella, D.; Saez, G.; Estruch, R.; de la Torre, R.; Frances, F.; Cabezas, C.; Lopez-Sabater Mdel, C.; Marrugat, J.; et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: A randomized controlled trial. Arch. Intern. Med. 2007, 167, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Canales, A.; Benedi, J.; Nus, M.; Librelotto, J.; Sanchez-Montero, J.M.; Sanchez-Muniz, F.J. Effect of walnut-enriched restructured meat in the antioxidant status of overweight/obese senior subjects with at least one extra CHD-risk factor. J. Am. Coll. Nutr. 2007, 26, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Nus, M.; Frances, F.; Librelotto, J.; Canales, A.; Corella, D.; Sanchez-Montero, J.M.; Sanchez-Muniz, F.J. Arylesterase activity and antioxidant status depend on PON1-Q192R and PON1-L55M polymorphisms in subjects with increased risk of cardiovascular disease consuming walnut-enriched meat. J. Nutr. 2007, 137, 1783–1788. [Google Scholar] [PubMed]
- Canales, A.; Sanchez-Muniz, F.J.; Bastida, S.; Librelotto, J.; Nus, M.; Corella, D.; Guillen, M.; Benedi, J. Effect of walnut-enriched meat on the relationship between VCAM, ICAM, and LTB4 levels and PON-1 activity in ApoA4 360 and PON-1 allele carriers at increased cardiovascular risk. Eur. J. Clin. Nutr. 2011, 65, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Muniz, F.J.; Canales, A.; Nus, M.; Bastida, S.; Guillen, M.; Corella, D.; Olmedilla-Alonso, B.; Granado-Lorencio, F.; Benedi, J. The antioxidant status response to low-fat and walnut paste-enriched meat differs in volunteers at high cardiovascular Risk carrying different PON-1 polymorphisms. J. Am. Coll. Nutr. 2012, 31, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Strunz, C.C.; Oliveira, T.V.; Vinagre, J.C.; Lima, A.; Cozzolino, S.; Maranhao, R.C. Brazil nut ingestion increased plasma selenium but had minimal effects on lipids, apolipoproteins, and high-density lipoprotein function in human subjects. Nutr. Res. 2008, 28, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, N.; Aksoy, M.; Bagci, C.; Gergerlioglu, H.S.; Celik, H.; Herken, E.; Yaman, A.; Tarakcioglu, M.; Soydinc, S.; Sari, I.; et al. Pistachio intake increases high density lipoprotein levels and inhibits low-density lipoprotein oxidation in rats. Tohoku J. Exp. Med. 2007, 212, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Surra, J.C.; Barranquero, C.; Torcal, M.P.; Orman, I.; Segovia, J.C.; Guillen, N.; Navarro, M.A.; Arnal, C.; Osada, J. In comparison with palm oil, dietary nut supplementation delays the progression of atherosclerotic lesions in female apoE-deficient mice. Br. J. Nutr. 2013, 109, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Manson, J.E.; Lee, I.M.; Cole, S.R.; Hennekens, C.H.; Willett, W.C.; Buring, J.E. Fruit and vegetable intake and risk of cardiovascular disease: The Women’s Health Study. Am. J. Clin. Nutr. 2000, 72, 922–928. [Google Scholar] [PubMed]
- Strandhagen, E.; Hansson, P.O.; Bosaeus, I.; Isaksson, B.; Eriksson, H. High fruit intake may reduce mortality among middle-aged and elderly men. The Study of Men Born in 1913. Eur. J. Clin. Nutr. 2000, 54, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Vupputuri, S.; Myers, L.; Whelton, P.K. Fruit and vegetable intake and risk of cardiovascular disease in US adults: The first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am. J. Clin. Nutr. 2002, 76, 93–99. [Google Scholar] [PubMed]
- Daniels, J.A.; Mulligan, C.; McCance, D.; Woodside, J.V.; Patterson, C.; Young, I.S.; McEneny, J. A randomised controlled trial of increasing fruit and vegetable intake and how this influences the carotenoid concentration and activities of PON-1 and LCAT in HDL from subjects with type 2 diabetes. Cardiovasc. Diabetol. 2014, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Proteggente, A.R.; Pannala, A.S.; Paganga, G.; van Buren, L.; Wagner, E.; Wiseman, S.; van de Put, F.; Dacombe, C.; Rice-Evans, C.A. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic. Res. 2002, 36, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Lixandru, D.; Mohora, M.; Coman, A.; Stoian, I.; van Gils, C.; Aerts, P.; Manuel, Y.K.B. Diet and paraoxonase 1 enzymatic activity in diabetic foot patients from Romania and Belgium: Favorable association of high flavonoid dietary intake with arylesterase activity. Ann. Nutr. Metab. 2010, 56, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Freese, R.; Alfthan, G.; Jauhiainen, M.; Basu, S.; Erlund, I.; Salminen, I.; Aro, A.; Mutanen, M. High intakes of vegetables, berries, and apples combined with a high intake of linoleic or oleic acid only slightly affect markers of lipid peroxidation and lipoprotein metabolism in healthy subjects. Am. J. Clin. Nutr. 2002, 76, 950–960. [Google Scholar] [PubMed]
- Rantala, M.; Silaste, M.L.; Tuominen, A.; Kaikkonen, J.; Salonen, J.T.; Alfthan, G.; Aro, A.; Kesaniemi, Y.A. Dietary modifications and gene polymorphisms alter serum paraoxonase activity in healthy women. J. Nutr. 2002, 132, 3012–3017. [Google Scholar] [PubMed]
- Rosenblat, M.; Volkova, N.; Aviram, M. Pomegranate juice (PJ) consumption antioxidative properties on mouse macrophages, but not PJ beneficial effects on macrophage cholesterol and triglyceride metabolism, are mediated via PJ-induced stimulation of macrophage PON2. Atherosclerosis 2010, 212, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M. Pomegranate for your cardiovascular health. Rambam Maimonides Med. J. 2013, 4, e0013. [Google Scholar] [CrossRef] [PubMed]
- Khateeb, J.; Gantman, A.; Kreitenberg, A.J.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (PON1) expression in hepatocytes is upregulated by pomegranate polyphenols: A role for PPAR-gamma pathway. Atherosclerosis 2010, 208, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Hayek, T.; Raz, A.; Coleman, R.; Dornfeld, L.; Vaya, J.; Aviram, M. Pomegranate juice supplementation to atherosclerotic mice reduces macrophage lipid peroxidation, cellular cholesterol accumulation and development of atherosclerosis. J. Nutr. 2001, 131, 2082–2089. [Google Scholar] [PubMed]
- Aviram, M.; Dornfeld, L.; Kaplan, M.; Coleman, R.; Gaitini, D.; Nitecki, S.; Hofman, A.; Rosenblat, M.; Volkova, N.; Presser, D.; et al. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: Studies in atherosclerotic mice and in humans. Drugs Exp. Clin. Res. 2002, 28, 49–62. [Google Scholar] [PubMed]
- Aviram, M.; Dornfeld, L.; Rosenblat, M.; Volkova, N.; Kaplan, M.; Coleman, R.; Hayek, T.; Presser, D.; Fuhrman, B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am. J. Clin. Nutr. 2000, 71, 1062–1076. [Google Scholar] [PubMed]
- Rosenblat, M.; Volkova, N.; Attias, J.; Mahamid, R.; Aviram, M. Consumption of polyphenolic-rich beverages (mostly pomegranate and black currant juices) by healthy subjects for a short term increased serum antioxidant status, and the serum’s ability to attenuate macrophage cholesterol accumulation. Food Funct. 2010, 1, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, M.; Hayek, T.; Aviram, M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis 2006, 187, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Parsaeyan, N.; Mozaffari-Khosravi, H.; Mozayan, M.R. Effect of pomegranate juice on paraoxonase enzyme activity in patients with type 2 diabetes. J. Diabetes Metab. Disord. 2012, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Rock, W.; Rosenblat, M.; Miller-Lotan, R.; Levy, A.P.; Elias, M.; Aviram, M. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J. Agric. Food Chem. 2008, 56, 8704–8713. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Volkova, N.; Aviram, M. Pomegranate juice polyphenols increase recombinant paraoxonase-1 binding to high-density lipoprotein: Studies in vitro and in diabetic patients. Nutrition 2010, 26, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Romain, C.; Gonzalez-Barrio, R.; Cristol, J.P.; Teissedre, P.L.; Crozier, A.; Rouanet, J.M. Raspberry juice consumption, oxidative stress and reduction of atherosclerosis risk factors in hypercholesterolemic golden Syrian hamsters. Food Funct. 2011, 2, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Ignatowicz, E.; Ewertowska, M.; Markowski, J.; Jodynis-Liebert, J. Cloudy apple juice protects against chemical-induced oxidative stress in rat. Eur. J. Nutr. 2011, 50, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Dalgard, C.; Christiansen, L.; Jonung, T.; Mackness, M.I.; de Maat, M.P.; Horder, M. No influence of increased intake of orange and blackcurrant juices and dietary amounts of vitamin E on paraoxonase-1 activity in patients with peripheral arterial disease. Eur. J. Nutr. 2007, 46, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Bub, A.; Barth, S.W.; Watzl, B.; Briviba, K.; Rechkemmer, G. Paraoxonase 1 Q192R (PON1–192) polymorphism is associated with reduced lipid peroxidation in healthy young men on a low-carotenoid diet supplemented with tomato juice. Br. J. Nutr. 2005, 93, 291–297. [Google Scholar] [CrossRef] [PubMed]
- McEneny, J.; Wade, L.; Young, I.S.; Masson, L.; Duthie, G.; McGinty, A.; McMaster, C.; Thies, F. Lycopene intervention reduces inflammation and improves HDL functionality in moderately overweight middle-aged individuals. J. Nutr. Biochem. 2013, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Rock, W.; Rosenblat, M.; Borochov-Neori, H.; Volkova, N.; Judeinstein, S.; Elias, M.; Aviram, M. Effects of date (Phoenix dactylifera L., Medjool or Hallawi Variety) consumption by healthy subjects on serum glucose and lipid levels and on serum oxidative status: A pilot study. J. Agric. Food Chem. 2009, 57, 8010–8017. [Google Scholar] [CrossRef] [PubMed]
- Pfeuffer, M.; Fielitz, K.; Laue, C.; Winkler, P.; Rubin, D.; Helwig, U.; Giller, K.; Kammann, J.; Schwedhelm, E.; Boger, R.H.; et al. CLA does not impair endothelial function and decreases body weight as compared with safflower oil in overweight and obese male subjects. J. Am. Coll. Nutr. 2011, 30, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanihaghjo, A.; Kolahi, S.; Seifirad, S.; Rashtchizadeh, N.; Argani, H.; Hajialilo, M.; Khabazi, A.; Alizadeh, S.; Bahreini, E. Effect of fish oil supplements on serum paraoxonase activity in female patients with rheumatoid arthritis: A double-blind randomized controlled trial. Arch. Iran Med. 2012, 15, 549–552. [Google Scholar] [PubMed]
- Stirban, A.; Nandrean, S.; Gotting, C.; Stratmann, B.; Tschoepe, D. Effects of n-3 polyunsaturated fatty acids (PUFAs) on circulating adiponectin and leptin in subjects with type 2 diabetes mellitus. Horm. Metab. Res. 2014, 46, 490–492. [Google Scholar] [PubMed]
- Boesch-Saadatmandi, C.; Niering, J.; Minihane, A.M.; Wiswedel, I.; Gardeman, A.; Wolffram, S.; Rimbach, G. Impact of apolipoprotein E genotype and dietary quercetin on paraoxonase 1 status in apoE3 and apoE4 transgenic mice. Atherosclerosis 2010, 211, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Bokkenheuser, V.D.; Shackleton, C.H.; Winter, J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem. J. 1987, 248, 953–956. [Google Scholar] [PubMed]
- Al-Rejaie, S.S.; Aleisa, A.M.; Sayed-Ahmed, M.M.; Al-Shabanah, O.A.; Abuohashish, H.M.; Ahmed, M.M.; Al-Hosaini, K.A.; Hafez, M.M. Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat. BMC Complement. Altern. Med. 2013, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, I.B.; Crozier, A. Dietary Flavonoids and Phenolic Compounds. In Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology; Fraga, C.G., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 1–49. [Google Scholar]
- Zhu, Y.; Huang, X.; Zhang, Y.; Wang, Y.; Liu, Y.; Sun, R.; Xia, M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014, 99, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, P.B.; Duke, J.A.; Brielmann, H.; Boik, J.; Hoyt, J.E. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: Implications for human nutrition and health. J. Altern. Complement. Med. 1997, 3, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ustundag, B.; Bahcecioglu, I.H.; Sahin, K.; Duzgun, S.; Koca, S.; Gulcu, F.; Ozercan, I.H. Protective effect of soy isoflavones and activity levels of plasma paraoxonase and arylesterase in the experimental nonalcoholic steatohepatitis model. Dig. Dis. Sci. 2007, 52, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Mohammadshahi, M.; Haidari, F.; Saei, A.A.; Rashidi, B.; Mahboob, S.; Rashidi, M.R. Soy protein, genistein, and daidzein improve serum paraoxonase activity and lipid profiles in rheumatoid arthritis in rats. J. Med. Food 2013, 16, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Schrader, C.; Ernst, I.M.; Sinnecker, H.; Soukup, S.T.; Kulling, S.E.; Rimbach, G. Genistein as a potential inducer of the anti-atherogenic enzyme paraoxonase-1: Studies in cultured hepatocytes in vitro and in rat liver in vivo. J. Cell. Mol. Med. 2012, 16, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Carrero-Gálvez, M.; García-Barroso, C.; Pérez-Bustamante, J.A. Analysis of polyphenolic compounds of different vinegar samples. Z Lebensm Unters Forsch 1994, 199, 29–31. [Google Scholar] [CrossRef]
- Cheng, G.W.; Crisosto, C.H. Browning potential, phenolic composition, and polyphenoloxidase activity of buffer extracts of peach and nectarine skin tissue. J. Am. Soc. Hortic. Sci. 1995, 120, 835–838. [Google Scholar]
- Quinde-Axtell, Z.; Baik, B.K. Phenolic compounds of barley grain and their implication in food product discoloration. J. Agric. Food Chem. 2006, 54, 9978–9984. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, N.; Rizvi, S.I. Onion extract (Allium cepa L.), quercetin and catechin up-regulate paraoxonase 1 activity with concomitant protection against low-density lipoprotein oxidation in male Wistar rats subjected to oxidative stress. J. Sci. Food Agric. 2014, 94, 2752–2757. [Google Scholar] [CrossRef] [PubMed]
- Amengual-Cladera, E.; Nadal-Casellas, A.; Gomez-Perez, Y.; Gomila, I.; Prieto, R.M.; Proenza, A.M.; Llado, I. Phytotherapy in a rat model of hyperoxaluria: The antioxidant effects of quercetin involve serum paraoxonase 1 activation. Exp. Biol. Med. (Maywood) 2011, 236, 1133–1138. [Google Scholar] [CrossRef]
- Kiyici, A.; Okudan, N.; Gokbel, H.; Belviranli, M. The effect of grape seed extracts on serum paraoxonase activities in streptozotocin-induced diabetic rats. J. Med. Food 2010, 13, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Aviram, M. Preservation of paraoxonase activity by wine flavonoids: Possible role in protection of LDL from lipid peroxidation. Ann. N. Y. Acad. Sci. 2002, 957, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Hayek, T.; Fuhrman, B.; Vaya, J.; Rosenblat, M.; Belinky, P.; Coleman, R.; Elis, A.; Aviram, M. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2744–2752. [Google Scholar] [CrossRef] [PubMed]
- Hamelet, J.; Demuth, K.; Dairou, J.; Ledru, A.; Paul, J.L.; Dupret, J.M.; Delabar, J.M.; Rodrigues-Lima, F.; Janel, N. Effects of catechin on homocysteine metabolism in hyperhomocysteinemic mice. Biochem. Biophys. Res. Commun. 2007, 355, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.M.; Choi, M.S.; Jung, U.J.; Kim, M.J.; Kim, H.J.; Jeon, S.M.; Shin, S.K.; Seong, C.N.; Lee, M.K. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Metabolism 2008, 57, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Schrader, C.; Schiborr, C.; Frank, J.; Rimbach, G. Curcumin induces paraoxonase 1 in cultured hepatocytes in vitro but not in mouse liver in vivo. Br. J. Nutr. 2011, 105, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Curtin, B.F.; Seetharam, K.I.; Dhoieam, P.; Gordon, R.K.; Doctor, B.P.; Nambiar, M.P. Resveratrol induces catalytic bioscavenger paraoxonase 1 expression and protects against chemical warfare nerve agent toxicity in human cell lines. J. Cell. Biochem. 2008, 103, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Boesch-Saadatmandi, C.; Breckwoldt, D.; Schrader, C.; Schmelzer, C.; Doring, F.; Hashida, K.; Hori, O.; Matsugo, S.; Rimbach, G. Ascorbic acid partly antagonizes resveratrol mediated heme oxygenase-1 but not paraoxonase-1 induction in cultured hepatocytes—Role of the redox-regulated transcription factor Nrf2. BMC Complement. Altern. Med. 2011, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Kandimalla, R.; Priyanka, K.; Singh, G.; Gill, K.D.; Singh, S. Effect of Resveratrol and Nicotine on PON1 Gene Expression: In Vitro Study. Indian J. Clin. Biochem. 2014, 29, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Gouedard, C.; Barouki, R.; Morel, Y. Induction of the paraoxonase-1 gene expression by resveratrol. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2378–2383. [Google Scholar] [CrossRef] [PubMed]
- Guyot, E.; Coumoul, X.; Chasse, J.F.; Khallouki, F.; Savouret, J.F.; Poirot, M.; Barouki, R. Identification of a new stilbene-derived inducer of paraoxonase 1 and ligand of the Aryl hydrocarbon Receptor. Biochem. Pharmacol. 2012, 83, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Noll, C.; Hamelet, J.; Ducros, V.; Belin, N.; Paul, J.L.; Delabar, J.M.; Janel, N. Resveratrol supplementation worsen the dysregulation of genes involved in hepatic lipid homeostasis observed in hyperhomocysteinemic mice. Food Chem. Toxicol. 2009, 47, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Do, G.M.; Kwon, E.Y.; Kim, H.J.; Jeon, S.M.; Ha, T.Y.; Park, T.; Choi, M.S. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem. Biophys. Res. Commun. 2008, 374, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Sarandol, E.; Tas, S.; Dirican, M.; Serdar, Z. Oxidative stress and serum paraoxonase activity in experimental hypothyroidism: Effect of vitamin E supplementation. Cell Biochem. Funct. 2005, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Motta, S.; Letellier, C.; Ropert, M.; Motta, C.; Thiebault, J.J. Protecting effect of vitamin E supplementation on submaximal exercise-induced oxidative stress in sedentary dogs as assessed by erythrocyte membrane fluidity and paraoxonase-1 activity. Vet. J. 2009, 181, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, S.; Karikas, G.A.; Parthimos, T.; Tsakiris, T.; Bakogiannis, C.; Schulpis, K.H. Alpha-tocopherol supplementation prevents the exercise-induced reduction of serum paraoxonase 1/arylesterase activities in healthy individuals. Eur. J. Clin. Nutr. 2009, 63, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Masciangelo, S.; Pallotta, G. Lipid peroxidation in hemodialysis patients: Effect of vitamin C supplementation. Clin. Biochem. 2008, 41, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Gatica, L.V.; Vega, V.A.; Zirulnik, F.; Oliveros, L.B.; Gimenez, M.S. Alterations in the lipid metabolism of rat aorta: Effects of vitamin a deficiency. J. Vasc. Res. 2006, 43, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Tanaka, N.; Matsufuji, H.; Chino, M. β-carotene reverses the IL-1 β-mediated reduction in paraoxonase-1 expression via induction of the CaMKKII pathway in human endothelial cells. Microvasc. Res. 2012, 84, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Baralic, I.; Djordjevic, B.; Dikic, N.; Kotur-Stevuljevic, J.; Spasic, S.; Jelic-Ivanovic, Z.; Radivojevic, N.; Andjelkovic, M.; Pejic, S. Effect of astaxanthin supplementation on paraoxonase 1 activities and oxidative stress status in young soccer players. Phytother. Res. 2013, 27, 1536–1542. [Google Scholar] [PubMed]
- Yegin, S.C.; Yur, F.; Ceylan, E. Effect of lycopene application in rats with experimental diabetes using lipoprotein, paraoxonase and cytokines. J. Membr. Biol. 2013, 246, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, E.S.; El-Sohemy, A.; Rao, A.V.; Rao, L.G. Paraoxonase 1 polymorphisms 172T-->A and 584A-->G modify the association between serum concentrations of the antioxidant lycopene and bone turnover markers and oxidative stress parameters in women 25–70 years of age. J. Nutrigenet. Nutrigenomics 2010, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ernster, L.; Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta 1995, 1271, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kamei, M.; Fujita, T.; Kanbe, T.; Sasaki, K.; Oshiba, K.; Otani, S.; Matsui-Yuasa, I.; Morisawa, S. The distribution and content of ubiquinone in foods. Int. J. Vitam. Nutr. Res. 1986, 56, 57–63. [Google Scholar] [PubMed]
- Mattila, P.; Kumpulainen, J. Coenzymes Q9 and Q10: Contents in foods and dietary intake. J. Food Comp. Anal. 2001, 14, 409–417. [Google Scholar] [CrossRef]
- Weber, C.; Bysted, A.; sHolmer, G. Coenzyme Q10 in the diet—daily intake and relative bioavailability. Mol. Asp. Med. 1997, 18, S251–S254. [Google Scholar] [CrossRef]
- Bruge, F.; Bacchetti, T.; Principi, F.; Scarpa, E.S.; Littarru, G.P.; Tiano, L. Olive oil supplemented with Coenzyme Q(10): Effect on plasma and lipoprotein oxidative status. Biofactors 2012, 38, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.K.; Sanders, T.A. Taurine concentrations in the diet, plasma, urine and breast milk of vegans compared with omnivores. Br. J. Nutr. 1986, 56, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Dirican, M.; Tas, S.; Sarandol, E. High-dose taurine supplementation increases serum paraoxonase and arylesterase activities in experimental hypothyroidism. Clin. Exp. Pharmacol. Physiol. 2007, 34, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.D.; Bansal, M.P. Studies on HDL associated enzymes under experimental hypercholesterolemia: Possible modulation on selenium supplementation. Lipids Health Dis. 2009, 8, 55. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou-Bonafonte, J.M.; Gabás-Rivera, C.; Navarro, M.A.; Osada, J. PON1 and Mediterranean Diet. Nutrients 2015, 7, 4068-4092. https://doi.org/10.3390/nu7064068
Lou-Bonafonte JM, Gabás-Rivera C, Navarro MA, Osada J. PON1 and Mediterranean Diet. Nutrients. 2015; 7(6):4068-4092. https://doi.org/10.3390/nu7064068
Chicago/Turabian StyleLou-Bonafonte, José M., Clara Gabás-Rivera, María A. Navarro, and Jesús Osada. 2015. "PON1 and Mediterranean Diet" Nutrients 7, no. 6: 4068-4092. https://doi.org/10.3390/nu7064068
APA StyleLou-Bonafonte, J. M., Gabás-Rivera, C., Navarro, M. A., & Osada, J. (2015). PON1 and Mediterranean Diet. Nutrients, 7(6), 4068-4092. https://doi.org/10.3390/nu7064068