Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases
Abstract
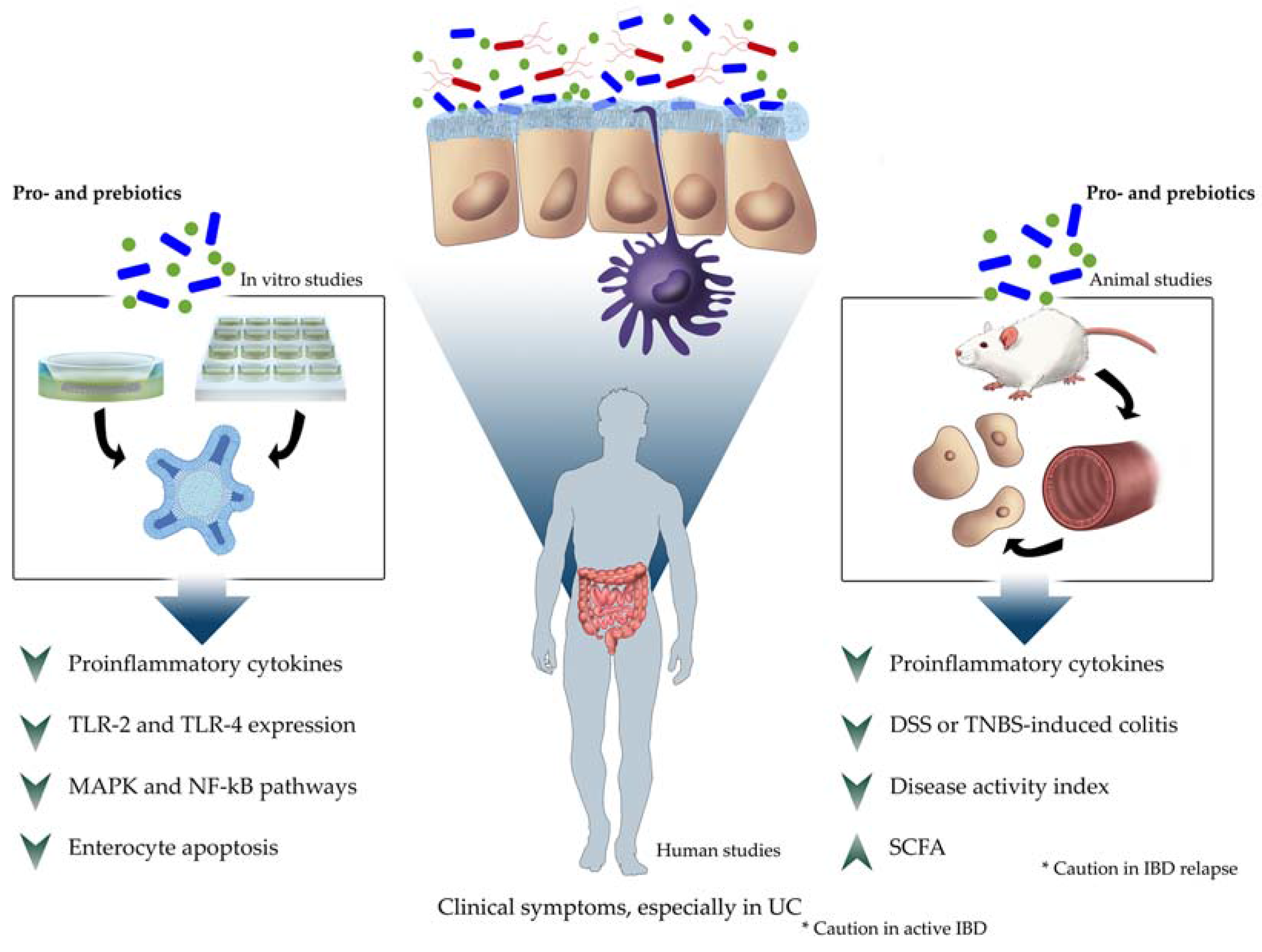
:1. Introduction
2. Materials and Methods
3. Results
3.1. In Vitro Studies
3.2. In Vivo Studies
3.2.1. Animals
Dextran Sulfate Sodium
2,4,6-Trinitrobenzenesulfonic Acid
Other Intestinal Inflammation Models
3.2.2. Humans
Ulcerative Colitis
Crohn’s Disease
4. Further Research and Directions
Author Contributions
Conflicts of Interest
References
- Metchnikoff, E. The Prolongation of Life: Optimistic Studies, 1st ed.; Mitchell, P.C., Ed.; G.P. Putnam’s Sons: New York, NY, USA, 1908. [Google Scholar]
- De Moreno de LeBlanc, A.; LeBlanc, J.G. Effect of probiotic administration on the intestinal microbiota, current knowledge and potential applications. World J. Gastroenterol. 2014, 20, 16518–16528. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Bermudez-Brito, M.; Plaza-Diaz, J.; Muñoz-Quezada, S.; Gil, A. Sources, isolation, characterisation and evaluation of probiotics. Br. J. Nutr. 2013, 109, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effects. Antonie Van Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Miller, J.M.; Gibson, G.R.; Bruck, W. Some insights into the derivation and early uses of the word ‘probiotic’. Br. J. Nutr. 2003, 90, 845. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization/World Health Organization. Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria; American Cordoba Park Hotel: Cordoba, Argentina, 2001; pp. 1–2. [Google Scholar]
- Roberfroid, M.B. Prebiotics and probiotics: are they functional foods? Am. J. Clin. Nutr. 2000, 71, 1682–1687. [Google Scholar]
- Giahi, L.; Aumueller, E.; Elmadfa, I.; Haslberger, A.G. Regulation of TLR4, p38 MAPkinase, IκB and miRNAs by inactivated strains of lactobacilli in human dendritic cells. Benef. Microbes 2012, 3, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Isolauri, E.; Salminen, S.; Sanz, Y. The impact of probiotic on gut health. Curr. Drug. Metab. 2009, 10, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Robles-Sánchez, C.; Abadía-Molina, F.; Morón-Calvente, V.; Sáez-Lara, M.J.; Ruiz-Bravo, A.; Jiménez-Valera, M.; Gil, A.; Gómez-Llorente, C.; Fontana, L. Adamdec1, Ednrb and Ptgs1/Cox1, inflammation genes upregulated in the intestinal mucosa of obese rats, are downregulated by three probiotic strains. Sci. Rep. 2017, 7, 1939. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Fernandez-Caballero, J.Á.; Chueca, N.; Garcia, F.; Gómez-Llorente, C.; Sáez-Lara, M.J.; Fontana, L.; Gil, A. Pyrosequencing analysis reveals changes in intestinal microbiota of healthy adults who received a daily dose of immunomodulatory probiotic strains. Nutrients 2015, 7, 3999–4015. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Abadia-Molina, F.; Saez-Lara, M.J.; Campaña-Martin, L.; Muñoz-Quezada, S.; Romero, F.; Gil, A.; Fontana, L. Effects of Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036 on hepatic steatosis in Zucker rats. PLoS ONE 2014, 9, e98401. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Gomez-Llorente, C.; Campaña-Martin, L.; Matencio, E.; Ortuño, I.; Martínez-Silla, R.; Gomez-Gallego, C.; Periago, M.J.; Ros, G.; Chenoll, E.; et al. Safety and immunomodulatory effects of three probiotic strains isolated from the feces of breast-fed infants in healthy adults: SETOPROB study. PLoS ONE 2013, 8, e78111. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, M.; Asp, N.G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical meeting on prebiotics. J. Clin. Gastroenterol. 2008, 42, S156–S159. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef] [PubMed]
- Tojo, R.; Suárez, A.; Clemente, M.G.; De los Reyes-Gavilán, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, N.; Moudgal, V. Probiotics: A Review. J. Clin. Outcomes Manag. 2012, 19, 76–84. [Google Scholar]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Wedlake, L.; Slack, N.; Andreyev, H.J.; Whelan, K. Fiber in the treatment and maintenance of inflammatory bowel disease: A systematic review of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Münch, A.; Aust, D.; Bohr, J.; Bonderup, O.; Fernández Bañares, F.; Hjortswang, H.; Madisch, A.; Munck, L.K.; Ström, M.; Tysk, C.; et al. Microscopic colitis: Current status, present and future challenges: Statements of the European Microscopic Colitis Group. J. Crohns Colitis. 2012, 6, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Derikx, L.A.; Dieleman, L.A.; Hoentjen, F. Probiotics and prebiotics in ulcerative colitis. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohns Colitis. 2017, 11, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Ringel, Y.; Carroll, I.M. Alterations in the intestinal microbiota and functional bowel symptoms. Gastrointest. Endosc. Clin. N. Am. 2009, 19, 141–150. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Cooney, J.C. Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip. Perspect. Infect. Dis. 2008, 2008. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Ng, C.Y.; Van Heel, D.A.; Lombardi, G.; Lechler, R.; Playford, R.J.; Ghosh, S. Modulation of dendritic cell phenotype and function in an in vitro model of the intestinal epithelium. Eur. J. Immunol. 2006, 36, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.R.; Bernardo, D.; Ng, S.C.; Rigby, R.J.; Al-Hassi, H.O.; Landy, J.; Peake, S.T.C.; Spranger, H.; English, N.R.; Thomas, L.V.; et al. Human gut dendritic cells drive aberrant gut-specific T-cell responses in ulcerative colitis, characterized by increased IL-4 production and loss of IL-22 and IFN-γ. Inflamm. Bowel Dis. 2014, 20, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.R.; You, J.; Horneffer-van der Sluis, V.; Bernardo, D.; Omar Al-Hassi, H.; Landy, J.; Peake, S.T.; Thomas, L.V.; Tee, C.T.; Lee, G.H.; et al. Dysregulated circulating dendritic cell function in ulcerative colitis is partially restored by probiotic strain Lactobacillus casei Shirota. Mediators Inflamm. 2013, 2013, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Llorente, C.; Muñoz, S.; Gil, A. Role of toll-like receptors in the development of immunotolerance mediated by probiotics. Proc. Nutr. Soc. 2010, 69, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhu, C.; Chen, Z.; Chen, Z.; Zhang, W.; Ma, X.; Wang, L.; Yang, X.; Jiang, Z. Protective effects of Lactobacillus plantarum on epithelial barrier disruption caused by enterotoxigenic Escherichia coli in intestinal porcine epithelial cells. Vet. Immunol. Immunopathol. 2016, 172, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wachi, S.; Kanmani, P.; Tomosada, Y.; Kobayashi, H.; Yuri, T.; Egusa, S.; Shimazu, T.; Suda, Y.; Aso, H.; Sugawara, M.; et al. Lactobacillus delbrueckii TUA4408L and its extracellular polysaccharides attenuate enterotoxigenic Escherichia coli-induced inflammatory response in porcine intestinal epitheliocytes via Toll-like receptor-2 and 4. Mol. Nutr. Food Res. 2014, 58, 2080–2093. [Google Scholar] [CrossRef] [PubMed]
- Prisciandaro, L.D.; Geier, M.S.; Chua, A.E.; Butler, R.N.; Cummins, A.G.; Sander, G.R.; Howarth, G.S. Probiotic factors partially prevent changes to caspases 3 and 7 activation and transepithelial electrical resistance in a model of 5-fluorouracil-induced epithelial cell damage. Support Care Cancer 2012, 20, 3205–3210. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Romero, F.; Gil, A. Lactobacillus rhamnosus and its cell-free culture supernatant differentially modulate inflammatory biomarkers in Escherichia coli-challenged human dendritic cells. Br. J. Nutr. 2014, 111, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Cell-free culture supernatant of Bifidobacterium breve CNCM I-4035 decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLoS ONE 2013, 8, e59370. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Human intestinal dendritic cells decrease cytokine release against Salmonella infection in the presence of Lactobacillus paracasei upon TLR activation. PLoS ONE 2012, 7, e43197. [Google Scholar] [CrossRef] [PubMed]
- Abdelouhab, K.; Rafa, H.; Toumi, R.; Bouaziz, S.; Medjeber, O.; Touil-Boukoffa, C. Mucosal intestinal alteration in experimental colitis correlates with nitric oxide production by peritoneal macrophages: Effect of probiotics and prebiotics. Immunopharmacol. Immunotoxicol. 2012, 34, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Toumi, R.; Abdelouhab, K.; Rafa, H.; Soufli, I.; Raissi-Kerboua, D.; Djeraba, Z.; Touil-Boukoffa, C. Beneficial role of the probiotic mixture Ultrabiotique on maintaining the integrity of intestinal mucosal barrier in DSS-induced experimental colitis. Immunopharmacol. Immunotoxicol. 2013, 35, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Atkins, H.L.; Geier, M.S.; Prisciandaro, L.D.; Pattanaik, A.K.; Forder, R.E.; Turner, M.S.; Howarth, G.S. Effects of a Lactobacillus reuteri BR11 mutant deficient in the cystine-transport system in a rat model of inflammatory bowel disease. Dig. Dis. Sci. 2012, 57, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wei, H.; Lu, F.; Liu, X.; Liu, D.; Gu, L.; Ouyang, C. Different effects of three selected Lactobacillus strains in dextran sulfate sodium-induced colitis in BALB/c mice. PLoS ONE 2016, 11, e0148241. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Zhang, L.; Li, Z.J.; Wu, W.K.; Ren, S.X.; Chen, Y.C.; Ng, T.B.; Cho, C.H. Protective effects of cathelicidin-encoding Lactococcus lactis in murine ulcerative colitis. J. Gastroenterol. Hepatol. 2012, 27, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Ahn, Y.T.; Park, J.C.; Lee, J.H.; Lee, H.; Huh, C.S.; Kim, D.H.; Ryu, D.H.; Hwang, G.S. 1H NMR-based metabonomic assessment of probiotic effects in a colitis mouse model. Arch. Pharm. Res. 2010, 33, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Utrilla, P.; Comalada, M.; Zorrilla, P.; Garrido-Mesa, J.; Zarzuelo, A.; Rodríguez-Cabezas, M.E.; Gálvez, J. The association of minocycline and the probiotic Escherichia coli Nissle 1917 results in an additive beneficial effect in a DSS model of reactivated colitis in mice. Biochem. Pharmacol. 2011, 82, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; van Bergenhenegouwen, J.; van de Kant, H.J.; Folkerts, G.; Garssen, J.; Vos, A.P.; Morgan, M.E.; Kraneveld, A.D. Specific probiotic dietary supplementation leads to different effects during remission and relapse in murine chronic colitis. Benef. Microbes 2016, 7, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Talero, E.; Bolivar, S.; Ávila-Román, J.; Alcaide, A.; Fiorucci, S.; Motilva, V. Inhibition of chronic ulcerative colitis-associated adenocarcinoma development in mice by VSL#3. Inflamm. Bowel. Dis. 2015, 21, 1027–1037. [Google Scholar] [PubMed]
- Dai, C.; Zheng, C.Q.; Meng, F.J.; Zhou, Z.; Sang, L.X.; Jiang, M. VSL#3 probiotics exerts the anti-inflammatory activity via PI3k/Akt and NF-κB pathway in rat model of DSS-induced colitis. Mol. Cell Biochem. 2013, 374, 1–11. [Google Scholar] [PubMed]
- Salim, S.Y.; Young, P.Y.; Lukowski, C.M.; Madsen, K.L.; Sis, B.; Churchill, T.A.; Khadaroo, R.G. VSL#3 probiotics provide protection against acute intestinal ischaemia/reperfusion injury. Benef. Microbes 2013, 4, 357–365. [Google Scholar] [PubMed]
- Zhang, H.L.; Li, W.S.; Xu, D.N.; Zheng, W.W.; Liu, Y.; Chen, J.; Qiu, Z.B.; Dorfman, R.G.; Zhang, J.; Liu, J. Mucosa-reparing and microbiota-balancing therapeutic effect of Bacillus subtilis alleviates dextrate sulfate sodium-induced ulcerative colitis in mice. Exp. Ther. Med. 2016, 12, 2554–2562. [Google Scholar] [CrossRef] [PubMed]
- Satish Kumar, C.; Kondal Reddy, K.; Reddy, A.G.; Vinoth, A.; Ch, S.R.; Boobalan, G.; Rao, G.S. Protective effect of Lactobacillus plantarum 21, a probiotic on trinitrobenzenesulfonic acid-induced ulcerative colitis in rats. Int. Immunopharmacol. 2015, 25, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fu, Y.; Liu, J.; Ren, H.Y. Impact of probiotics on toll-like receptor 4 expression in an experimental model of ulcerative colitis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Machiels, K.; Perrier, C.; Romero, C.; Maes, S.; Flahou, B.; Steppe, M.; Haesebrouck, F.; Sas, B.; Ducatelle, R.; et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 2013, 62, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.; Werling, D.; Allenspach, K. Effects of ex-vivo and in-vivo treatment with probiotics on the inflammasome in dogs with chronic enteropathy. PLoS ONE 2015, 10, e0120779. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.; Glanemann, B.; Garden, O.A.; Brooks, H.; Chang, Y.M.; Werling, D.; Allenspach, K. A prospective, randomized, blinded, placebo-controlled pilot study on the effect of Enterococcus faecium on clinical activity and intestinal gene expression in canine food-responsive chronic enteropathy. J. Vet. Intern. Med. 2015, 29, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Shi, Y.; Wang, F.; Han, D.; Lei, H.; Zhao, Y.; Sun, Q. Study on the effects of microencapsulated Lactobacillus delbrueckii on the mouse intestinal flora. J. Microencapsul. 2015, 32, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Štofilová, J.; Szabadosová, V.; Hrčková, G.; Salaj, R.; Bertková, I.; Hijová, E.; Strojný, L.; Bomba, A. Co-administration of a probiotic strain Lactobacillus plantarum LS/07 CCM7766 with prebiotic inulin alleviates the intestinal inflammation in rats exposed to N,N-dimethylhydrazine. Int. Immunopharmacol. 2015, 24, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Ogita, T.; Bergamo, P.; Maurano, F.; D′Arienzo, R.; Mazzarella, G.; Bozzella, G.; Luongo, D.; Sashihara, T.; Suzuki, T.; Tanabe, S.; et al. Modulatory activity of Lactobacillus rhamnosus OLL2838 in a mouse model of intestinal immunopathology. Immunobiology 2015, 220, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yoon, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Wan, J.; Petrof, E.O.; Claud, E.C.; Chen, D.; Sun, J. Vitamin D receptor pathway is required for probiotic protection in colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, H.; Nakase, H.; Inoue, S.; Kawanami, C.; Itani, T.; Ohana, M.; Kusaka, T.; Uose, S.; Hisatsune, H.; Tojo, M.; et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig. Endosc. 2016, 28, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, Y.; Yamada, A.; Furukawa, R.; Sono, K.; Osamura, A.; Nakamura, K.; Aoki, H.; Tsuda, Y.; Hosoe, N.; Takada, N.; et al. Effectiveness of probiotic therapy for the prevention of relapse in patients with inactive ulcerative colitis. World J. Gastroenterol. 2015, 21, 5985–5994. [Google Scholar] [PubMed]
- Krag, A.; Munkholm, P.; Israelsen, H.; von Ryberg, B.; Andersen, K.K.; Bendtsen, F. Profermin is efficacious in patients with active ulcerative colitis-a randomized controlled trial. Inflamm. Bowel Dis. 2013, 19, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
- Meini, S.; Laureano, R.; Fani, L.; Tascini, C.; Galano, A.; Antonelli, A.; Rossolini, G.M. Breakthrough Lactobacillus rhamnosus GG bacteremia associated with probiotic use in an adult patient with severe active ulcerative colitis: case report and review of the literature. Infection 2015, 43, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Suskind, D.L.; Namita Singh, N.; Nielson, H.; Wahbeh, G. Fecal microbial transplant via nasogastric tube for active pediatric ulcerative colitis. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; Leong, R.W.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 2017, 389, 1218–1228. [Google Scholar] [CrossRef]
- Brace, C.; Gloor, G.B.; Ropeleski, M.; Allen-Verco, E.; Petrof, E.O. Microbial composition analysis of Clostridium difficile infections in an ulcerative colitis patient treated with multiple fecal microbiota transplantations. J. Crohns Colitis 2014, 8, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Vahabnezhad, E.; Mochon, A.B.; Wozniak, L.Y.; Ziring, D.A. Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. Clin. Gastroenterol. 2013, 47, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Gouriet, F.; Million, M.; Henri, M.; Fournier, P.E.; Raoult, D. Lactobacillus rhamnosus bacteremia: An emerging clinical entity. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2469–2480. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.; Mirsepasi, H.; Halkjær, S.I.; Mortensen, E.M.; Nordgaard-Lassen, I.; Krogfelt, K.A. Ciprofloxacin and probiotic Escherichia coli Nissle add-on treatment in active ulcerative colitis: A double-blind randomized placebo controlled clinical trial. J. Crohns Colitis 2014, 8, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, R.N.; Feagan, B.G.; Hotte, N.; Leddin, D.; Dieleman, L.A.; Petrunia, D.M.; Enns, R.; Bitton, A.; Chiba, N.; Paré, P. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for crohn’s disease. Clin. Gastroenterol. Hepatol. 2015, 13, 928–935. [Google Scholar] [PubMed]
- Hevia, A.; López, P.; Suárez, A.; Jacquot, C.; Urdaci, M.C.; Margolles, A.; Sánchez, B. Association of levels of antibodies from patients with inflammatory bowel disease with extracellular proteins of food and probiotic bacteria. Biomed. Res. Int. 2014, 2014, 351204. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Reddy, B.S.; Mølbak, L.; Leser, T.D.; MacFie, J. Impact of probiotics on colonic microflora in patients with colitis: A prospective double blind randomised crossover study. Int. J. Surg. 2013, 11, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Persborn, M.; Gerritsen, J.; Wallon, C.; Carlsson, A.; Akkermans, L.M.A.; Söderholm, J.D. The effects of probiotics on barrier function and mucosal pouch microbiota during maintenance treatment for severe pouchitis in patients with ulcerative colitis. Aliment Pharmacol. Ther. 2013, 38, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Bourreille, A.; Cadiot, G.; Le Dreau, G.; Laharie, D.; Beaugerie, L.; Dupas, J.L.; Marteau, P.; Rampal, P.; Moyse, D.; Saleh, A. Saccharomyces boulardii does not prevent relapse of Crohn′s disease. Clin. Gastroenterol. Hepatol. 2013, 11, 982–987. [Google Scholar] [CrossRef] [PubMed]
| Reference | Cell Type | Probiotic Strain | Type of Study | Main Outcome |
|---|---|---|---|---|
| Mann et al. 2014, 2013 [32,33] | human DC | L. casei Shirota | In vitro | DC from UC patients samples have an increase of IL-4 production and loss of IL-22 and IFN-γ secretion. L. casei Shirota treatment restored the normal stimulatory capacity through a reduction in the TLR-2 and TLR4 expression |
| Wu et al., 2016 [35] | IPEC-J2 model | L. plantarum strain CGMCC1258 | In vitro | L. plantarum decreased transcript abundances of IL-8, TNF-α, and negative regulators of TLRs. Moreover, L. plantarum treatment decreased the gene and protein expression of occludin |
| Wachi et al., 2014 [36] | PIE cells | L. delbrueckii subsp. delbrueckii TUA4408L | In vitro | The activation of MAPK and NF-κB pathways induced by E. coli 987P were downregulated through upregulation of TLR negative regulators, principally by TLR2 |
| Prisciandaro et al., 2012 [37] | IEC-6 | E. coli Nissle 1917 and L. rhamnosus GG | In vitro | Pre-treatment with these probiotics could prevent or inhibit enterocyte apoptosis and loss of intestinal barrier function induced by 5-FU |
| Bermudez-Brito et al., 2012, 2013, 2014 [13,38,39] | DC | L. paracasei CNCM I-4034, B. breve CNCM I-4035, and L. rhamnosus CNCM I-4036 | In vitro | Induction of TLR-9 expression and TGF-β2 secretion. CFS treatment decreased the pro-inflammatory cytokines and chemokines |
| Reference | Animal Species | Probiotic Strain/Treatment | Type of Study | Main Outcome | Adverse Event/Adverse Effects |
|---|---|---|---|---|---|
| Abdelouhab et al., 2012 [41] | Swiss mice | L. acidophilus, L. plantarum, B. lactis, B. breve, and inulin | In vivo, DSS-induced colitis | Oral administration of probiotic strains and inulin decreased severity colitis | - |
| Toumi et al., 2013 [42] | Swiss mice | L. acidophilus, L. plantarum, B. lactis, B. breve | In vivo, DSS-induced colitis | Probiotic administration improved clinical symptoms, histological alterations, and mucus production | - |
| Atkins et al., 2012 [43] | Male Sprague–Dawley rats | L. reuteri BR11 | In vivo, DSS-induced colitis | Probiotic administration partially alleviated the DSS effects, with a minor improvement in crypt area | - |
| Cui et al., 2016 [44] | BALB/c mice | L. fermentum CCTCC M206110, L. crispatus CCTCC M206119, and L. plantarum NCIMB8826 | In vivo, DSS-induced colitis | L. fermentum CCTCC M206110 proved to be effective at attenuating DSS-induced colitis. Administration of L. crispatus CCTCC M206119 aggravated DSS-induced colitis | Administration of L. crispatus CCTCC M206119 aggravated DSS-induced colitis |
| Wong et al., 2012 [45] | BALB/c mice | A mutant of L. lactis | In vivo, DSS-induced colitis | L. lactis could improve the clinical symptoms, maintain crypt integrity and preserve mucus content. The number of apoptotic cells, MPO activity and malondialdehyde level were also significantly reduced | - |
| Zhang et al., 2016 [52] | Male C57 mice | B. subtilis | In vivo, DSS-induced colitis | B. subtilis treatment ameliorated gut inflammation and dysbiosis | - |
| Hong et al., 2010 [46] | Male ICR mice | L. brevis HY7401, L. sp. HY7801 and B. longum HY8004 | In vivo, DSS-induced colitis | Increased levels of acetate, butyrate, and glutamine and decreased levels of trimethylamine | - |
| Garrido-Mesa et al., 2011 [47] | C57BL/6J mice | E. coli Nissle 1917 | In vivo, DSS-induced colitis | E. coli Nissle 1917 administration exerted intestinal anti-inflammatory effect and attenuated the reactivation of the colitis | - |
| Zheng et al., 2016 [48] | Female C57BL/6 mice | L. rhamnosus NutRes 1 and B. breve NutRes 204 | In vivo, DSS-induced colitis | An increased expression of inflammation markers were found in DSS-treated mice with L. rhamnosus supplementation | - |
| Talero et al., 2015 [49] | Female C57BL/6 mice | Capsules with bifidobacteria, lactobacilli, and S. thermophilus | In vivo, DSS-induced colitis | Probiotic mixture attenuated the disease activity index score and colon inflammation and also inflammation markers | - |
| Dai et al., 2013 [50] | Male Wistar rats | Capsules with bifidobacteria, lactobacilli, and S. thermophilus | In vivo, DSS-induced colitis | The probiotic mixture have anti-inflammatory properties reducing the disease activity index, MPO activity, inflammation biomarkers, and also increasing of IL-10 expression | - |
| Salim et al., 2013 [51] | Adult male 129/SvEv mice | Capsules with bifidobacteria, lactobacilli, and S. thermophilus | In vivo, acute intestinal ischemia/reperfusion injury | Levels of active NF-κB were significantly higher in the control group, corroborating with the inflammation of the tissue, which was attenuated by probiotic administration | - |
| Satish Kumar et al., 2015 [53] | Wistar female rats | L. plantarum 21 | In vivo, TNBS-induced colitis | Treatment with L. plantarum 21 for 14 days after induction of colitis decreased TBARS, NO, IL-1β and TNF-α and increased glutathione concentration and IL-10 expression | - |
| Yang et al., 2013 [54] | Sprague-Dawley Rats | Lactobacilli and bifidobacteria | In vivo, TNBS-induced colitis | TLR4 and TNF-α expression were reduced with probiotics | - |
| Eeckhaut et al., 2013 [55] | Male Wistar rats | B. pullicaecorum | In vivo, TNBS-induced colitis | B. pullicaecorum administration resulted in a decreased intestinal MPO, TNF-α and IL-12 levels | - |
| Schmitz et al., 2015 [56,57] | Dogs | E. faecium NCIMB 10415 E1707 | Chronic enteropathies | There was no difference between groups or treatments regarding clinical efficacy, histology scores | - |
| Sun et al., 2015 [58] | BALB/c mice | L. delbrueckii | Intestinal malfunction induced by Lincomycin hydrochloride | L. delbrueckii administration increased secretory immunoglobulin A and decreased the intestine pathological damage | - |
| Štofilová et al., 2015 [59] | Female Sprague Dawley rats | L. plantarum LS/07 CCM7766 | In vivo, N,N-dimethylhydrazine-induced colitis | L. plantarum LS/07 CCM7766 and its combination with inulin abolished inflammatory process in the jejunal mucosa | - |
| Ogita et al., 2015 [60] | DQ8 transgenic mice | L. rhamnosus OLL2838, B. infantis ATCC 15697, and S. thermophilus Sfi 39 | In vivo, model of gluten-specific enteropathy | Probiotic administration enhanced total glutathione and glutathione S-transferase activity, whereas caspase-3 activity was reduced | - |
| Wu et al., 2015 [61] | Female C57BL/6 mice | L. rhamnosus GG and L. plantarum | In vivo, vitamin D receptor knockout mice | Probiotic treatment conferred physiological and histologic protection from colitis | - |
| Reference | Subjects | Probiotic Strains/Treatment | Time | Main Outcome | Adverse Event/Adverse Effects |
|---|---|---|---|---|---|
| Tamaki et al., 2016 [62] | 56 with mild to moderate UC | B. longum 536 | 8 weeks | Probiotics administration improved clinical symptoms in the patients with mild to moderately active UC | - |
| Yoshimatsu et al., 2015 [63] | 60 outpatients with UC in remission | S. faecalis, C. butyricum and B. mesentericus | 12 months | Probiotic may be effective for maintaining clinical remission in patients with UC | - |
| Krag et al., 2013 [64] | 74 patients with a mild-to-moderate UC | L. plantarum 299v | 8 weeks | Probiotic supplementation was safe, well tolerated, palatable, and able to reduce disease index scores in patients with mild-to-moderate UC | - |
| Petersen et al., 2014 [71] | 100 patients with UC | E. coli Nissle 1917 | 7 weeks | There is no benefit in the use of E. coli Nissle as an add-on treatment to conventional therapies for active UC | - |
| Fedorak et al., 2015 [72] | 119 patients with CD (within 30 days of ileocolonic resection and re-anastomosis | Capsules with bifidobacteria, lactobacilli, and S. thermophilus | 90 days | There were no differences in endoscopic recurrence, but mucosal levels of inflammatory cytokines such as IL-8, IL-1β were lower among patients who received the probiotic | - |
| Hevia et al., 2014 [73] | 50 healthy individuals, 37 CD patients and 15 UC patients | L. casei subsp. rhamnosus GG | 90 days | Levels of IgA antibodies developed against a cell-wall hydrolase from L. casei subsp. rhamnosus GG were significantly higher in the IBD group | - |
| Ahmed et al., 2013 [74] | 8 patients with CD and 8 patients with UC | L. acidophilus LA-5, L. delbrueckii subsp. bulgaricus LBY-27, B. animalis subsp. lactis BB-12, S. thermophilus STY-31 and 15 g oligofructose | 1 month | There were no differences in colonic microbiota between patients with CD or UC and the spectrum a bacterium was not altered by synbiotics administration | - |
| Persborn et al., 2013 [75] | 16 patients with chronic pouchitis and 13 individuals as a control | L. acidophilus Ecologic 825: B. bifidum (W23), B. lactis (W51), B. lactis (W52), L. acidophilus (W22), L. casei (W56), L. paracasei (W20), L. plantarum (W62), L. salivarius (W24) and L. lactis (W19) | 8 weeks | Probiotics restored the mucosal barrier to E. coli in patients with pouchitis | - |
| Groeger et al., 2013 [76] | 22 UC patients, 48 patients with chronic fatigue syndrome and 26 psoriasis patients | B. infantis 35,624 | 6–8 weeks | Probiotics administration reduced the systemic pro-inflammatory biomarkers in both gastrointestinal and non-gastrointestinal conditions | - |
| Bourreille et al., 2013 [77] | 165 patients with CD | S. boulardii | 52 weeks | Probiotics were well tolerated but it did not show any effect. Twenty-one AEs occurred during the treatment, these affected 17 patients, 9 in the S. boulardii group and 8 in placebo group | Twenty-one AEs occurred during the treatment, these affected 17 patients, 9 in the S. boulardii group and 8 in placebo group |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. https://doi.org/10.3390/nu9060555
Plaza-Díaz J, Ruiz-Ojeda FJ, Vilchez-Padial LM, Gil A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients. 2017; 9(6):555. https://doi.org/10.3390/nu9060555
Chicago/Turabian StylePlaza-Díaz, Julio, Francisco Javier Ruiz-Ojeda, Laura Maria Vilchez-Padial, and Angel Gil. 2017. "Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases" Nutrients 9, no. 6: 555. https://doi.org/10.3390/nu9060555







