Cleaving Ergot Alkaloids by Hydrazinolysis—A Promising Approach for a Sum Parameter Screening Method
Abstract
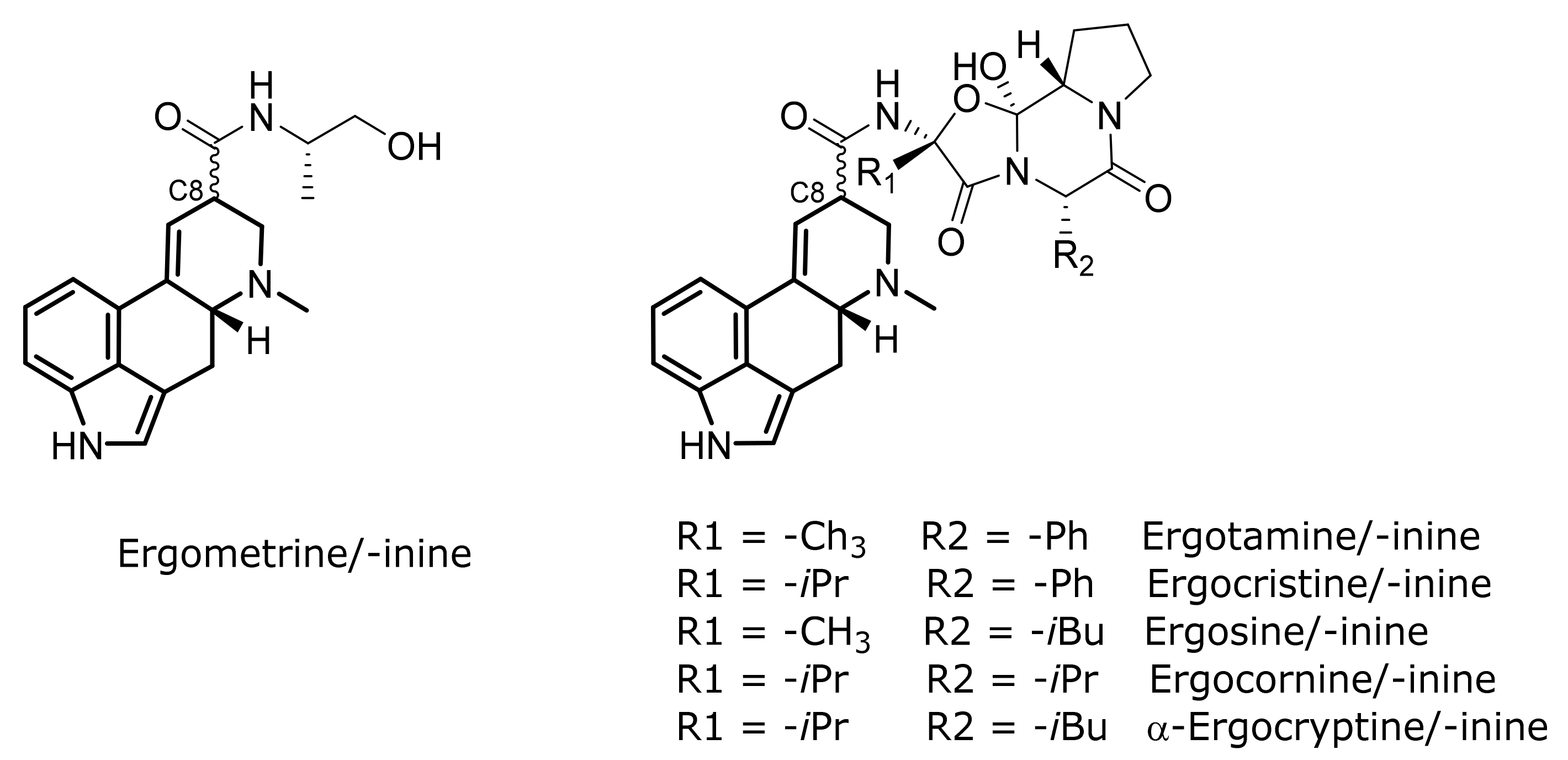
1. Introduction
2. Results
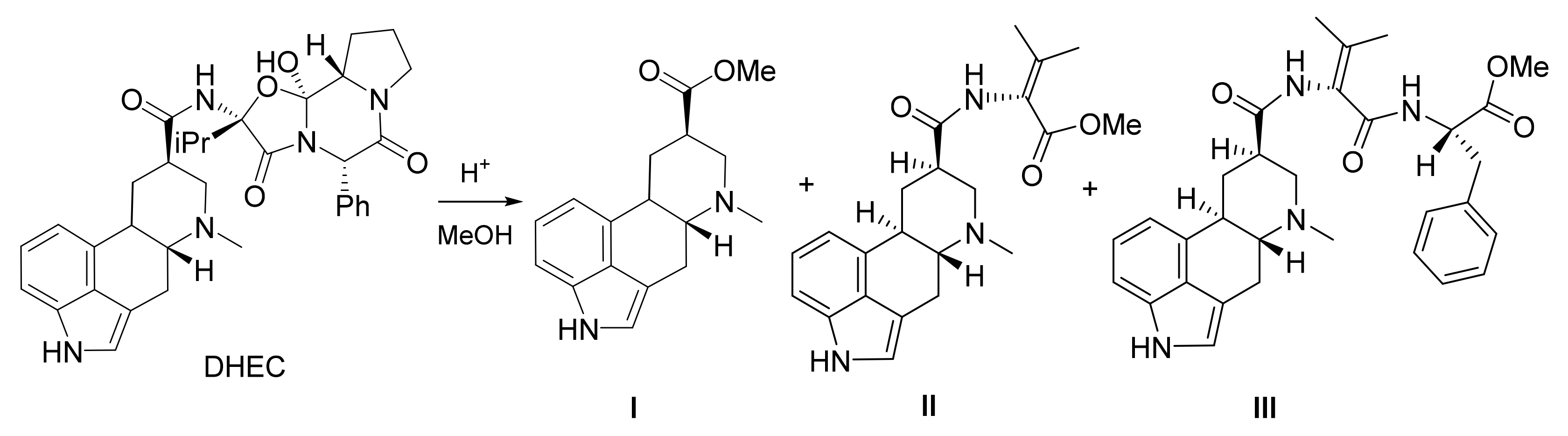
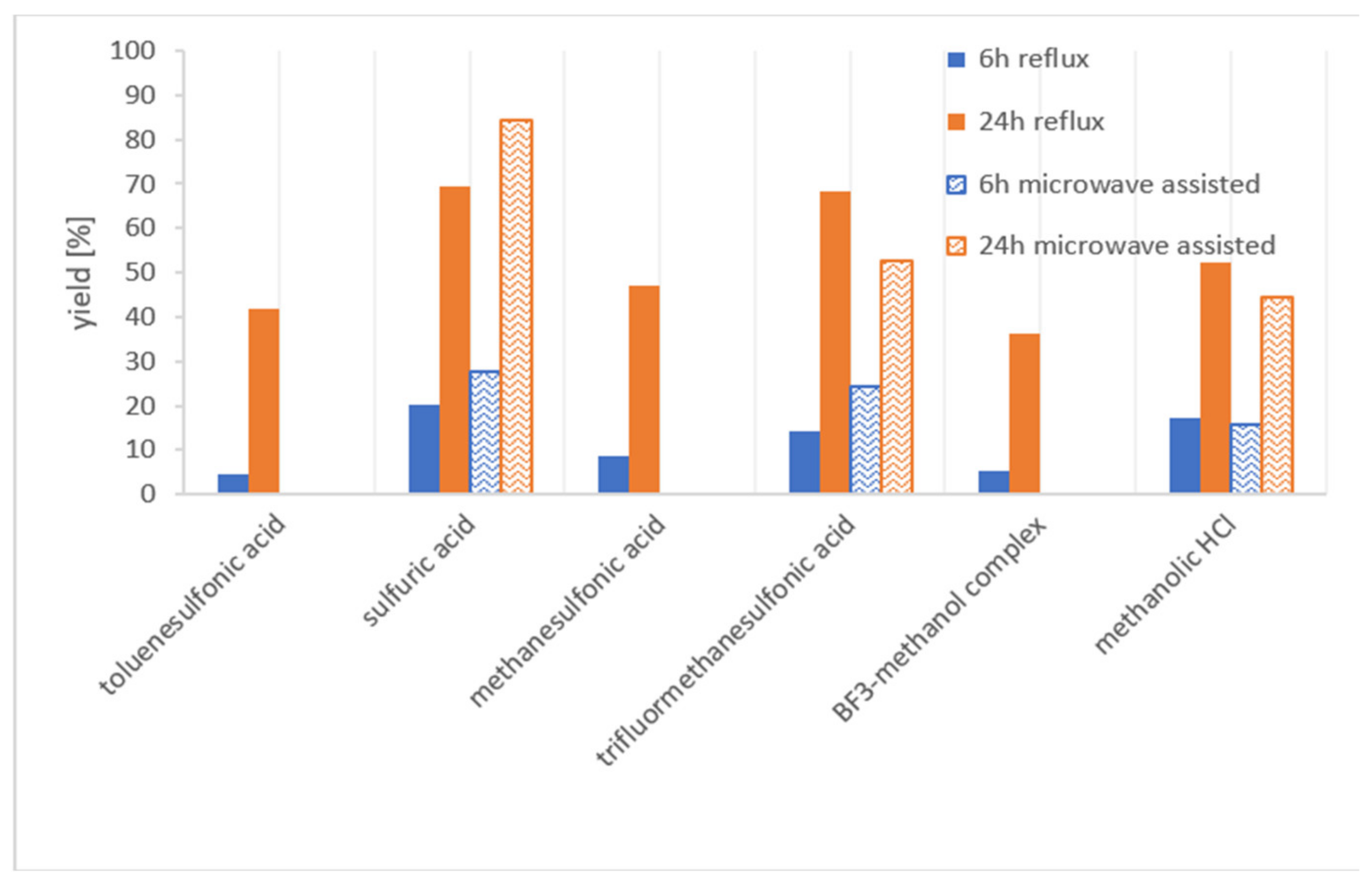
2.1. Acidic Esterification
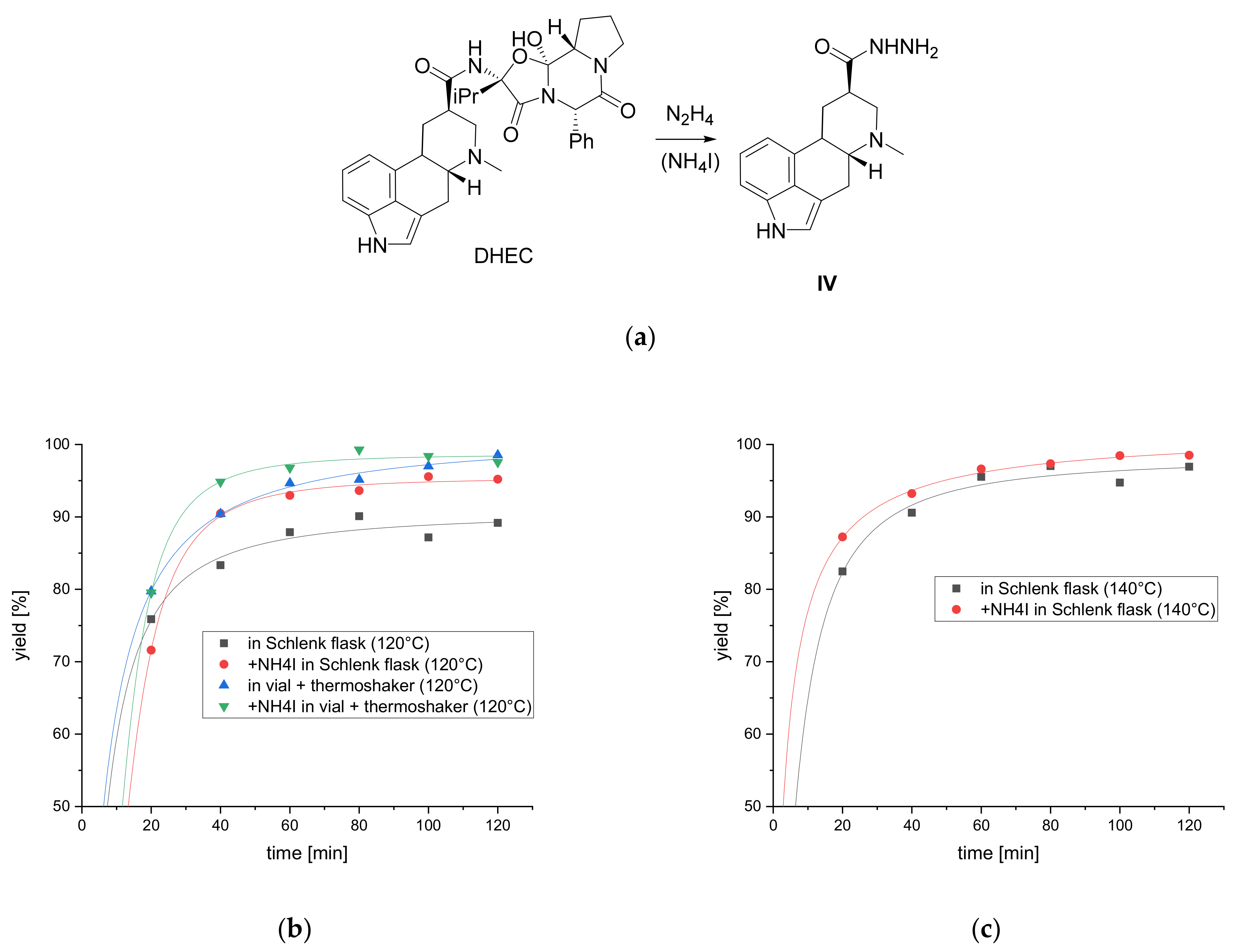
2.2. Hydrazinolysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Equipment
4.2. Synthesis of Dihydrolysergic Acid Methylester (I)
4.3. Yield Determination of the Acidic Esterification
4.4. Synthesis of Dihydrolysergic Acid Hydrazide (IV)
4.5. Yield Determination of the Hydrazinolysis
4.6. Cleavage of the Native Ergot Alkaloids
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krska, R.; Crews, C. Significance, chemistry and determination of ergot alkaloids: A review. Food Addit. Contam. Part A 2008, 25, 722–731. [Google Scholar] [CrossRef]
- Beuerle, T.; Benford, D.; Brimer, L.; Cottrill, B.; Doerge, D.; Dusemund, B.; Farmer, P.; Fürst, P.; Humpf, H.; Mulder, P.P.J. Scientific Opinion on Ergot alkaloids in food and feed. EFSA J. 2012, 10, 2798. [Google Scholar] [CrossRef]
- Lorenz, K.; Hoseney, R.C. Ergot on cereal grains. Crit. Rev. Food Sci. Nutr. 1979, 11, 311–354. [Google Scholar] [CrossRef]
- Hofmann, A. Historical view on ergot alkaloids. Pharmacology 1978, 16 (Suppl. S1). [Google Scholar] [CrossRef]
- Streller, S.; Roth, K. Der gehörnte Roggen. Ein chemischer Blick auf den Isenheimer Altar. Chem. Unserer Zeit 2009, 43, 272–287. [Google Scholar] [CrossRef]
- Gabbai, L. Ergot Poisoning at Pont St. Esprit. Br. Med. J. 1951, 2, 650–651. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flieger, M.; Wurst, M.; Shelby, R. Ergot alkaloids—Sources, structures and analytical methods. Folia Microbiol. 1997, 42, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.M. Analysis of ergot alkaloids—A review. Mycotoxin Res. 2007, 23, 113–121. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Cramer, B.; Dall’Asta, C.; Iha, M.H.; Lattanzio, V.M.T.; Malone, R.J.; Maragos, C.; Solfrizzo, M.; Stranska-Zachariasova, M.; Stroka, J. Developments in mycotoxin analysis: An update for 2017–2018. World Mycotoxin J. 2019, 12, 3–29. [Google Scholar] [CrossRef]
- Crews, C. Analysis of Ergot Alkaloids. Toxins 2015, 7, 2024–2050. [Google Scholar] [CrossRef] [PubMed]
- Oellig, C. Lysergic acid amide as chemical marker for the total ergot alkaloids in rye flour—Determination by high-performance thin-layer chromatography–fluorescence detection. J. Chromatogr. A 2017, 1507, 124–131. [Google Scholar] [CrossRef]
- Van Urk, H.W. A new sensitive reaction for the ergot alkaloids, ergotamine, ergotoxine and ergotinine and its adaptation to the examination and colorimetric determination of ergot preparations. Pharm. Weekbl. 1929, 66, 473–481. [Google Scholar]
- Vermeulen, P.; Pierna, J.A.F.; van Egmond, H.P.; Zegers, J.; Dardenne, P.; Baeten, V. Validation and transferability study of a method based on near-infrared hyperspectral imaging for the detection and quantification of ergot bodies in cereals. Anal. Bioanal. Chem. 2013, 405, 7765–7772. [Google Scholar] [CrossRef]
- Pindur, U. 2,2′-Diindolylmethane, 7. Mitt. Diindolylmethan-Leukobasen bei der van Urk-Reaktion mit physiologisch aktiven Indolen. Arch. Pharm. 1984, 317, 502–505. [Google Scholar] [CrossRef]
- Kodisch, A.; Oberforster, M.; Raditschnig, A.; Rodemann, B.; Tratwal, A.; Danielewicz, J.; Korbas, M.; Schmiedchen, B.; Eifler, J.; Gordillo, A.; et al. Covariation of Ergot Severity and Alkaloid Content Measured by HPLC and One ELISA Method in Inoculated Winter Rye across Three Isolates and Three European Countries. Toxins 2020, 12, 676. [Google Scholar] [CrossRef]
- Tunali, B.; Shelby, R.A.; Morgan-Jones, G.; Kodan, M. Endophytic fungi and ergot alkaloids in native Turkish grasses. Phytoparasitica 2000, 28, 375–377. [Google Scholar] [CrossRef]
- Schnitzius, J.M.; Hill, N.S.; Thompson, C.S.; Craig, A.M. Semiquantitative Determination of Ergot Alkaloids in Seed, Straw, and Digesta Samples Using a Competitive Enzyme-Linked Immunosorbent Assay. J. Vet. Diagn. Invest. 2001, 13, 230–237. [Google Scholar] [CrossRef]
- Hill, N.S.; Agee, C.S. Detection of Ergoline Alkaloids in Endophyte-Infected Tall Fescue by Immunoassay. Crop Sci. 1994, 34, 530–534. [Google Scholar] [CrossRef]
- Komarova, E.L.; Tolkachev, O.N. The Chemistry of Peptide Ergot Alkaloids. Part 1. Classification and Chemistry of Ergot Peptides. Pharm. Chem. J. 2001, 35, 504–513. [Google Scholar] [CrossRef]
- Stoll, A.; Petrzilka, T.; Rutschmann, J.; Hofmann, A.; Günthard, H.H. Über die Stereochemie der Lysergsäuren und der Dihydro-lysergsäuren. 37. Mitteilung über Mutterkornalkaloide. Helv. Chim. Acta 1954, 37, 2039–2057. [Google Scholar] [CrossRef]
- Stoll, A.; Hofmann, A. Zur Kenntnis des Polypeptidteils der Mutterkornalkaloide II. (partielle alkalische Hydrolyse der Mutterkornalkaloide). 20. Mitteilung über Mutterkornalkaloide. Helv. Chim. Acta 1950, 33, 1705–1711. [Google Scholar] [CrossRef]
- Sauer, G.; Haffer, G. Process for the Preparation of Lysergic Acid Esters. U.S. Patent 4,524,208, 18 June 1985. [Google Scholar]
- Sauer, G. Verfahren zur Herstellung von Dihydrolysergsäureestern. D.E. Patent 3,220,200 A1, 8 December 1983. [Google Scholar]
- Stoll, A.; Hofmann, A.; Petrzilka, T. Die Konstitution der Mutterkornalkaloide. Struktur des Peptidteils. III. 24. Mitteilung über Mutterkornalkaloide. Helv. Chim. Acta 1951, 34, 1544–1576. [Google Scholar] [CrossRef]
- Stoll, A.; Petrzilka, T.; Becker, B. Beitrag zur Kenntnis des Polypeptidteils von Mutterkornalkaloiden (Spaltung der Mutterkornalkaloide mit Hydrazin). 16. Mitteilung über Mutterkornalkaloide. Helv. Chim. Acta 1950, 33, 57–67. [Google Scholar] [CrossRef]
- Stoll, A.; Hofmann, A. Lysergic Acid Hydrazide and a Process for Its Manufacture. U.S. Patent 2,090,429, 17 August 1937. [Google Scholar]
- Dankházi, T.; Fekete, É.; Paál, K.; Farsang, G. Electrochemical oxidation of lysergic acid-type ergot alkaloids in acetonitrile. Part 1. Stoichiometry of the anodic oxidation electrode reaction. Anal. Chim. Acta 1993, 282, 289–296. [Google Scholar] [CrossRef]
- Shimizu, Y.; Noshita, M.; Mukai, Y.; Morimoto, H.; Ohshima, T. Cleavage of unactivated amide bonds by ammonium salt-accelerated hydrazinolysis. Chem. Commun. 2014, 50, 12623–12625. [Google Scholar] [CrossRef]
- Shafer, J.A.; Morawetz, H. Participation of a Neighboring Amide Group in the Decomposition of Esters and Amides of Substituted Phthalamic Acids1. J. Org. Chem. 1963, 28, 1899–1901. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuner, M.; Kühn, S.; Haase, H.; Meyer, K.; Koch, M. Cleaving Ergot Alkaloids by Hydrazinolysis—A Promising Approach for a Sum Parameter Screening Method. Toxins 2021, 13, 342. https://doi.org/10.3390/toxins13050342
Kuner M, Kühn S, Haase H, Meyer K, Koch M. Cleaving Ergot Alkaloids by Hydrazinolysis—A Promising Approach for a Sum Parameter Screening Method. Toxins. 2021; 13(5):342. https://doi.org/10.3390/toxins13050342
Chicago/Turabian StyleKuner, Maximilian, Susanne Kühn, Hajo Haase, Klas Meyer, and Matthias Koch. 2021. "Cleaving Ergot Alkaloids by Hydrazinolysis—A Promising Approach for a Sum Parameter Screening Method" Toxins 13, no. 5: 342. https://doi.org/10.3390/toxins13050342
APA StyleKuner, M., Kühn, S., Haase, H., Meyer, K., & Koch, M. (2021). Cleaving Ergot Alkaloids by Hydrazinolysis—A Promising Approach for a Sum Parameter Screening Method. Toxins, 13(5), 342. https://doi.org/10.3390/toxins13050342






