B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review
Abstract
:1. Background
What Are Vitamins?
| Vitamin | Generally Known as | Good Dietary Sources | RDA 1 (mg) | UL 2 | Principal Bioactive Coenzymes (and Principal Coenzyme Role [8]) | Symptoms of Deficiency | Brain Specific Symptoms of Deficiency | Specific Risk Factors for Deficiency |
|---|---|---|---|---|---|---|---|---|
| B1 | Thiamin(e) | Cereals (esp. whole grain), brown rice, green vegetables, potatoes, pasta, liver, pork, eggs | 1.2/1.1 | - | Thiamine pyrophosphate (Generation of leaving group potential) | Mild deficiency: general fatigue/weakness gastro-intestinal symptoms [9]. Deficiency: “Beri-beri”— Peripheral nerve damage and cardiovascular dysfunction leading to: pain, impaired sensory perception; swelling, weakness and pain in the limbs; shortness of breath, irregular heart rate, heart failure [10] | Mild deficiency: irritability, emotional disturbances, confusion, disturbed sleep, memory loss [9]. Deficiency: Wernicke-Korsakoff syndrome (neurodegeneration, within the medial thalamus and cerebellum). Ataxia, abnormal motor function and eye movement, amnesia, apathy, confabulation [10] | Alcohol abuse, obesity [9] |
| B2 | Riboflavin | Dairy products, leafy vegetables, legumes, liver, kidneys, yeast, mushrooms | 1.3/1.1 | - | Flavoproteins: flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN) (redox reactions) | Weakness, oral pain/tenderness, burning/itching of the eyes, dermatitis, anaemia [11] | Fatigue, personality change, brain dysfunction [11] | inherited riboflavin malabsorption/utilisation (10%–15% prevalence) [12] |
| B3 | Niacin | Meat, fish, whole grain cereal, legumes, mushrooms, nuts | 16/14 | 35 mg | Nicotinamide adenine dinucleotide (NAD) and its phosphate (NADP) (redox reactions) | Pellagra: dermatitis/photo dermatitis, alopecia, muscle weakness, twitching/burning in the extremities, altered gait, diarrhoea [13] | Depression, anxiety, progressing to vertigo, memory loss, paranoia, psychotic symptoms, aggression (Pellagrous insanity) [13] | Alcohol abuse |
| B5 | Pantothenic acid | Meat, whole grain cereals, broccoli | 5 | - | Co-enzyme A (CoA) (acyl activation and transfer) | Numbness/burning sensations in extremities, dermatitis, diarrhoea [14] | Encephalopathy, behaviour change, demyelination [14] | |
| B6 | Vitamin B6 (referring to: pyridoxal, pyridoxamine, pyridoxine) | Meat, fish, legumes, nuts, bananas, potatoes | 1.3/1.3 (1.7/1.5 >50 year) | 100 mg | pyridoxal-5′-phosphate (PLP) and pyridoxamine-5′-phosphate (PMP) (Generation of leaving group potential) | Anaemia | Irritability, impaired alertness, depression, cognitive decline, dementia, autonomic dysfunction, convulsions [15] | Alcohol abuse, age-related malabsorption, contraceptive medications [16] |
| B7 | Biotin | Eggs, liver, pork, leafy vegetables | 30 (µg) | - | biotin (carboxylation reactions) | Seborrheic eczematous rash, tingling/burning of the extremities [17] | Depression, lethargy, hallucinations, seizures [17] | Type II diabetes, poor gluco-regulation [18] |
| B9 | Folic acid/folate | Leafy vegetables, legumes, citrus fruits | 400 (µg) | 1000 µg | tetrahydrofolates inc. methyltetrahydrofolate (One carbon transfer) | megaloblastic anaemia, peripheral neuropathy 3, spinal cord lesions, metabolic abnormalities [19,20] | Affective disorders 4, behaviour changes, psychosis, cognitive impairment/decline, dementia (inc Alzheimer’s disease and vascular dementia) [19] | Common genetic polymorphisms (inc. MTHFR C667T) [21] Low Riboflavin and B12 [22] |
| B12 | Vitamin B12 (referring to: the cobalamins) | Meat, fish and other animal products | 2.4 (µg) | - | Methylcobalamin, adenosylcobalamin (vicinal rearrangements) | age-related malabsorption [23], vegetarians, vegans [24] Genetic polymorphisms [21] |
2. Mechanisms of Action and Functions of B Vitamins
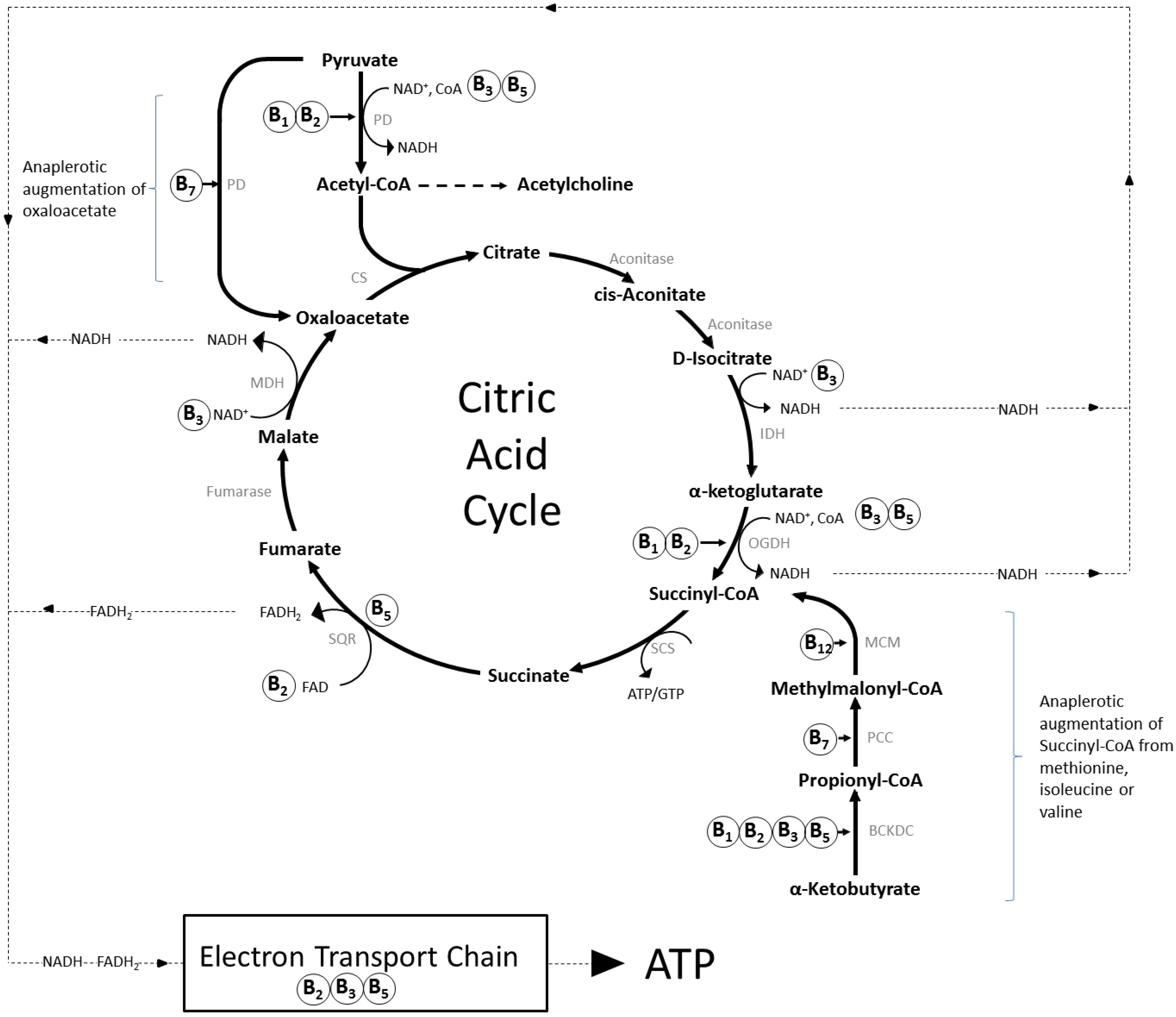

2.1. Brain Specific Roles of B Vitamins
2.1.1. Thiamine (Vitamin B1)
2.1.2. Riboflavin (Vitamin B2)
2.1.3. Niacin (Vitamin B3)
2.1.4. Pantothenic Acid (Vitamin B5)
2.1.5. Vitamin B6 (Pyridoxine, Pyridoxal, Pyridoxamine)
2.1.6. Biotin (Vitamin B7)
2.1.7. Folate (Vitamin B9) and Vitamin B12 (Cobolamin)
3. The Homocysteine Hypothesis
4. B Vitamin Deficiencies in Developed Societies
5. How Much Is Enough?
6. Do B Vitamins Have an Impact on Brain Function?
6.1. Observational Studies
6.2. Controlled Intervention Trials
6.2.1. Folate, Vitamin B12 and Vitamin B6
6.2.2. Thiamine, Riboflavin, Biotin, Pantothenic Acid, Niacin
6.2.3. Multivitamins and Brain Function
6.2.4. Acute Effects of Multivitamins
6.2.5. Chronic Effects of Multi-Vitamins in Children
6.2.6. Chronic Effects of Multi-Vitamins in Adults
7. Summary and Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| AADC, aromatic l-amino acid decarboxylase |
| AAAH, aromatic amino acid hydroxylases |
| ATP, adenosine triphosphate |
| BCKDC, branched-chain α-ketoacid dehydrogenase complex |
| BH2, dihydrobiopterin |
| BH4, tetrahydrobiopterin |
| CBS, cystathionine beta synthase |
| CGL, cystathionine gamma-lyase |
| CoA, coenzyme A |
| CS, citrate synthase |
| DHFR, dihydrofolate reductase |
| dTMP, thymidine monophosphate |
| dUMP, deoxyuridine monophosphate |
| EEG, electroencephalography |
| EGRAC, erythrocyte glutathione reductase activation test |
| FAD/FADH2, flavin adenine dinucleotide (oxidised/reduced) |
| fMRI, functional magnetic resonance imaging |
| GABA, gamma-aminobutyric acid |
| GSSG, glutathione disulphide |
| IDH, isocitrate dehydrogenase |
| MAT, methionine adenosyltransferase |
| MDH, malate dehydrogenase |
| MCM, methylmalonyl-CoA mutase |
| MMSE, Mini Mental State Exam |
| MS, methionine synthase |
| MTHFR, methyltetrahydrofolate reductase |
| MTRR, methionine synthase reductase |
| NAD, nicotinamide adenine dinucleotide (+/H = oxidised/reduced) |
| NIACR, Niacin receptor |
| NOS, nitric oxide synthase |
| OGDH, α-ketoglutarate dehydrogenase |
| PCC, propionyl-CoA Carboxylase |
| PC, pyruvate carboxylase |
| PD, pyruvate dehydrogenase |
| RDA, Recommended Daily Allowance |
| RDI, Recommended Daily Intake |
| SAH, S-adenosylhomocysteine |
| SAHH, S-adenosylhomocysteine hydrolase |
| SAM, S-adenosyl methionine |
| SCS, succinyl-CoA synthetase |
| SH, serine hydroxymethyltransferase |
| SQR, succinate-coenzyme Q reductase |
| THF, tetrahydrofolate |
| TS, thymidylate synthase |
References
- Smith, A.G.; Croft, M.T.; Moulin, M.; Webb, M.E. Plants need their vitamins too. Curr. Opin. Plant Biol. 2007, 10, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O. Plants and the Human Brain; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Banhegyi, G.; Braun, L.; Csala, M.; Puskas, F.; Mandl, J. Ascorbate metabolism and its regulation in animals. Free Radic. Biol. Med. 1997, 23, 793–803. [Google Scholar] [CrossRef]
- Pauling, L. Evolution and the need for ascorbic acid. Proc. Natl. Acad. Sci. USA 1970, 67, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Kawai, T.; Yagi, K. Guinea pigs possess a highly mutated gene for l-gulono-gamma-lactone oxidase, the key enzyme for l-ascorbic acid biosynthesis missing in this species. J. Biol. Chem. 1992, 267, 21967–21972. [Google Scholar] [PubMed]
- Tanaka, T.; Tateno, Y.; Gojobori, T. Evolution of vitamin B-6 (pyridoxine) metabolism by gain and loss of genes. Mol. Biol. Evol. 2005, 22, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Maguire, F.; Henriquez, F.L.; Leonard, G.; Dacks, J.B.; Brown, M.W.; Richards, T.A. Complex patterns of gene fission in the eukaryotic folate biosynthesis pathway. Genome Biol. Evol. 2014, 6, 2709–2720. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.B. Bioorganic mechanisms important to coenzyme functions. In Handbook of Vitamins, 4th ed.; Zempleni, J., Rucker, R.B., McCormick, D.B., Suttie, J.W., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kerns, J.C.; Arundel, C.; Chawla, L.S. Thiamin deficiency in people with obesity. Adv. Nutr. Int. Rev. J. 2015, 6, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.J. Thiamine. In Handbook of Vitamins, 4th ed.; Zempleni, J., Rucker, R.B., McCormick, D.B., Suttie, J.W., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Rivlin, R.S. Riboflavin (vitamin B2). In Handbook of Vitamins, 4th ed.; Zempleni, J., Rucker, R.B., McCormick, D.B., Suttie, J.W., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Sinigaglia-Coimbra, R.; Lopes, A.C.; Coimbra, C.G. Riboflavin deficiency, brain function, and health. In Handbook of Behavior, Food and Nutrition; Springer: Berlin, Germany, 2011; pp. 2427–2449. [Google Scholar]
- Kirkland, J.B. Niacin. In Handbook of Vitamins, 4th ed.; Zempleni, J., Rucker, R.B., McCormick, D.B., Suttie, J.W., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Rucker, R.B.; Bauerly, K. Pantothenic acid. In Handbook of Vitamins, 5th ed.; Zempleni, J., Suttie, J.W., Gregory, J.F., III, Stover, P.J., Eds.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Dakshinamurti, S.; Dakshinamurti, K. Vitamin b6. In Handbook of Vitamins, 5th ed.; Zempleni, J., Suttie, J.W., Gregory, J.F., III, Stover, P.J., Eds.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Morris, M.S.; Picciano, M.F.; Jacques, P.F.; Selhub, J. Plasma pyridoxal 5′-phosphate in the us population: The national health and nutrition examination survey, 2003–2004. Am. J. Clin. Nutr. 2008, 87, 1446–1454. [Google Scholar] [PubMed]
- Mock, D.M. Biotin. In Handbook of Vitamins, 4th ed.; Zempleni, J., Rucker, R.B., McCormick, D.B., Suttie, J.W., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Via, M. The malnutrition of obesity: Micronutrient deficiencies that promote diabetes. ISRN Endocrinol. 2012, 2012, 103472. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006, 5, 949–960. [Google Scholar] [CrossRef]
- Green, R.; Miller, J. Vitamin B12. In Handbook of Vitamins, 4th ed.; Zempleni, J., Rucker, R.B., McCormick, D.B., Suttie, J.W., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Mitchell, E.S.; Conus, N.; Kaput, J. B vitamin polymorphisms and behavior: Evidence of associations with neurodevelopment, depression, schizophrenia, bipolar disorder and cognitive decline. Neurosci. Biobehav. Rev. 2014, 47, 307–320. [Google Scholar] [CrossRef] [PubMed]
- García-Minguillán, C.J.; Fernandez-Ballart, J.D.; Ceruelo, S.; Ríos, L.; Bueno, O.; Berrocal-Zaragoza, M.I.; Molloy, A.M.; Ueland, P.M.; Meyer, K.; Murphy, M.M. Riboflavin status modifies the effects of methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR) polymorphisms on homocysteine. Genes Nutr. 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Rich, S.; Rosenberg, I.; Jacques, P.; Dallal, G.; Wilson, P.W.; Selhub, J. Plasma vitamin B-12 concentrations relate to intake source in the framingham offspring study. Am. J. Clin. Nutr. 2000, 71, 514–522. [Google Scholar] [PubMed]
- Pawlak, R.; Parrott, S.J.; Raj, S.; Cullum-Dugan, D.; Lucus, D. How prevalent is vitamin B12 deficiency among vegetarians? Nutr. Rev. 2013, 71, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [PubMed]
- Benzie, I.F.F. Evolution of dietary antioxidants. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 136, 113–126. [Google Scholar] [CrossRef]
- Milton, K. Back to basics: Why foods of wild primates have relevance for modern human health. Nutrition 2000, 16, 480–483. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Haskell, C.F. Vitamins and cognition: What is the evidence? Drugs 2011, 71, 1957–1971. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Bes-Rastrollo, M.; Román-Vinas, B.; Pfrimer, K.; Sánchez-Villegas, A.; Martínez-González, M.A. Dietary patterns and nutritional adequacy in a mediterranean country. Br. J. Nutr. 2009, 101, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Castro-Quezada, I.; Román-Viñas, B.; Serra-Majem, L. The mediterranean diet and nutritional adequacy: A review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, M.; Polanuyer, B.; Farrell, M.; Scholle, M.; Lykidis, A.; de Crécy-Lagard, V.; Osterman, A. Complete reconstitution of the human coenzyme a biosynthetic pathway via comparative genomics. J. Biol. Chem. 2002, 277, 21431–21439. [Google Scholar] [CrossRef] [PubMed]
- Huskisson, E.; Maggini, S.; Ruf, M. The role of vitamins and minerals in energy metabolism and well-being. J. Int. Med. Res. 2007, 35, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of b vitamins on the one-carbon transfer pathways. Chem. Biol. Interact. 2006, 163, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Depeint, F.; Bruce, W.R.; Shangari, N.; Mehta, R.; O’Brien, P.J. Mitochondrial function and toxicity: Role of the b vitamin family on mitochondrial energy metabolism. Chem. Biol. Interact. 2006, 163, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Ba, A. Metabolic and structural role of thiamine in nervous tissues. Cell. Mol. Neurobiol. 2008, 28, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, M.J.; Tatham, A.L.; Hale, A.B.; Alp, N.J.; Channon, K.M. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling relative importance of the de novo biopterin synthesis versus salvage pathways. J. Biol. Chem. 2009, 284, 28128–28136. [Google Scholar] [CrossRef] [PubMed]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.M.; Crabtree, M.J. Tetrahydrobiopterin in cardiovascular health and disease. Antioxid. Redox Signal. 2014, 20, 3040–3077. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. Two views of brain function. Trends Cogn. Sci. 2010, 14, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Spector, R. Vitamin transport diseases of brain: Focus on folates, thiamine and riboflavin. Brain Disord. Ther. 2014, 3, 120. [Google Scholar] [CrossRef]
- Spector, R.; Johanson, C.E. Vitamin transport and homeostasis in mammalian brain: Focus on vitamins B and E. J. Neurochem. 2007, 103, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Ito, K.; Ohtsuki, S.; Kubo, Y.; Suzuki, T.; Terasaki, T. Major involvement of na+-dependent multivitamin transporter (SLC5A6/SMVT) in uptake of biotin and pantothenic acid by human brain capillary endothelial cells. J. Neurochem. 2015, 134, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.A.; Parrott, J. New considerations on the neuromodulatory role of thiamine. Pharmacology 2012, 89, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Powers, H.J.; Hill, M.H.; Mushtaq, S.; Dainty, J.R.; Majsak-Newman, G.; Williams, E.A. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the united kingdom (ribofem). Am. J. Clin. Nutr. 2011, 93, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Ashoori, M.; Saedisomeolia, A. Riboflavin (vitamin B2) and oxidative stress: A review. Br. J. Nutr. 2014, 111, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.B. Folic acid. In Handbook of Vitamins, 4th ed.; Zempleni, J., Rucker, R.B., McCormick, D.B., Suttie, J.W., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Miller, C.L.; Dulay, J.R. The high-affinity niacin receptor HM74A is decreased in the anterior cingulate cortex of individuals with schizophrenia. Brain Res. Bull. 2008, 77, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Digby, J.E.; McNeill, E.; Dyar, O.J.; Lam, V.; Greaves, D.R.; Choudhury, R.P. Anti-inflammatory effects of nicotinic acid in adipocytes demonstrated by suppression of fractalkine, rantes, and mcp-1 and upregulation of adiponectin. Atherosclerosis 2010, 209, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Schmidt, R.J.; Foxworthy, P.; Emkey, R.; Oler, J.K.; Large, T.H.; Wang, H.; Su, E.W.; Mosior, M.K.; Eacho, P.I. Niacin mediates lipolysis in adipose tissue through its g-protein coupled receptor HM74A. Biochem. Biophys. Res. Commun. 2005, 334, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Sonnabend, M.; Fasshauer, M.; Höllriegel, R.; Schuler, G.; Niebauer, J.; Stumvoll, M.; Blüher, M. Effects of extended-release niacin on lipid profile and adipocyte biology in patients with impaired glucose tolerance. Atherosclerosis 2009, 205, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wakade, C.; Chong, R.; Bradley, E.; Thomas, B.; Morgan, J. Upregulation of GPR109A in parkinson’s disease. PLoS ONE 2014, 9, e109818. [Google Scholar] [CrossRef] [PubMed]
- Wakade, C.; Chong, R.; Bradley, E.; Morgan, J.C. Low-dose niacin supplementation modulates GPR109A, niacin index and ameliorates parkinson’s disease symptoms without side effects. Clin. Case Rep. 2015, 3, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Anitha, M.; Abraham, P.M.; Paulose, C.S. Striatal dopamine receptors modulate the expression of insulin receptor, Igf-1 and Glut-3 in diabetic rats: Effect of pyridoxine treatment. Eur. J. Pharmacol. 2012, 696, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Sakakeeny, L.; Roubenoff, R.; Obin, M.; Fontes, J.D.; Benjamin, E.J.; Bujanover, Y.; Jacques, P.F.; Selhub, J. Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of us adults. J. Nutr. 2012, 142, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Sakakeeny, L.; Jacques, P.F.; Picciano, M.F.; Selhub, J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J. Nutr. 2010, 140, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tracy, R. Emerging relationships of inflammation, cardiovascular disease and chronic diseases of aging. Int. J. Obes. 2003, 27, S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. L-methylfolate: A vitamin for your monoamines. J. Clin. Psychiatry 2008, 69, 1352–1353. [Google Scholar] [CrossRef] [PubMed]
- Moat, S.J.; Clarke, Z.L.; Madhavan, A.K.; Lewis, M.J.; Lang, D. Folic acid reverses endothelial dysfunction induced by inhibition of tetrahydrobiopterin biosynthesis. Eur. J. Pharmacol. 2006, 530, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Sturman, J.A.; Rivlin, R.S. Pathogenesis of brain dysfunction in deficiency of thiamine, riboflavin, pantothenic acid, or vitamin B6. In Biology of Brain Dysfunction; Springer: Berlin, Germany, 1975; pp. 425–475. [Google Scholar]
- Thomson, A.D.; Marshall, E.J. The natural history and pathophysiology of wernicke’s encephalopathy and korsakoff’s psychosis. Alcohol Alcohol. 2006, 41, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Collaboration, H.S. Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA 2002, 288, 2015–2022. [Google Scholar] [CrossRef]
- Smulders, Y.M.; Blom, H.J. The homocysteine controversy. J. Inherit. Metab. Dis. 2011, 34, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Gottfries, C.; Regland, B. Identification of cognitive impairment in the elderly: Homocysteine is an early marker. Dement. Geriatr. Cogn. Disord. 1999, 10, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D. The worldwide challenge of the dementias: A role for b vitamins and homocysteine? Food Nutr. Bull. 2008, 29, S143–S172. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.; Rong, Y.; Jinming, L.; Yongjun, L.; Hui, Z. Effect of homocysteine interventions on the risk of cardiocerebrovascular events: A meta-analysis of randomised controlled trials. Int. J. Clin. Pract. 2010, 64, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Martí-Carvajal, A.J.; Solà, I.; Lathyris, D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Datebase Syst. Rev. 2015, 1. [Google Scholar] [CrossRef]
- Moorthy, D.; Peter, I.; Scott, T.M.; Parnell, L.D.; Lai, C.-Q.; Crott, J.W.; Ordovás, J.M.; Selhub, J.; Griffith, J.; Rosenberg, I.H. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J. Nutr. 2012, 142, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Bennett, D.; Parish, S.; Lewington, S.; Skeaff, M.; Eussen, S.J.; Lewerin, C.; Stott, D.J.; Armitage, J.; Hankey, G.J. Effects of homocysteine lowering with b vitamins on cognitive aging: Meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am. J. Clin. Nutr. 2014, 100, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K.; Gustafson, L.; Hultberg, B. Elevated plasma homocysteine level in vascular dementia reflects the vascular disease process. Dement. Geriatr. Cogn. Disord. Extra 2013, 3, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Luft, F.C. Fitting homocysteine to disease models, as well as adjusting the models to the disease. J. Mol. Med. 2015, 93, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Ganji, V.; Kafai, M.R. Frequent consumption of milk, yogurt, cold breakfast cereals, peppers, and cruciferous vegetables and intakes of dietary folate and riboflavin but not vitamins B-12 and B-6 are inversely associated with serum total homocysteine concentrations in the us population. Am. J. Clin. Nutr. 2004, 80, 1500–1507. [Google Scholar] [PubMed]
- Moat, S.J.; Ashfield-Watt, P.A.; Powers, H.J.; Newcombe, R.G.; McDowell, I.F. Effect of riboflavin status on the homocysteine-lowering effect of folate in relation to the MTHFR (C677T) genotype. Clin. Chem. 2003, 49, 295–302. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.; le Roy, C.D.; Strain, J.; Dunne, A.; Ward, M.; Molloy, A.M.; McAnena, L.B.; Hughes, J.P.; Hannon-Fletcher, M.; Scott, J.M. Riboflavin lowers homocysteine in individuals homozygous for the MTHFR 677C -> T polymorphism. Circulation 2006, 113, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Horigan, G.; McNulty, H.; Ward, M.; Strain, J.; Purvis, J.; Scott, J.M. Riboflavin lowers blood pressure in cardiovascular disease patients homozygous for the 677C -> T polymorphism in mthfr. J. Hypertens. 2010, 28, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older americans in the age of folic acid fortification. Am. J. Clin. Nutr. 2007, 85, 193–200. [Google Scholar] [PubMed]
- Morris, M.S.; Selhub, J.; Jacques, P.F. Vitamin B-12 and folate status in relation to decline in scores on the mini-mental state examination in the framingham heart study. J. Am. Geriatr. Soc. 2012, 60, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.E.; Hornstra, J.M.; Kok, R.M.; Blom, H.J.; Smulders, Y.M. Folic acid supplementation does not reduce intracellular homocysteine, and may disturb intracellular one-carbon metabolism. Clin. Chem. Lab. Med. 2013, 51, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.R.; Martel, F.; Borges, N.; Araújo, J.M.; Keating, E. Folates and aging: Role in mild cognitive impairment, dementia and depression. Ageing Res. Rev. 2015, 22, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wen, J.; Wang, X.; Xiao, C. High-dose folic acid improves endothelial function by increasing tetrahydrobiopterin and decreasing homocysteine levels. Mol. Med. Rep. 2014, 10, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I.; Shelton, R.C.; Zajecka, J.M.; Etemad, B.; Rickels, K.; Clain, A.; Baer, L.; Dalton, E.D.; Sacco, G.R.; Schoenfeld, D. l-methylfolate as adjunctive therapy for ssri-resistant major depression: Results of two randomized, double-blind, parallel-sequential trials. Am. J. Psychiatry 2014, 169, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R. Evidence for a recommended dietary allowance for vitamin C from pharmacokinetics: A comment and analysis. Proc. Natl. Acad. Sci. USA 1996, 93, 14344–14348. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Caudill, M.A. Folate bioavailability: Implications for establishing dietary recommendations and optimizing status. Am. J. Clin. Nutr. 2009, 91, 1455S–1460S. [Google Scholar] [CrossRef] [PubMed]
- Kauwell, G.P.A.; Wilsky, C.E.; Cerda, J.J.; Herrlinger-Garcia, K.; Hutson, A.D.; Theriaque, D.W.; Boddie, A.; Rampersaud, G.C.; Bailey, L.B. Methylenetetrahydrofolate reductase mutation (677C -> T) negatively influences plasma homocysteine response to marginal folate intake in elderly women. Metab. Clin. Exp. 2000, 49, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Fukuwatari, T.; Ohta, M.; Okamoto, H.; Watanabe, T.; Fukui, T.; Nishimuta, M.; Totani, M.; Kimura, M.; Ohishi, N.; et al. Values of water-soluble vitamins in blood and urine of japanese young men and women consuming a semi-purified diet based on the Japanese dietary reference intakes. J. Nutr. Sci. Vitaminol. 2005, 51, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Fukuwatari, T.; Watanabe, T.; Nishimuta, M. Intra- and inter-individual variations of blood and urinary water-soluble vitamins in japanese young adults consuming a semi-purified diet for 7 days. J. Nutr. Sci. Vitaminol. 2009, 55, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Challem, J.J. Toward a new definition of essential nutrients: Is it now time for a third “vitamin” paradigm? Med. Hypotheses 1999, 52, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative western countries. Br. J. Nutr. 2012, 108, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Ruston, D.; Hoare, J.; Henderson, L.; Gregory, J.; Bates, C.; Prentice, A.; Birch, M.; Swan, G.; Farron, M. National Diet and Nutrition Survey: Adults Aged 19–64 Years. Volume 4: Nutritional Status (Anthropometry and Blood Analytes), Blood Pressure and Physical Activity; TSO: London, UK, 2004; Volume 5. [Google Scholar]
- Nelson, M.; Erens, B.; Bates, B.; Church, S.; Boshier, T. Low Income Diet and Nutrition Survey; TSO: London, UK, 2007; Volume 3. [Google Scholar]
- CDC. Second National Report on Biochemical Indicators of Diet and Nutrition in the US Population; US Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2012.
- Aparicio-Ugarriza, R.; Palacios, G.; Alder, M.; González-Gross, M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin. Chem. Lab. Med. (CCLM) 2014, 53. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H. Do we need to reconsider the desirable blood level of vitamin B12? J. Intern. Med. 2012, 271, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.J.; Hanger, H.C.; Elmslie, J.; George, P.M.; Sainsbury, R. The response to treatment of subclinical thiamine deficiency in the elderly. Am. J. Clin. Nutr. 1997, 66, 925–928. [Google Scholar] [PubMed]
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Kimmons, J.E.; Blanck, H.M.; Tohill, B.C.; Zhang, J.; Khan, L.K. Associations between body mass index and the prevalence of low micronutrient levels among US adults. Medscape Gen. Med. 2006, 8, 59. [Google Scholar]
- Lotto, V.; Choi, S.-W.; Friso, S. Vitamin b6: A challenging link between nutrition and inflammation in cvd. Br. J. Nutr. 2011, 106, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin c based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [PubMed]
- Qi, Y.P.; Do, A.N.; Hamner, H.C.; Pfeiffer, C.M.; Berry, R.J. The prevalence of low serum vitamin B-12 status in the absence of anemia or macrocytosis did not increase among older us adults after mandatory folic acid fortification. J. Nutr. 2014, 144, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Long, S.-J.; Benton, D. Effects of vitamin and mineral supplementation on stress, mild psychiatric symptoms, and mood in nonclinical samples: A meta-analysis. Psychosom. Med. 2013, 75, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S. The role of B vitamins in preventing and treating cognitive impairment and decline. Adv. Nutr. Int. Rev. J. 2012, 3, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin and Choline; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Smith, A.D.; Kim, Y.-I.; Refsum, H. Is folic acid good for everyone? Am. J. Clin. Nutr. 2008, 87, 517–533. [Google Scholar] [PubMed]
- Smithline, H.A.; Donnino, M.; Greenblatt, D.J. Pharmacokinetics of high-dose oral thiamine hydrochloride in healthy subjects. BMC Pharmacol. Toxicol. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, A.J.; Shi, Y.; Greene-Finestone, L.S. High-dose compared with low-dose vitamin B-12 supplement use is not associated with higher vitamin B-12 status in children, adolescents, and older adults. J. Nutr. 2014, 144, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Dullemeijer, C.; Souverein, O.W.; Doets, E.L.; van der Voet, H.; van Wijngaarden, J.P.; de Boer, W.J.; Plada, M.; Dhonukshe-Rutten, R.A.; In’t Veld, P.H.; Cavelaars, A.E. Systematic review with dose-response meta-analyses between vitamin B-12 intake and european micronutrient recommendations aligned’s prioritized biomarkers of vitamin B-12 including randomized controlled trials and observational studies in adults and elderly persons. Am. J. Clin. Nutr. 2013, 97, 390–402. [Google Scholar] [PubMed]
- Eussen, S.J.; de Groot, L.C.; Clarke, R.; Schneede, J.; Ueland, P.M.; Hoefnagels, W.H.; van Staveren, W.A. Oral cyanocobalamin supplementation in older people with vitamin B12 deficiency: A dose-finding trial. Arch. Intern. Med. 2005, 165, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, K.E.; Williams, S.M.; Green, T.J.; McMahon, J.A.; Mann, J.I.; Knight, R.G.; Skeaff, C.M. Differences in erythrocyte folate concentrations in older adults reached steady-state within one year in a two-year, controlled, 1 mg/d folate supplementation trial. J. Nutr. 2012, 142, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Homocysteine Lowering Trialists’Collaboration. Dose-dependent effects of folic acid on blood concentrations of homocysteine: A meta-analysis of the randomized trials. Am. J. Clin. Nutr. 2005, 82, 806–812. [Google Scholar]
- Miner, S.E.S.; Cole, D.E.C.; Evrovski, J.; Forrest, Q.; Hutchison, S.; Holmes, K.; Ross, H.J. Pyridoxine improves endothelial function in cardiac transplant recipients. J. Heart Lung Transplant. 2001, 20, 964–969. [Google Scholar] [CrossRef]
- Moat, S.J.; Lang, D.; McDowell, I.F.; Clarke, Z.L.; Madhavan, A.K.; Lewis, M.J.; Goodfellow, J. Folate, homocysteine, endothelial function and cardiovascular disease. J. Nutr. Biochem. 2004, 15, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Wakade, C.; Chong, R. A novel treatment target for parkinson’s disease. J. Neurol. Sci. 2014, 347, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Ho, R.C.; Cheung, M.W.; Fu, E.; Win, H.H.; Zaw, M.H.; Ng, A.; Mak, A. Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. Am. J. Geriatr. Psychiatry 2011, 19, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.S.; Kasturiratne, A.; Simmonds, M. Serum homocysteine and dementia: Meta-analysis of eight cohort studies including 8669 participants. Alzheimer’s Dement. 2011, 7, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Nie, T.; Lu, T.; Xie, L.; Huang, P.; Lu, Y.; Jiang, M. Hyperhomocysteinemia and risk of cognitive decline: A meta-analysis of prospective cohort studies. Eur. Neurol. 2014, 72, 241–248. [Google Scholar] [PubMed]
- Nilsson, T.K.; Yngve, A.; Böttiger, A.K.; Hurtig-Wennlöf, A.; Sjöström, M. High folate intake is related to better academic achievement in swedish adolescents. Pediatrics 2011, 128, e358–e365. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.T.; Kousoulis, A.A.; Michelakos, T.; Papathoma, P.; Dessypris, N.; Papadopoulos, F.C.; Stefanadis, C. Folate and B12 serum levels in association with depression in the aged: A systematic review and meta-analysis. Aging Ment. Health 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Michelakos, T.; Kousoulis, A.A.; Katsiardanis, K.; Dessypris, N.; Anastasiou, A.; Katsiardani, K.-P.; Kanavidis, P.; Stefanadis, C.; Papadopoulos, F.C.; Petridou, E.T. Serum folate and B12 levels in association with cognitive impairment among seniors results from the velestino study in greece and meta-analysis. J. Aging Health 2013, 25, 589–616. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, F.; Allman-Farinelli, M.; Samman, S. Vitamin B12 status, cognitive decline and dementia: A systematic review of prospective cohort studies. Br. J. Nutr. 2012, 108, 1948–1961. [Google Scholar] [CrossRef] [PubMed]
- Doets, E.L.; van Wijngaarden, J.P.; Szczecińska, A.; Dullemeijer, C.; Souverein, O.W.; Dhonukshe-Rutten, R.A.; Cavelaars, A.E.; van’t Veer, P.; Brzozowska, A.; de Groot, L.C. Vitamin B12 intake and status and cognitive function in elderly people. Epidemiol. Rev. 2013, 35, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Lopes da Silva, S.; Vellas, B.; Elemans, S.; Luchsinger, J.; Kamphuis, P.; Yaffe, K.; Sijben, J.; Groenendijk, M.; Stijnen, T. Plasma nutrient status of patients with alzheimer’s disease: Systematic review and meta-analysis. Alzheimer’s Dement. 2014, 10, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Malouf, R.; Grimley Evans, J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst. Rev. 2008, 4. [Google Scholar] [CrossRef]
- Malouf, R.; Areosa Sastre, A. Vitamin B12 for cognition. Cochrane Database Syst. Rev. 2003, 3. [Google Scholar] [CrossRef] [Green Version]
- Malouf, R.; Grimley Evans, J. Vitamin B6 for cognition. Cochrane Database Syst. Rev. 2003, 4. [Google Scholar] [CrossRef] [Green Version]
- Dangour, A.D.; Whitehouse, P.J.; Rafferty, K.; Mitchell, S.A.; Smith, L.; Hawkesworth, S.; Vellas, B. B-vitamins and fatty acids in the prevention and treatment of alzheimer’s disease and dementia: A systematic review. J. Alzheimer’s Dis. 2010, 22, 205–224. [Google Scholar]
- Balk, E.M.; Raman, G.; Tatsioni, A.; Chung, M.; Lau, J.; Rosenberg, I.H. Vitamin B6, B12, and folic acid supplementation and cognitive function: A systematic review of randomized trials. Arch. Intern. Med. 2007, 167, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Wald, D.S.; Kasturiratne, A.; Simmonds, M. Effect of folic acid, with or without other B vitamins, on cognitive decline: Meta-analysis of randomized trials. Am. J. Med. 2010, 123, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.H.; Almeida, O.P. Effect of homocysteine lowering treatment on cognitive function: A systematic review and meta-analysis of randomized controlled trials. J. Alzheimer’s Dis. 2012, 29, 133–149. [Google Scholar]
- Garrard, P.; Jacoby, R. B-vitamin trials meta-analysis: Less than meets the eye. Am. J. Clin. Nutr. 2015, 101, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; de Jager, C.A.; Refsum, H.; Rosenberg, I.H. Homocysteine lowering, B vitamins, and cognitive aging. Am. J. Clin. Nutr. 2015, 101, 415–416. [Google Scholar] [CrossRef] [PubMed]
- McCaddon, A.; Miller, J.W. Assessing the association between homocysteine and cognition: Reflections on bradford hill, meta-analyses, and causality. Nutr. Rev. 2015, 73, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Durga, J.; van Boxtel, M.P.J.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the facit trial: A randomised, double blind, controlled trial. Lancet 2007, 369, 208–216. [Google Scholar] [CrossRef]
- Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.; Aggarwal, N.; Li, H.; Wilson, R.; Decarli, C.; Evans, D.; Morris, M. Vitamin B12, cognition, and brain mri measures a cross-sectional examination. Neurology 2011, 77, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Refsum, H.; Johnston, C.; Smith, S.; Bradley, K.; de Jager, C.; Budge, M.; Smith, A. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology 2008, 71, 826–832. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.; Smith, A.; Refsum, H.; Johnston, C.; Breteler, M. Plasma vitamin B12 status and cerebral white-matter lesions. J. Neurol. Neurosurg. Psychiatry 2009, 80, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Smith, S.M.; de Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by b vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Refsum, H.; de Jager, C.A.; Jacoby, R.; Nichols, T.E.; Smith, S.M.; Smith, A.D. Preventing alzheimer’s disease-related gray matter atrophy by b-vitamin treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Ford, A.H.; Flicker, L. Systematic review and meta-analysis of randomized placebo-controlled trials of folate and vitamin B12 for depression. Int. Psychogeriatr. 2015, 27, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Strain, J.; Hughes, C.F.; McNulty, H.; Ward, M. Riboflavin lowers blood pressure: A review of a novel gene-nutrient interaction. Nutr. Food Sci. Res. 2015, 2, 3–6. [Google Scholar]
- Singer, G.M.; Geohas, J. The effect of chromium picolinate and biotin supplementation on glycemic control in poorly controlled patients with type 2 diabetes mellitus: A placebo-controlled, double-blinded, randomized trial. Diabetes Technol. Ther. 2006, 8, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, C.; Fuqua, B.; Geohas, J.; Juturu, V.; Finch, M.R.; Komorowski, J.R. Combination of chromium and biotin improves coronary risk factors in hypercholesterolemic type 2 diabetes mellitus: A placebo-controlled, double-blind randomized clinical trial. J. Cardiometab. Syndr. 2007, 2, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mejia, C.; Zendejas-Ruiz, I.; Revilla-Monsalve, C.; Islas-Andrade, S.; Baez-Saldana, A.; Cardenas, A.; Rojas-Ochoa, A. Biotin treatment increases insulin sensitivity in type 2 diabetics. Diabetes 2003, 52, A459. [Google Scholar]
- Hemmati, M.; Babaei, H.; Abdolsalehei, M. Survey of the effect of biotin on glycemic control and plasma lipid concentrations in type 1 diabetic patients in kermanshah in Iran (2008–2009). Oman Med. J. 2013, 28, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mejia, C. Pharmacological effects of biotin. J. Nutr. Biochem. 2005, 16, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, P.M.; Karas, R.H. The current state of niacin in cardiovascular disease prevention: A systematic review and meta-regression. J. Am. Coll. Cardiol. 2013, 61, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Kuvin, J.T.; Dave, D.M.; Sliney, K.A.; Mooney, P.; Patel, A.R.; Kimmelstiel, C.D.; Karas, R.H. Effects of extended-release niacin on lipoprotein particle size, distribution, and inflammatory markers in patients with coronary artery disease. Am. J. Cardiol. 2006, 98, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Griffiths, R.; Haller, J. Thiamine supplementation mood and cognitive functioning. Psychopharmacology 1997, 129, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Doshi, S.N.; McDowell, I.F.W.; Moat, S.J.; Payne, N.; Durrant, H.J.; Lewis, M.J.; Goodfellow, J. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation 2002, 105, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Obad, A.; Palada, I.; Valic, Z.; Ivancev, V.; Bakovic, D.; Wisloff, U.; Brubakk, A.O.; Dujic, Z. The effects of acute oral antioxidants on diving-induced alterations in human cardiovascular function. J. Physiol. Lond. 2007, 578, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.L.; Nawaz, H.; Boukhalil, J.; Giannamore, V.; Chan, W.; Ahmadi, R.; Sarrel, P.M. Acute effects of oats and vitamin E on endothelial responses to ingested fat. Am. J. Prev. Med. 2001, 20, 124–129. [Google Scholar] [CrossRef]
- Title, L.M.; Cummings, P.M.; Giddens, K.; Nassar, B.A. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: An effect prevented by vitamins c and e. J. Am. Coll. Cardiol. 2000, 36, 2185–2191. [Google Scholar] [CrossRef]
- Usui, M.; Matsuoka, H.; Miyazaki, H.; Ueda, S.; Okuda, S.; Imaizumi, T. Endothelial dysfunction by acute hyperhomocyst (e) inaemia: Restoration by folic acid. Clin. Sci. 1999, 96, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Hartvig, P.; Lindner, K.; Bjurling, P.; Långström, B.; Tedroff, J. Pyridoxine effect on synthesis rate of serotonin in the monkey brain measured with positron emission tomography. J. Neural Transm. Gen. Sect. JNT 1995, 102, 91–97. [Google Scholar] [CrossRef]
- Ebben, M.; Lequerica, A.; Spielman, A. Effects of pyridoxine on dreaming: A preliminary study. Percept. Mot. Skills 2002, 94, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Haskell, C.F.; Scholey, A.B.; Jackson, P.A.; Elliott, J.M.; Defeyter, M.A.; Greer, J.; Robertson, B.C.; Buchanan, T.; Tiplady, B.; Kennedy, D.O. Cognitive and mood effects in healthy children during 12 weeks’ supplementation with multi-vitamin/minerals. Br. J. Nutr. 2008, 100, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.; Bauer, I.; Neale, C.; Savage, K.; Camfield, D.; White, D.; Maggini, S.; Pipingas, A.; Stough, C.; Hughes, M. Acute effects of different multivitamin mineral preparations with and without guaraná on mood, cognitive performance and functional brain activation. Nutrients 2013, 5, 3589–3604. [Google Scholar] [CrossRef] [PubMed]
- White, D.J.; Camfield, D.A.; Maggini, S.; Pipingas, A.; Silberstein, R.; Stough, C.; Scholey, A. The effect of a single dose of multivitamin and mineral combinations with and without guaraná on functional brain activity during a continuous performance task. Nutr. Neurosci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Stevenson, E.; Jackson, P.; Wishart, K.; Bieri, G.; Barella, L.; Carne, A.; Dunn, S.; Robertson, B.; Forster, J.; et al. Multivitamins/minerals modulate cerebral blood-flow and whole-body energy metabolism during cognitive tasks of graded difficulty. J. Nutr. Metab. 2016, in press. [Google Scholar]
- Benton, D. Micro-nutrient supplementation and the intelligence of children. Neurosci. Biobehav. Rev. 2001, 25, 297–309. [Google Scholar] [CrossRef]
- Eilander, A.; Gera, T.; Sachdev, H.S.; Transler, C.; van der Knaap, H.C.M.; Kok, F.J.; Osendarp, S.J.M. Multiple micronutrient supplementation for improving cognitive performance in children: Systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2010, 91, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Frensham, L.J.; Bryan, J.; Parletta, N. Influences of micronutrient and omega-3 fatty acid supplementation on cognition, learning, and behavior: Methodological considerations and implications for children and adolescents in developed societies. Nutr. Rev. 2012, 70, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Grima, N.A.; Pase, M.P.; Macpherson, H.; Pipingas, A. The effects of multivitamins on cognitive performance: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2012, 29, 561–569. [Google Scholar]
- White, D.J.; Cox, K.H.; Peters, R.; Pipingas, A.; Scholey, A.B. Effects of four-week supplementation with a multi-vitamin/mineral preparation on mood and blood biomarkers in young adults: A randomised, double-blind, placebo-controlled trial. Nutrients 2015, 7, 9005–9017. [Google Scholar] [CrossRef] [PubMed]
- Haskell, C.F.; Robertson, B.; Jones, E.; Forster, J.; Jones, R.; Wilde, A.; Maggini, S.; Kennedy, D.O. Effects of a multi-vitamin/mineral supplement on cognitive function and fatigue during extended multi-tasking. Hum. Psychopharmacol. Clin. Exp. 2010, 25, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Grodstein, F.; O’Brien, J.; Kang, J.H.; Dushkes, R.; Cook, N.R.; Okereke, O.; Manson, J.E.; Glynn, R.J.; Buring, J.E.; Gaziano, J.M. Long-term multivitamin supplementation and cognitive function in mena randomized trial. Ann. Intern. Med. 2013, 159, 806–814. [Google Scholar] [CrossRef]
- Harris, E.; Macpherson, H.; Vitetta, L.; Kirk, J.; Sali, A.; Pipingas, A. Effects of a multivitamin, mineral and herbal supplement on cognition and blood biomarkers in older men: A randomised, placebo-controlled trial. Hum. Psychopharmacol. Clin. Exp. 2012, 27, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, H.; Ellis, K.A.; Sali, A.; Pipingas, A. Memory improvements in elderly women following 16 weeks treatment with a combined multivitamin, mineral and herbal supplement. Psychopharmacology 2012, 220, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.; Kirk, J.; Rowsell, R.; Vitetta, L.; Sali, A.; Scholey, A.B.; Pipingas, A. The effect of multivitamin supplementation on mood and stress in healthy older men. Hum. Psychopharmacol. Clin. Exp. 2011, 26, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Pipingas, A.; Camfield, D.A.; Stough, C.; Scholey, A.B.; Cox, K.H.; White, D.; Sarris, J.; Sali, A.; Macpherson, H. Effects of multivitamin, mineral and herbal supplement on cognition in younger adults and the contribution of B group vitamins. Hum. Psychopharmacol. Clin. Exp. 2014, 29, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Pipingas, A.; Camfield, D.; Stough, C.; Cox, K.; Fogg, E.; Tiplady, B.; Sarris, J.; White, D.; Sali, A.; Wetherell, M. The effects of multivitamin supplementation on mood and general well-being in healthy young adults. A laboratory and at-home mobile phone assessment. Appetite 2013, 69, 123–136. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. https://doi.org/10.3390/nu8020068
Kennedy DO. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients. 2016; 8(2):68. https://doi.org/10.3390/nu8020068
Chicago/Turabian StyleKennedy, David O. 2016. "B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review" Nutrients 8, no. 2: 68. https://doi.org/10.3390/nu8020068
APA StyleKennedy, D. O. (2016). B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients, 8(2), 68. https://doi.org/10.3390/nu8020068




