Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation
Abstract
:1. Introduction
2. Vitamin B6 Metabolism
Vitamers and Their Metabolism
3. Vitamin B6 Metabolic Functions
4. Transmethylation vs. Transsulfuration
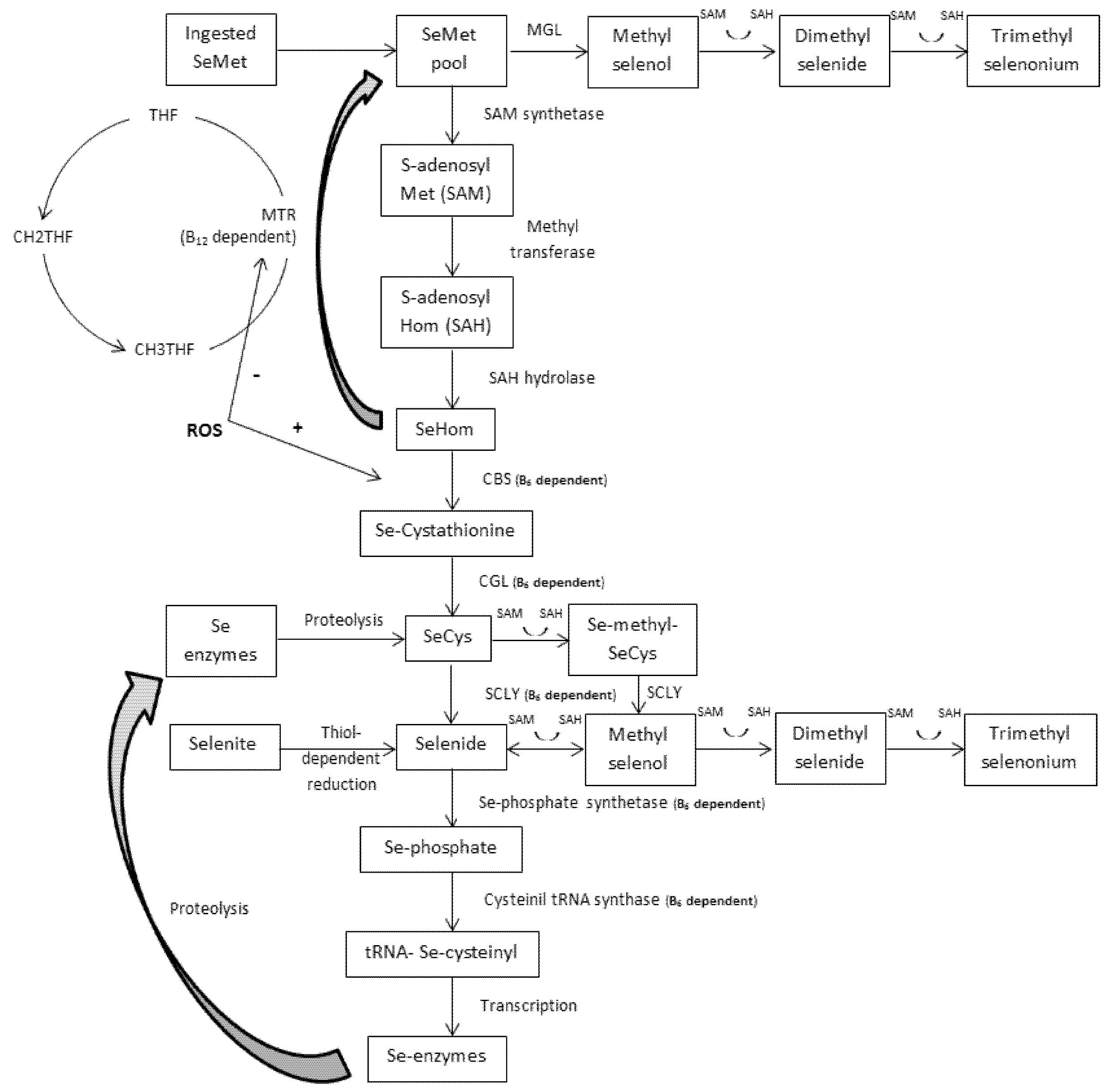
4.1. Transmethylation and the One-Carbon Metabolism
4.2. Transsulfuration and the GPX System
5. The Role of B6
Differential Effects on Organic and Mineral Se Metabolisms in Gilts
6. Embryo Metabolism
6.1. 5-Days Porcine Embryos
6.2. 30-Days Porcine Embryos
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Joseph, J.; Loscalzo, J. Methoxistasis: Integrating the roles of homocysteine and folic acid in cardiovascular pathobiology. Nutrients 2013, 5, 3235–3256. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C. One carbon metabolism in pregnancy: Impact on maternal, fetal and neonatal health. Mol. Cell. Endocrinol. 2016, 435, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; W.H. Freeman and Company: New York, NY, USA, 2002; pp. 571–572, 674–678, 686, 694, 698–700, 704, 771–772. [Google Scholar]
- Chen, Z.; Chakraborty, S.; Banerjee, R. Demonstration that mammalian methionine synthases are predominantly cobalamin-loaded. J. Biol. Chem. 1995, 270, 19246–19249. [Google Scholar] [PubMed]
- Castro, C.; Millian, N.S.; Garrow, T.A. Liver betaine-homocysteine S-methyltransferase activity undergoes a redox switch at the active site zinc. Arch. Biochem. Biophys. 2008, 472, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Gulati, S.; Baker, P.J.; Brody, L.C.; Banerjee, R.; Kruger, W.D. Cloning, mapping and RNA analysis of the human methionine synthase gene. Hum. Mol. Genet. 1996, 5, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Mosharov, E.; Cranford, M.R.; Banerjee, R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 2000, 39, 13005–13011. [Google Scholar] [CrossRef] [PubMed]
- Windisch, W. Interaction of chemical species with biological regulation of the metabolism of essential trace elements. Anal. Bioanal. Chem. 2002, 372, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.S.; Reed, D.J. Glutathione redox cycle-driven recovery of reduced glutathione after oxidation by tertiary-butyl hydroperoxide in preimplantation mouse embryos. Arch. Biochem. Biophys. 1995, 321, 6–12. [Google Scholar] [CrossRef]
- Davis, S.; Scheer, J.; Quinlivan, E.; Coats, B.; Stacpoole, P.; Gregory, J. Dietary vitamin B6 restriction does not alter rates of homocysteine remethylation or synthesis in healthy young women and men. Am. J. Clin. Nutr. 2005, 81, 648–655. [Google Scholar] [PubMed]
- Burk, R.F.; Olson, G.E.; Hill, K.E.; Winfrey, V.P.; Motley, A.K.; Kurokawa, S. Maternal-fetal transfer of selenium in the mouse. FASEB J. 2013, 27, 3249–3256. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Levonen, A.L.; Lapatto, R.; Saksela, M.; Raivio, K.O. Human cystathionine gamma-lyase: Developmental and in vitro expression of two isoforms. Biochem. J. 2000, 347, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.A. Nutritional Biochemistry of the Vitamins, 2nd ed.; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Combs, G.F., Jr. The Vitamins, 4th ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Combs, G.F. The Vitamins: Fundamental Aspects in Nutrition and Health; Elsevier: San Diego, CA, USA, 2008. [Google Scholar]
- Berdanier, C.D. Water-Soluble Vitamins. In Advanced Nutrition-Micronutrients; Berdanier, C.D., Ed.; CRC Press: New York, NY, USA, 1998. [Google Scholar]
- Matte, J.J.; Girard, C.L.; Sève, B. Effects of long-term parenteral administration of vitamin B6 on B6 status and some aspects of the glucose and protein metabolism of early-weaned piglets. Br. J. Nutr. 2001, 85, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Helmreich, E.J. How pyridoxal 5′-phosphate could function in glycogen phosphorylase catalysis. Biofactors 1992, 3, 159–172. [Google Scholar] [PubMed]
- Bilski, P.; Li, M.Y.; Ehrenshaft, M.; Daub, M.E.; Chignell, C.F. Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem. Photobiol. 2000, 71, 129–134. [Google Scholar] [CrossRef]
- Oka, T. Modulation of gene expression by vitamin B6. Nutr. Res. Rev. 2001, 14, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Drewke, C.; Leistner, E. Biosynthesis of vitamin B6 and structurally related derivatives. Vitam. Horm. 2001, 61, 121–155. [Google Scholar] [PubMed]
- Mittenhuber, G. Phylogenetic analyses and comparative genomics of vitamin B6 (pyridoxine) and pyridoxal phosphate biosynthesis pathways. J. Mol. Microbiol. Biotechnol. 2001, 3, 1–20. [Google Scholar] [PubMed]
- Mudd, S.H.; Cantoni, G.L. Activation of methionine for transmethylation. III. The methionine-activating enzyme of Bakers’ yeast. J. Biol. Chem. 1958, 231, 481–492. [Google Scholar] [PubMed]
- Ulrey, C.L.; Liu, L.; Andrews, L.G.; Tollefsbol, T.O. The impact of metabolism on DNA methylation. Hum. Mol. Genet. 2005, 14. [Google Scholar] [CrossRef] [PubMed]
- Födinger, M.; Hörl, W.; Sunder-Plassmann, G. Molecular biology of 5,10-methylenetetrahydrofolate reductase. J. Nephrol. 2000, 13, 20–33. [Google Scholar] [PubMed]
- Tibbetts, A.S.; Appling, D.R. Compartmentalization of mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 2010, 30, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Cuskelly, G.J.; Williamson, J.; Toth, J.P.; Gregory, J.F. Vitamin B6 deficiency in rats reduces hepatic serine hydroxymethyltransferase and cystathionine beta-synthase activities and rates of in vivo protein turnover, homocysteine remethylation and transsulfuration. J. Nutr. 2000, 130, 1115–1123. [Google Scholar] [PubMed]
- Perry, C.; Yu, S.; Chen, J.; Matharu, K.; Stover, P. Effect of vitamin B6 availability on serine hydroxymethyltransferase in MCF-7 cells. Arch. Biochem. Biophys. 2007, 462, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.G.; Smith, A.E.; Zhou, Z.S.; Taurog, R.E.; Bandarian, V.; Evans, J.C.; Ludwig, M. Cobalamin-dependent and cobalamin-independent methionine synthases: Are there two solutions to the same chemical problem? Helv. Chim. Acta 2003, 86, 3939–3954. [Google Scholar] [CrossRef]
- Aitken, S.M.; Lodha, P.H.; Morneau, D.J.K. The enzymes of the transsulfuration pathways: Active-site characterizations. Biochim. Biophys. Acta Proteins Proteom. 2011, 1814, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Ereno-Orbea, J.; Majtan, T.; Oyenarte, I.; Kraus, J.P.; Martinez-Cruz, L.A. Structural insight into the molecular mechanism of allosteric activation of human cystathionine beta-synthase by S-adenosylmethionine. Proc. Natl. Acad. Sci. USA 2014, 111, E3845–E3852. [Google Scholar] [CrossRef] [PubMed]
- Flavin, M.; Slaughter, C. Cystathionine cleavage enzymes of neurospora. J. Biol. Chem. 1964, 239, 2212–2219. [Google Scholar] [PubMed]
- Franklin, C.C.; Backos, D.S.; Mohar, I.; White, C.C.; Forman, H.J.; Kavanagh, T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Aspects Med. 2009, 30, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Taoka, S.; Ohja, S.; Shan, X.; Kruger, W.D.; Banerjee, R. Evidence for heme-mediated redox regulation of human cystathionine β-synthase activity. J. Biol. Chem. 1998, 273, 25179–25184. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. The nutritional significance, metabolism and toxicology of selenomethionine. Adv. Food Nutr. Res. 2003, 47, 73–112. [Google Scholar] [PubMed]
- Dalto, D.B.; Roy, M.; Audet, I.; Palin, M.-F.; Guay, F.; Lapointe, J.; Matte, J.J. Interaction between vitamin B6 and source of selenium on the response of the selenium-dependent glutathione peroxidase system to oxidative stress induced by oestrus in pubertal pig. J. Trace Elem. Med. Biol. 2015, 32, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Dalto, D.B.; Audet, I.; Lapointe, J.; Matte, J.J. The importance of pyridoxine for the impact of the dietary selenium sources on redox balance, embryo development, and reproductive performance in gilts. J. Trace Elem. Med. Biol. 2016, 34, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Birringer, M.; Pilawa, S.; Flohe, L. Trends in selenium biochemistry. Nat. Prod. Rep. 2002, 19, 693–718. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Doi, C.; Suzuki, N. Metabolism of 76Se-methylselenocysteine compared with that of 77Se-selenomethionine and 82Se-selenite. Toxicol. Appl. Pharmacol. 2006, 217, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Kurasaki, K.; Suzuki, N. Selenocysteine β-lyase and methylselenol demethylase in the metabolism of Se-methylated selenocompounds into selenide. Biochim. Biophys. Acta 2007, 1770, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Esaki, N.; Nakamura, T.; Tanaka, H.; Soda, K. Selenocysteine lyase, a novel enzyme that specifically acts on selenocysteine. Mammalian distribution and purification and properties of pig liver enzyme. J. Biol. Chem. 1982, 257, 4386–4391. [Google Scholar] [PubMed]
- Foster, L.H.; Sumar, S. Selenium in health and disease: A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Carlson, B.A.; Mix, H.; Zhang, Y.; Saira, K.; Glass, R.S.; Berry, M.J.; Gladyshev, V.N.; Hatfield, D.L. Biosynthesis of selenocysteine on its tRNA in eukaryotes. PLoS Biol. 2007, 5, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Sunde, R.A.; Thompson, B.M.; Palm, M.D.; Weiss, S.L.; Thompson, K.M.; Evenson, J.K. Selenium regulation of selenium-dependent glutathione peroxidases in animals and transfected CHO cells. Biomed. Environ. Sci. 1997, 10, 346–355. [Google Scholar] [PubMed]
- Johansson, L.; Gafvelin, G.; Arnér, E.S.J. Selenocysteine in proteins—Properties and biotechnological use. Biochim. Biophys. Acta Gen. Subj. 2005, 1726, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.H.; Ganther, H.E. Biosynthesis of dimethyl selenide from sodium selenite in rat liver and kidney cell-free systems. Biochim. Biophys. Acta Gen. Subj. 1977, 497, 205–217. [Google Scholar] [CrossRef]
- Ganther, H.E. Enzymic synthesis of dimethyl selenide from sodium selenite in mouse liver extracts. Biochemistry 1966, 5, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Mahan, D.C. Comparative effects of high dietary levels of organic and inorganic selenium on selenium toxicity of growing-finishing pigs. J. Anim. Sci. 2001, 79, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Fortier, M.-E.; Audet, I.; Giguère, A.; Laforest, J.-P.; Bilodeau, J.-F.; Quesnel, H.; Matte, J.J. Effect of dietary organic and inorganic selenium on antioxidant status, embryo development, and reproductive performance in hyperovulatory first-parity gilts. J. Anim. Sci. 2012, 90, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, B.; Cooper, M.L.; Green, F.R.; McNulty, H.; Pentieva, K.; Scott, J.M.; Molloy, A.M.; Rayman, M.P. Effect of selenium status and supplementation with high-selenium yeast on plasma homocysteine and B vitamin concentrations in the UK elderly. Mol. Nutr. Food Res. 2008, 52, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Quinlivan, E.P.; Stacpoole, P.W.; Gregory, J.F., 3rd. Plasma glutathione and cystathionine concentrations are elevated but cysteine flux is unchanged by dietary vitamin B-6 restriction in young men and women. J. Nutr. 2006, 136, 373–378. [Google Scholar] [PubMed]
- Lima, C.P.; Davis, S.R.; Mackey, A.D.; Scheer, J.B.; Williamson, J.; Gregory, J.F., 3rd. Vitamin B-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed AIN-76A or AIN-93G diets. J. Nutr. 2006, 136, 2141–2147. [Google Scholar] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 5, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Dalto, D.B.; Tsoi, S.; Audet, I.; Dyck, M.K.; Foxcroft, G.R.; Matte, J.J. Gene expression of porcine blastocysts from gilts fed organic or inorganic selenium and pyridoxine. Reproduction 2015, 149, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, M.; Ficek, R.; Drabek, J. Efficacy of selenium from Se-enriched yeast on selenium transfer from sows to piglets. Acta Vet. Brno 2008, 77, 515–521. [Google Scholar] [CrossRef]
- Ma, Y.L.; Lindemann, M.D.; Pierce, J.L.; Unrine, J.M.; Cromwell, G.L. Effect of inorganic or organic selenium supplementation on reproductive performance and tissue trace mineral concentrations in gravid first-parity gilts, fetuses, and nursing piglets. J. Anim. Sci. 2014, 92, 5540–5550. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soda, K.; Oikawa, T.; Esaki, N. Vitamin B6 enzymes participating in selenium amino acid metabolism. BioFactors 1999, 10, 257–262. [Google Scholar] [CrossRef] [PubMed]

| Day 3 Post-Estrus a | 30 Days Gestation b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CONT | MSe B60 c | MSe B610 c | OSe B60 c | OSe B610 c | CONT | MSe B60 c | MSe B610 c | OSe B60 c | OSe B610 c | |
| GPX1 | 0.44 | 1.02 | 1.09 | 0.77 | 1.57 | 1.15 | 1.19 | 1.37 | 1.27 | 1.15 |
| GPX3 | 0.43 | 1.02 | 1.22 | 0.67 | 1.17 | 0.77 | 1.03 | 0.87 | 0.96 | 0.93 |
| GPX4 | 0.59 | 1.00 | 1.08 | 0.66 | 1.19 | 1.05 | 1.05 | 1.19 | 1.07 | 0.98 |
| SCLY | 0.72 | 0.82 | 0.84 | 0.89 | 1.45 | 0.82 | 0.92 | 0.84 | 0.71 | 0.82 |
| Day 3 Post-Estrus a | 30 Days Gestation b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CONT c | MSe B60 c | MSe B610 c | OSe B60 c | OSe B610 c | CONT c | MSe B60 c | MSe B610 c | OSe B60 c | OSe B610 c | |
| GPX1 | 0.72 | 1.16 | 1.25 | 1.19 | 1.83 | 0.97 | 1.11 | 0.95 | 1.03 | 1.14 |
| GPX3 | 0.73 | 1.14 | 1.33 | 1.41 | 1.75 | 1.09 | 1.17 | 1.09 | 1.11 | 1.03 |
| GPX4 | 1.06 | 1.12 | 1.18 | 1.18 | 1.47 | 1.10 | 1.22 | 1.12 | 1.18 | 1.17 |
| SCLY | 1.20 | 1.18 | 1.20 | 1.48 | 2.05 | 1.28 | 1.15 | 1.17 | 1.07 | 1.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalto, D.B.; Matte, J.-J. Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation. Nutrients 2017, 9, 189. https://doi.org/10.3390/nu9030189
Dalto DB, Matte J-J. Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation. Nutrients. 2017; 9(3):189. https://doi.org/10.3390/nu9030189
Chicago/Turabian StyleDalto, Danyel Bueno, and Jean-Jacques Matte. 2017. "Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation" Nutrients 9, no. 3: 189. https://doi.org/10.3390/nu9030189
APA StyleDalto, D. B., & Matte, J.-J. (2017). Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation. Nutrients, 9(3), 189. https://doi.org/10.3390/nu9030189




