Cholecalciferol Additively Reduces Serum Parathyroid Hormone and Increases Vitamin D and Cathelicidin Levels in Paricalcitol-Treated Secondary Hyperparathyroid Hemodialysis Patients
Abstract
:1. Background
2. Methods
2.1. Study Design
2.2. Study Approval and Informed Consent
2.3. Patient Eligibility and Randomization
2.4. Treatment Intervention
2.5. Objectives, Outcomes and Measures
2.6. Data Collection and Statistical Analysis
3. Results
3.1. Patient Recruitment and Analysis Sets
3.2. Changes in Serum PTH Levels within and between Groups during Study Period
3.3. Changes in Serum 25(OH)D3 Levels within and between Groups during Study Period
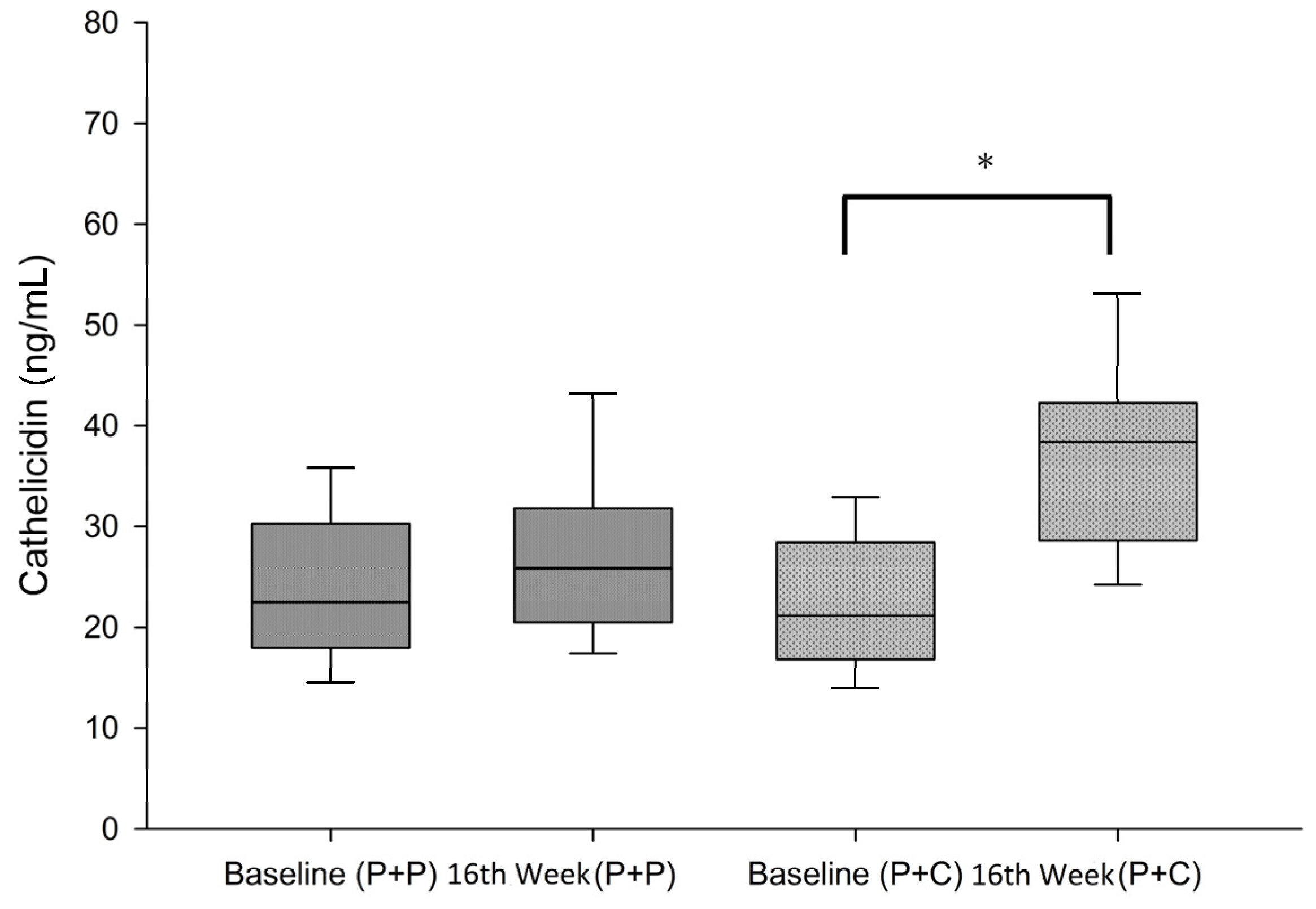
3.4. Changes in Serum Cathelicidin (hCAP-18) Levels within and between Groups during the Study Period
3.5. Correlation between the Change in Serum 25(OH)D3 and hCAP-18 Levels after 16 Weeks of Study in HD Patients with SHPT
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lomashvili, K.A.; Wang, X.; O’Neill, W.C. Role of local versus systemic vitamin D receptors in vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 146–151. [Google Scholar] [CrossRef]
- Briese, S.; Wiesner, S.; Will, J.C.; Lembcke, A.; Opgen-Rhein, B.; Nissel, R.; Wernecke, K.D.; Andreae, J.; Haffner, D.; Querfeld, U. Arterial and cardiac disease in young adults with childhood-onset end-stage renal disease-impact of calcium and vitamin D therapy. Nephrol. Dial. Transplant. 2006, 21, 1906–1914. [Google Scholar] [CrossRef]
- Gomez-Alonso, C.; Naves-Diaz, M.L.; Fernandez-Martin, J.L.; Diaz-Lopez, J.B.; Fernandez-Coto, M.T.; Cannata-Andia, J.B. Vitamin D status and secondary hyperparathyroidism: The importance of 25-hydroxyvitamin D cut-off levels. Kidney Int. 2003, 63, S44–S48. [Google Scholar] [CrossRef]
- Harkness, L.; Cromer, B. Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos. Int. 2005, 16, 109–113. [Google Scholar] [CrossRef]
- Hollis, B.W. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 2005, 135, 317–322. [Google Scholar]
- Vieth, R.; Ladak, Y.; Walfish, P.G. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J. Clin. Endocrinol. Metab. 2003, 88, 185–191. [Google Scholar] [CrossRef]
- Ooms, M.E.; Lips, P.; Roos, J.C.; van der Vijgh, W.J.; Popp-Snijders, C.; Bezemer, P.D.; Bouter, L.M. Vitamin D status and sex hormone binding globulin: Determinants of bone turnover and bone mineral density in elderly women. J. Bone Miner. Res. 1995, 10, 1177–1184. [Google Scholar] [CrossRef]
- Dusso, A.; Brown, A.; Slatopolsky, E. Extrarenal production of calcitriol. Semin. Nephrol. 1994, 14, 144–155. [Google Scholar]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin D(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar]
- Wolf, M.; Shah, A.; Gutierrez, O.; Ankers, E.; Monroy, M.; Tamez, H.; Steele, D.; Chang, Y.; Camargo, C.A., Jr.; Tonelli, M.; et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007, 72, 1004–1013. [Google Scholar] [CrossRef]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2007, 71, 31–38. [Google Scholar] [CrossRef]
- Ishimura, E.; Nishizawa, Y.; Inaba, M.; Matsumoto, N.; Emoto, M.; Kawagishi, T.; Shoji, S.; Okuno, S.; Kim, M.; Miki, T.; et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure. Kidney Int. 1999, 55, 1019–1027. [Google Scholar] [CrossRef]
- Drechsler, C.; Verduijn, M.; Pilz, S.; Dekker, F.W.; Krediet, R.T.; Ritz, E.; Wanner, C.; Boeschoten, E.W.; Brandenburg, V.; Group, N.S. Vitamin D status and clinical outcomes in incident dialysis patients: Results from the necosad study. Nephrol. Dial. Transplant. 2011, 26, 1024–1032. [Google Scholar] [CrossRef]
- Segersten, U.; Correa, P.; Hewison, M.; Hellman, P.; Dralle, H.; Carling, T.; Akerstrom, G.; Westin, G. 25-hydroxyvitamin D(3)-1alpha-hydroxylase expression in normal and pathological parathyroid glands. J. Clin. Endocrinol. Metab. 2002, 87, 2967–2972. [Google Scholar]
- Correa, P.; Segersten, U.; Hellman, P.; Akerstrom, G.; Westin, G. Increased 25-hydroxyvitamin D3 1alpha-hydroxylase and reduced 25-hydroxyvitamin D3 24-hydroxylase expression in parathyroid tumors—New prospects for treatment of hyperparathyroidism with vitamin D. J. Clin. Endocrinol. Metab. 2002, 87, 5826–5829. [Google Scholar] [CrossRef]
- Ritter, C.S.; Armbrecht, H.J.; Slatopolsky, E.; Brown, A.J. 25-hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int. 2006, 70, 654–659. [Google Scholar] [CrossRef]
- Rojas-Rivera, J.; De La Piedra, C.; Ramos, A.; Ortiz, A.; Egido, J. The expanding spectrum of biological actions of vitamin D. Nephrol. Dial. Transplant. 2010, 25, 2850–2865. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kovesdy, C.P. Clinical outcomes with active versus nutritional vitamin D compounds in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 1529–1539. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Yim, S.; Dhawan, P.; Ragunath, C.; Christakos, S.; Diamond, G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J. Cyst. Fibros. 2007, 6, 403–410. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and immune function: Autocrine, paracrine or endocrine? Scand. J. Clin. Lab. Investig. 2012, 72 (Suppl. S243), 92–102. [Google Scholar]
- Yuk, J.M.; Shin, D.M.; Lee, H.M.; Yang, C.S.; Jin, H.S.; Kim, K.K.; Lee, Z.W.; Lee, S.H.; Kim, J.M.; Jo, E.K. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe 2009, 6, 231–243. [Google Scholar] [CrossRef]
- Smolders, J.; Thewissen, M.; Peelen, E.; Menheere, P.; Tervaert, J.W.; Damoiseaux, J.; Hupperts, R. Vitamin D status is positively correlated with regulatory t cell function in patients with multiple sclerosis. PLoS ONE 2009, 4, e6635. [Google Scholar] [CrossRef]
- Gombart, A.F.; Bhan, I.; Borregaard, N.; Tamez, H.; Camargo, C.A., Jr.; Koeffler, H.P.; Thadhani, R. Low plasma level of cathelicidin antimicrobial peptide (HCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin. Infect. Dis. 2009, 48, 418–424. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Ding, C.; Gao, D.; Wilding, J.; Trayhurn, P.; Bing, C. Vitamin D signalling in adipose tissue. Br. J. Nutr. 2012, 108, 1915–1923. [Google Scholar] [CrossRef]
- Bell, N.H.; Shaw, S.; Turner, R.T. Evidence that 1,25-dihydroxyvitamin D3 inhibits the hepatic production of 25-hydroxyvitamin D in man. J. Clin. Investig. 1984, 74, 1540–1544. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B. Regulation of mouse Cyp24a1 expression via promoter-proximal and downstream-distal enhancers highlights new concepts of 1,25-dihydroxyvitamin D(3) action. Arch. Biochem. Biophys. 2012, 523, 2–8. [Google Scholar] [CrossRef]
- Echida, Y.; Mochizuki, T.; Uchida, K.; Tsuchiya, K.; Nitta, K. Risk factors for vitamin D deficiency in patients with chronic kidney disease. Intern. Med. 2012, 51, 845–850. [Google Scholar] [CrossRef]
- Metzger, M.; Houillier, P.; Gauci, C.; Haymann, J.P.; Flamant, M.; Thervet, E.; Boffa, J.J.; Vrtovsnik, F.; Froissart, M.; Stengel, B.; et al. Relation between circulating levels of 25(OH) vitamin D and parathyroid hormone in chronic kidney disease: Quest for a threshold. J. Clin. Endocrinol. Metab. 2013, 98, 2922–2928. [Google Scholar] [CrossRef]
- Fournier, A.; Harbouche, L.; Mansour, J.; Shahapuni, I. Impact of calcium and vitamin D therapy on arterial and cardiac disease in young adults with childhood-onset end stage renal disease. Nephrol. Dial. Transplant. 2007, 22, 956–957. [Google Scholar] [CrossRef]
- Liu, W.C.; Wu, C.C.; Hung, Y.M.; Liao, M.T.; Shyu, J.F.; Lin, Y.F.; Lu, K.C.; Yeh, K.C. Pleiotropic effects of vitamin D in chronic kidney disease. Clin. Chim. Acta 2016, 453, 1–12. [Google Scholar] [CrossRef]
- Armas, L.A.; Andukuri, R.; Barger-Lux, J.; Heaney, R.P.; Lund, R. 25-hydroxyvitamin D response to cholecalciferol supplementation in hemodialysis. Clin. J. Am. Soc. Nephrol. 2012, 7, 1428–1434. [Google Scholar] [CrossRef]
- Bansal, B.; Bansal, S.B.; Mithal, A.; Kher, V.; Marwaha, R.; Singh, P.; Irfan, N. A randomized controlled trial of cholecalciferol supplementation in patients on maintenance hemodialysis. Indian J. Endocrinol. Metab. 2014, 18, 655–661. [Google Scholar]
- Delanaye, P.; Weekers, L.; Warling, X.; Moonen, M.; Smelten, N.; Medart, L.; Krzesinski, J.M.; Cavalier, E. Cholecalciferol in haemodialysis patients: A randomized, double-blind, proof-of-concept and safety study. Nephrol. Dial. Transplant. 2013, 28, 1779–1786. [Google Scholar] [CrossRef]
- Dusilova-Sulkova, S.; Safranek, R.; Vavrova, J.; Horacek, J.; Pavlikova, L.; Palicka, V. Low-dose cholecalciferol supplementation and dual vitamin D therapy in haemodialysis patients. Int. Urol. Nephrol. 2015, 47, 169–176. [Google Scholar] [CrossRef]
- Dogan, E.; Erkoc, R.; Sayarlioglu, H.; Soyoral, Y.; Dulger, H. Effect of depot oral cholecalciferol treatment on secondary hyperparathyroidism in stage 3 and stage 4 chronic kidney diseases patients. Ren. Fail. 2008, 30, 407–410. [Google Scholar] [CrossRef]
- Bhan, I.; Dobens, D.; Tamez, H.; Deferio, J.J.; Li, Y.C.; Warren, H.S.; Ankers, E.; Wenger, J.; Tucker, J.K.; Trottier, C.; et al. Nutritional vitamin D supplementation in dialysis: A randomized trial. Clin. J. Am. Soc. Nephrol. 2015, 10, 611–619. [Google Scholar] [CrossRef]
- Liu, W.C.; Zheng, C.M.; Lu, C.L.; Lin, Y.F.; Shyu, J.F.; Wu, C.C.; Lu, K.C. Vitamin D and immune function in chronic kidney disease. Clin. Chim. Acta 2015, 450, 135–144. [Google Scholar] [CrossRef]
- DiNubile, M.J. Understanding the high infectious disease mortality among patients receiving hemodialysis: Potential risk of concurrently low cathelicidin and gelsolin levels. Clin. Infect. Dis. 2009, 49, 163–164. [Google Scholar]
- Leaf, D.E.; Croy, H.E.; Abrahams, S.J.; Raed, A.; Waikar, S.S. Cathelicidin antimicrobialprotein, vitamin D, and risk of death in critically ill patients. Crit. Care 2015, 19, 80. [Google Scholar] [CrossRef]
- McMahon, L.; Schwartz, K.; Yilmaz, O.; Brown, E.; Ryan, L.K.; Diamond, G. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect. Immun. 2011, 79, 2250–2256. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Tang, D.H.; Modlin, R.L. Cutting edge: Vitamin D-mediated human antimicrobial activity against mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007, 179, 2060–2063. [Google Scholar] [CrossRef]
- Gombart, A.F.; Saito, T.; Koeffler, H.P. Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genom. 2009, 10, 321. [Google Scholar] [CrossRef]
- Larcombe, L.; Mookherjee, N.; Slater, J.; Slivinski, C.; Dantouze, J.; Singer, M.; Whaley, C.; Denechezhe, L.; Matyas, S.; Decter, K.; et al. Vitamin D, serum 25(OH)D, LL-37 and polymorphisms in a Canadian first nation population with endemic tuberculosis. Int. J. Circumpolar Health 2015, 74, 28952. [Google Scholar] [CrossRef]
- Bacchetta, J.; Chun, R.F.; Gales, B.; Zaritsky, J.J.; Leroy, S.; Wesseling-Perry, K.; Boregaard, N.; Rastogi, A.; Salusky, I.B.; Hewison, M. Antibacterial responses byperitoneal macrophages are enhanced following vitamin D supplementation. PLoS ONE 2014, 30, e116530. [Google Scholar]
- Lachmann, R.; Bevan, M.A.; Kim, S.; Patel, N.; Hawrylowicz, C.; Vyakarnam, A.; Peters, B.S. A comparative phase 1 clinical trial to identify anti-infective mechanisms of vitamin D in people with HIV infection. AIDS 2015, 19, 1127–1135. [Google Scholar] [CrossRef]
- Quraishi, S.A.; De Pascale, G.; Needleman, J.S.; Nakazawa, H.; Kaneki, M.; Bajwa, E.K.; Camargo, C.A., Jr.; Bhan, I. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: A randomized, placebo-controlled trial. Crit. Care Med. 2015, 43, 1928–1937. [Google Scholar] [CrossRef]




| Characteristics | Paricalcitol + Placebo (n = 30) | Paricalcitol + Cholecalciferol (n = 30) | p Value |
|---|---|---|---|
| Age, mean ± SD (years) | 65.2 ± 12.4 | 64.8 ± 13.2 | 0.904 |
| Male, n (%) | 16 (53.3%) | 14 (46.7%) | 0.941 |
| BMI (kg/m2) | 22.92 ± 3.48 | 23.37 ± 4.02 | 0.65 |
| HD vintage (months) | 49.85 ± 35.33 | 54.03 ± 26.67 | 0.52 |
| GN, n (%) | 5 (16.7%) | 6 (20%) | 0.959 |
| DM, n (%) | 13 (43.3%) | 12 (40%) | 0.944 |
| HTN, n (%) | 4 (13.3%) | 3 (10%) | 0.99 |
| Others, n (%) | 8 (26.6%) | 9 (30%) | 0.952 |
| Prior calcitriol usage, n (%) | 14 (46.7%) | 15 (50%) | 0.937 |
| iPTH (pg/mL) | 681.9 ± 173.3 | 689.0 ± 180.1 | 0.877 |
| 25(OH)D3 (ng/mL) | 19.53 ± 8.2 | 19.6 ± 7.3 | 0.972 |
| hCAP-18 (ng/mL) | 24.05 ± 7.99 | 22.25 ± 6.71 | 0.349 |
| Albumin (g/dL) | 3.95 ± 0.34 | 3.82 ± 0.37 | 0.162 |
| Alkaline phosphatase (U/L) | 121.2 ± 48.4 | 128.8 ± 52.4 | 0.562 |
| cCa (mg/dL) | 9.19 ± 0.63 | 9.22 ± 0.51 | 0.840 |
| P (mg/dL) | 5.02 ± 0.78 | 5.14 ± 0.74 | 0.543 |
| Parameters | Paricalcitol + Placebo (Control) | Paricalcitol + Cholecalciferol (Study) | ||||
|---|---|---|---|---|---|---|
| Before | After | p Value | Before | After | p Value | |
| iPTH (pg/mL) | 681.9 ± 173.3 | 299.37 ± 74.62 | <0.05 | 689.0 ± 180.08 | 262.9 ± 57.14 | <0.05 |
| 25(OH)D3 (ng/mL) | 19.53 ± 8.2 | 19.63 ± 7.56 | 0.96 | 19.63 ± 7.26 | 30.4 ± 7.7 | <0.05 |
| hCAP-18 (ng/mL) | 24.05 ± 7.99 | 26.59 ± 65.68 | 0.075 | 22.25 ± 6.71 | 82.13 ± 68.67 | <0.01 |
| cCa (mg/dL) | 9.19 ± 0.63 | 9.28 ± 0.72 | 0.61 | 9.22 ± 0.51 | 9.18 ± 0.62 | 0.46 |
| P (mg/dL) | 5.02 ± 0.78 | 5.08 ± 0.82 | 0.77 | 5.14 ± 0.74 | 5.07 ± 0.62 | 0.69 |
| Primary Outcome | 4th Week | 8th Week | 12th Week | 16th Week |
|---|---|---|---|---|
| iPTH ≤ 300 pg/mL | n/30 (%) | n/30 (%) | n/30 (%) | n/30 (%) |
| Paricalcitol | 2/30 (6.7) | 7/30 (23) | 12/30 (40) | 15/30 (50) |
| Paricalcitol + Cholecalciferol | 2/30 (6.7) | 7/30 (23) | 18/30 (60) | 23/30 (76.7) |
| p = 1.000 | p = 1.000 | p = 0.121 | p = 0.032 | |
| Secondaty Outcome | 4th Week | 8th Week | 12th Week | 16th Week |
| 25(OH)D3 ≥ 30 ng/dL | ||||
| Paricalcitol | 6/30 (20) | 2/30 (6.7) | 3/30 (10) | |
| Paricalcitol + Cholecalciferol | 4/30 (13.3) | 15/30 (50) | 18/30 (60) | |
| p = 0.488 | p = 0.001 | p = 0.001 | ||
| Double of hCAP-18 | ||||
| Paricalcitol | 2/30 (6.7) | |||
| Paricalcitol + Cholecalciferol | 12/30 (40) | |||
| p = 0.006 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.-Q.; Hou, Y.-C.; Zheng, C.-M.; Lu, C.-L.; Liu, W.-C.; Wu, C.-C.; Huang, M.-T.; Lin, Y.-F.; Lu, K.-C. Cholecalciferol Additively Reduces Serum Parathyroid Hormone and Increases Vitamin D and Cathelicidin Levels in Paricalcitol-Treated Secondary Hyperparathyroid Hemodialysis Patients. Nutrients 2016, 8, 708. https://doi.org/10.3390/nu8110708
Zheng J-Q, Hou Y-C, Zheng C-M, Lu C-L, Liu W-C, Wu C-C, Huang M-T, Lin Y-F, Lu K-C. Cholecalciferol Additively Reduces Serum Parathyroid Hormone and Increases Vitamin D and Cathelicidin Levels in Paricalcitol-Treated Secondary Hyperparathyroid Hemodialysis Patients. Nutrients. 2016; 8(11):708. https://doi.org/10.3390/nu8110708
Chicago/Turabian StyleZheng, Jing-Quan, Yi-Chou Hou, Cai-Mei Zheng, Chien-Lin Lu, Wen-Chih Liu, Chia-Chao Wu, Ming-Te Huang, Yuh-Feng Lin, and Kuo-Cheng Lu. 2016. "Cholecalciferol Additively Reduces Serum Parathyroid Hormone and Increases Vitamin D and Cathelicidin Levels in Paricalcitol-Treated Secondary Hyperparathyroid Hemodialysis Patients" Nutrients 8, no. 11: 708. https://doi.org/10.3390/nu8110708
APA StyleZheng, J.-Q., Hou, Y.-C., Zheng, C.-M., Lu, C.-L., Liu, W.-C., Wu, C.-C., Huang, M.-T., Lin, Y.-F., & Lu, K.-C. (2016). Cholecalciferol Additively Reduces Serum Parathyroid Hormone and Increases Vitamin D and Cathelicidin Levels in Paricalcitol-Treated Secondary Hyperparathyroid Hemodialysis Patients. Nutrients, 8(11), 708. https://doi.org/10.3390/nu8110708





