Epsilon Toxin from Clostridium perfringens Causes Inhibition of Potassium inward Rectifier (Kir) Channels in Oligodendrocytes
Abstract
1. Introduction
2. Results
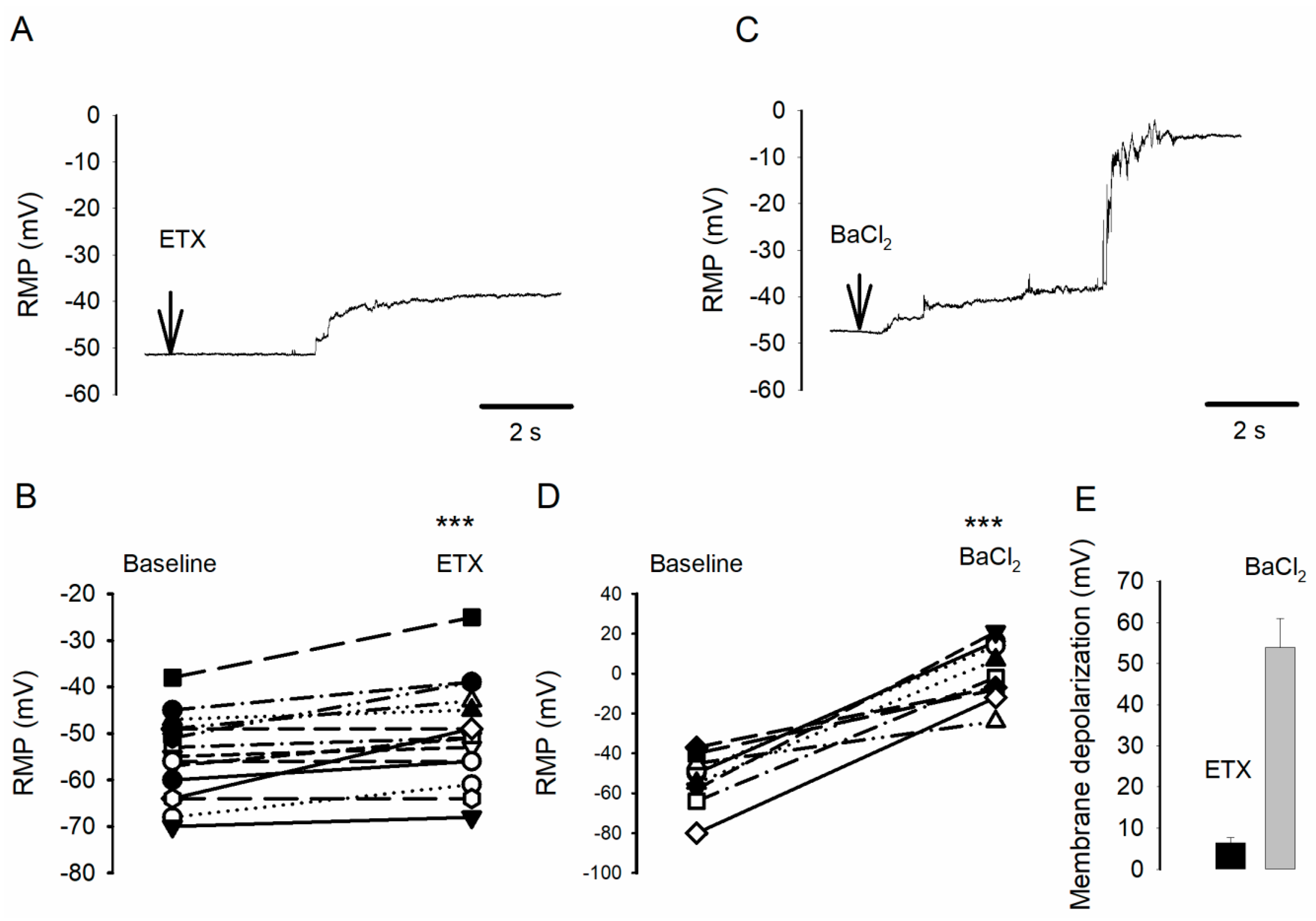
2.1. ETX Causes Small Oligodendrocyte Membrane Potential Depolarization
2.2. ETX Reduces Amplitude of Voltage-Dependent Kir Current in Cultured Rat Oligodendrocytes
2.3. Further Characterization of the Fraction of Kir Current Inhibited by ETX
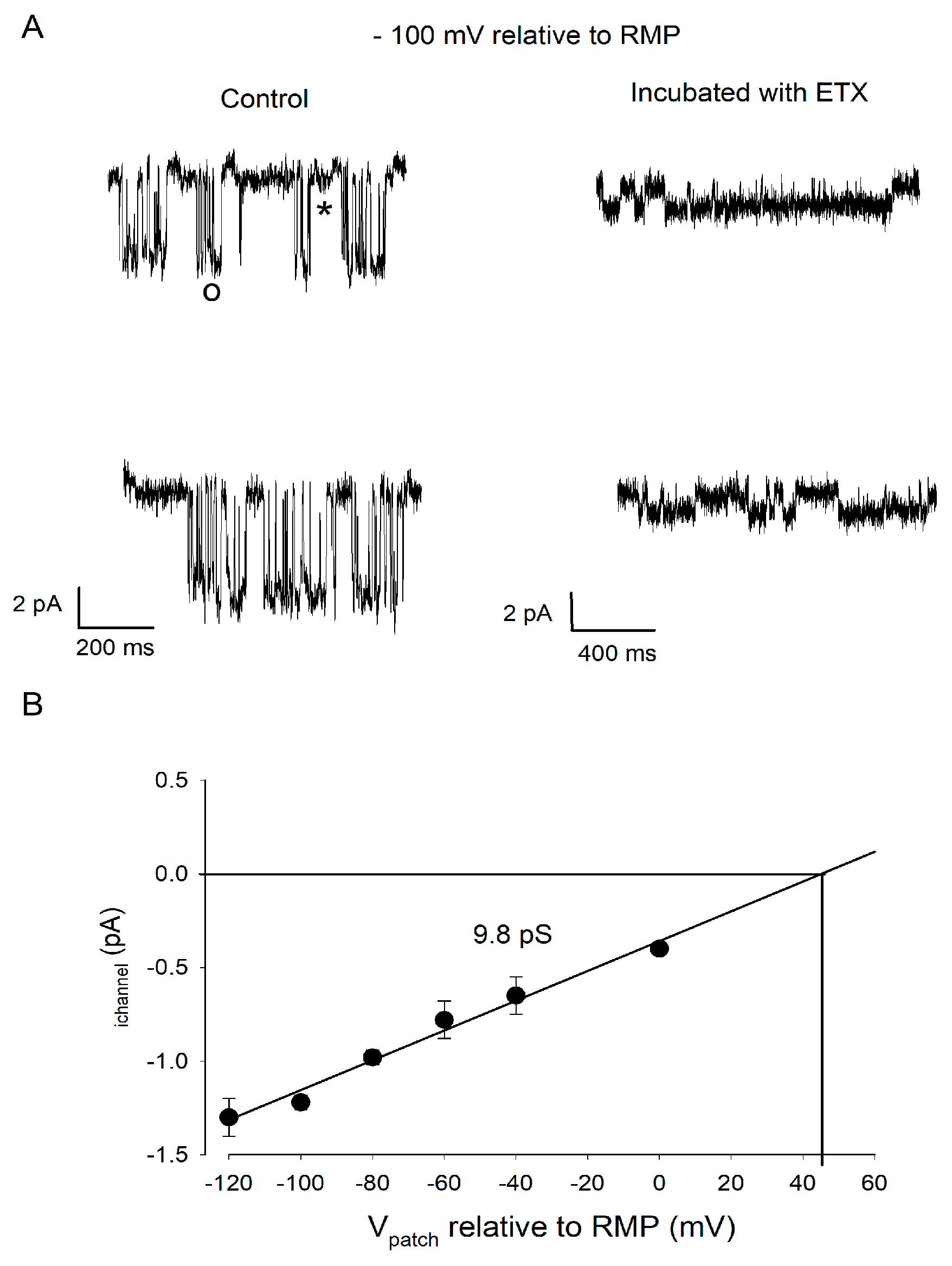
2.4. ETX Blocks a Large Conductance Kir Channel Type
3. Discussion
3.1. Do the Observed Effects Result from a Direct Action of ETX on Oligodendrocytes?
3.2. Relationship between Membrane Depolarization and Kir Channel Inhibition
3.3. What Might Be the Kir Channel Type Inhibited by ETX?
3.4. ETX Inhibits Large Amplitude Unitary Kir Channel Activity via An Intracellular Mechanism
3.5. Heterogeneity of ETX Effects on Oligodendrocytes
3.6. Possible Contribution of Kir Inihibition by ETX to Myelin Dysfunction
4. Conclusions
5. Experimental Procedures
5.1. Ethics Statement
5.2. Epsilon Toxin
5.3. Cerebellar Primary Cultures Containing Oligodendrocytes and Astrocytes
5.4. Electrophysiological Recordings
5.5. Graphs, Fitting Procedures and Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Stiles, B.G.; Barth, G.; Barth, H.; Popoff, M.R. Clostridium perfringens epsilon toxin: A malevolent molecule for animals and man? Toxins 2013, 5, 2138–2160. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.A.; McClane, B.A.; Uzal, F.A. Mechanisms of Action and Cell Death Associated with Clostridium perfringens Toxins. Toxins 2018, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Finnie, J.W. Neurological disorders produced by Clostridium perfringens type D epsilon toxin. Anaerobe 2004, 10, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Wioland, L.; Dupont, J.L.; Bossu, J.L.; Popoff, M.R.; Poulain, B. Attack of the nervous system by Clostridium perfringens Epsilon toxin: From disease to mode of action on neural cells. Toxicon 2013, 75, 122–135. [Google Scholar] [CrossRef]
- Soler-Jover, A.; Dorca, J.; Popoff, M.R.; Gibert, M.; Saura, J.; Tusell, J.M.; Serratosa, J.; Blasi, J.; Martín-Satué, M. Distribution of Clostridium perfringens epsilon toxin in the brains of acutely intoxicated mice and its effect upon glial cells. Toxicon 2007, 50, 530–540. [Google Scholar] [CrossRef]
- Dorca-Arévalo, J.; Soler-Jover, A.; Gibert, M.; Popoff, M.R.; Martín-Satué, M.; Blasi, J. Binding of epsilon-toxin from Clostridium perfringens in the nervous system. Vet. Microbiol. 2008, 131, 14–25. [Google Scholar] [CrossRef]
- Linden, J.R.; Flores, C.; Schmidt, E.F.; Uzal, F.A.; Michel, A.O.; Valenzuela, M.; Dobrow, S.; Vartanian, T. Clostridium perfringens epsilon toxin induces blood brain barrier permeability via caveolae-dependent transcytosis and requires expression of MAL. PLoS Pathog. 2019, 15, e1008014. [Google Scholar] [CrossRef]
- Lonchamp, E.; Dupont, J.L.; Wioland, L.; Courjaret, R.; Mbebi-Liegeois, C.; Jover, E.; Doussau, F.; Popoff, M.R.; Bossu, J.L.; de Barry, J.; et al. Clostridium perfringens epsilon toxin targets granule cells in the mouse cerebellum and stimulates glutamate release. PLoS ONE 2010, 5, e13046. [Google Scholar] [CrossRef]
- Wioland, L.; Dupont, J.L.; Doussau, F.; Gaillard, S.; Heid, F.; Isope, P.; Pauillac, S.; Popoff, M.R.; Bossu, J.L.; Poulain, B. Epsilon toxin from Clostridium perfringens acts on oligodendrocytes without forming pores, and causes demyelination. Cell Microbiol. 2015, 17, 369–388. [Google Scholar] [CrossRef]
- Doussau, F.; Dupont, J.L.; Neel, D.; Schneider, A.; Poulain, B.; Bossu, J.L. Organotypic cultures of cerebellar slices as a model to investigate demyelinating disorders. Expert Opin. Drug Discov. 2017, 12, 1011–1022. [Google Scholar] [CrossRef]
- Rumah, K.R.; Ma, Y.; Linden, J.R.; Oo, M.L.; Anrather, J.; Schaeren-Wiemers, N.; Alonso, M.A.; Fischetti, V.A.; McClain, M.S.; Vartanian, T. The myelin and lymphocyte protein MAL is required for binding and activity of Clostridium perfringens ε-Toxin. PLoS Pathog. 2015, 11, e1004896. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.R.; Ma, Y.; Zhao, B.; Harris, J.M.; Rumah, K.R.; Schaeren-Wiemers, N.; Vartanian, T. Clostridium perfringens Epsilon Toxin Causes Selective Death of Mature Oligodendrocytes and Central Nervous System Demyelination. MBio 2015, 6, e02513. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.E.; Goldstein, J.; Redondo, L.M.; Cangelosi, A.; Geoghegan, P.; Brocco, M.; Loidl, F.C.; Fernandez-Miyakawa, M.E. Clostridium perfringens epsilon toxin induces permanent neuronal degeneration and behavioral changes. Toxicon 2017, 130, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Cases, M.; Llobet, A.; Terni, B.; Gómez de Aranda, I.; Blanch, M.; Doohan, B.; Revill, A.; Brown, A.M.; Blasi, J.; Solsona, C. Acute Effect of Pore-Forming Clostridium perfrin gens ε-Toxin on Compound Action Potentials of Optic Nerve of Mouse. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Rumah, K.R.; Linden, J.; Fischetti, V.A.; Vartanian, T. Isolation of Clostridium perfringens type B in an individual at first clinical presentation of multiple sclerosis provides clues for environmental triggers of the disease. PLoS ONE 2013, 8, e76359. [Google Scholar] [CrossRef]
- Wagley, S.; Bokori-Brown, M.; Morcrette, H.; Malaspina, A.; D’Arcy, C.; Gnanapavan, S.; Lewis, N.; Popoff, M.R.; Raciborska, D.; Nicholas, R.; et al. Evidence of Clostridium perfringens epsilon toxin associated with multiple sclerosis. Mult. Scler. 2019, 25, 653–660. [Google Scholar] [CrossRef]
- Butt, A.M.; Kalsi, A. Inwardly rectifying potassium channels (Kir) in central nervous system glia: A special role for Kir4.1 in glial functions. J. Cell Mol. Med. 2006, 10, 33–44. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- Neusch, C.; Rozengurt, N.; Jacobs, R.E.; Lester, H.A.; Kofuji, P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J. Neurosci. 2001, 21, 5429–5438. [Google Scholar] [CrossRef]
- Bolton, S.; Butt, A.M. Cyclic AMP-mediated regulation of the resting membrane potential in myelin-forming oligodendrocytes in the isolated intact rat optic nerve. Exp. Neurol. 2006, 202, 36–43. [Google Scholar] [CrossRef]
- Sontheimer, H.; Kettenmann, H. Heterogeneity of potassium currents in cultured oligodendrocytes. Glia 1988, 1, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.; Bossu, J.L. Voltage-gated ionic currents in mature oligodendrocytes isolated from rat cerebellum. Neurosci. Lett. 1995, 190, 191–194. [Google Scholar] [CrossRef]
- Chassin, C.; Bens, M.; de Barry, J.; Courjaret, R.; Bossu, J.L.; Cluzeaud, F.; Ben Mkaddem, S.; Gibert, M.; Poulain, B.; Popoff, M.R.; et al. Pore-forming epsilon toxin causes membrane permeabilization and rapid ATP depletion-mediated cell death in renal collecting duct cells. Am. J. Physiol. Renal Physiol. 2007, 293, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2001; Available online: https://www.sinauer.com/media/wysiwyg/tocs/IonChannels3.pdf (accessed on 6 January 2020).
- Brasko, C.; Hawkins, V.; Chacon De La Rocha, I.; Butt, A.M. Expression of Kir4.1 and Kir5.1 inwardly rectifying potassium channels in oligodendrocytes, the myelinating cells of the CNS. Brain Struct. Funct. 2017, 222, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Samartín, A.; Garay, E.; Moctezuma, J.P.H.; Cisneros-Mejorado, A.; Sánchez-Gómez, M.V.; Martel-Gallegos, G.; Robles-Martínez, L.; Canedo-Antelo, M.; Matute, C.; Arellano, R.O. Inwardly rectifying K+ currents in cultured oligodendrocytes from rat optic nerve are insensitive to pH. Neurochem. Res. 2017, 42, 2443–2455. [Google Scholar] [CrossRef]
- Fennessey, C.M.; Ivie, S.E.; McClain, M.S. Coenzyme depletion by members of the aerolysin family of pore-forming toxins leads to diminished ATP levels and cell death. Mol. Biosyst. 2012, 8, 2097–2105. [Google Scholar] [CrossRef]
- Dorca-Arévalo, J.; Blanch, M.; Pradas, M.; Blasi, J. Epsilon toxin from Clostridium perfringens induces cytotoxicity in FRT thyroid epithelial cells. Anaerobe 2018, 53, 43–49. [Google Scholar] [CrossRef]
- Larson, V.A.; Mironova, Y.; Vanderpool, K.G.; Waisman, A.; Rash, J.E.; Agarwal, A.; Bergles, D.E. Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. Elife 2018, 7, e34829. [Google Scholar] [CrossRef]
- Miyamoto, O.; Sumitani, K.; Nakamura, T.; Yamagami, S.; Miyata, S.; Itano, T.; Negi, T.; Okabe, A. Clostridium perfringens epsilon toxin causes excessive release of glutamate in the mouse hippocampus. FEMS Microbiol. Lett. 2000, 189, 109–113. [Google Scholar] [CrossRef]
- Djukic, B.; Casper, K.B.; Philpot, B.D.; Chin, L.-S.; McCarthy, K.D. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J. Neurosci. 2007, 27, 11354–11365. [Google Scholar] [CrossRef]
- Inyushin, M.; Kucheryavykh, L.Y.; Kucheryavykh, Y.V.; Nichols, C.G.; Buono, R.J.; Ferraro, T.N.; Skatchkov, S.N.; Eaton, M.J. Potassium channel activity and glutamate uptake are impaired in astrocytes of seizure-susceptible DBA/2 mice. Epilepsia 2010, 51, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Petit, L.; Gibert, M.; Gillet, D.; Laurent-Winter, C.; Boquet, P.; Popoff, M.R. Clostridium perfringens epsilon-toxin acts on MDCK cells by forming a large membrane complex. J. Bacteriol. 1997, 179, 6480–6487. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.; Lohof, A.; Ben-Ari, Y.; Poulain, B.; Bossu, J.L. Maturation of GABAergic Transmission in Cerebellar Purkinje Cells Is Sex Dependent and Altered in the Valproate Model of Autism. Front. Cell Neurosci. 2018, 12, 232. [Google Scholar] [CrossRef]
- Bossu, J.L.; Gähwiler, B.H. Distinct modes of channel gating underlie inactivation of somatic K+ current in rat hippocampal pyramidal cells in vitro. J. Physiol. 1996, 495, 383–397. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bossu, J.L.; Wioland, L.; Doussau, F.; Isope, P.; Popoff, M.R.; Poulain, B. Epsilon Toxin from Clostridium perfringens Causes Inhibition of Potassium inward Rectifier (Kir) Channels in Oligodendrocytes. Toxins 2020, 12, 36. https://doi.org/10.3390/toxins12010036
Bossu JL, Wioland L, Doussau F, Isope P, Popoff MR, Poulain B. Epsilon Toxin from Clostridium perfringens Causes Inhibition of Potassium inward Rectifier (Kir) Channels in Oligodendrocytes. Toxins. 2020; 12(1):36. https://doi.org/10.3390/toxins12010036
Chicago/Turabian StyleBossu, Jean Louis, Laetitia Wioland, Frédéric Doussau, Philippe Isope, Michel R. Popoff, and Bernard Poulain. 2020. "Epsilon Toxin from Clostridium perfringens Causes Inhibition of Potassium inward Rectifier (Kir) Channels in Oligodendrocytes" Toxins 12, no. 1: 36. https://doi.org/10.3390/toxins12010036
APA StyleBossu, J. L., Wioland, L., Doussau, F., Isope, P., Popoff, M. R., & Poulain, B. (2020). Epsilon Toxin from Clostridium perfringens Causes Inhibition of Potassium inward Rectifier (Kir) Channels in Oligodendrocytes. Toxins, 12(1), 36. https://doi.org/10.3390/toxins12010036





