Jelleine, a Family of Peptides Isolated from the Royal Jelly of the Honey Bees (Apis mellifera), as a Promising Prototype for New Medicines: A Narrative Review
Abstract
:1. Introduction
2. History and Origin of Jelleines

3. Chemical Properties of Jelleines
4. Pharmacological Proprieties
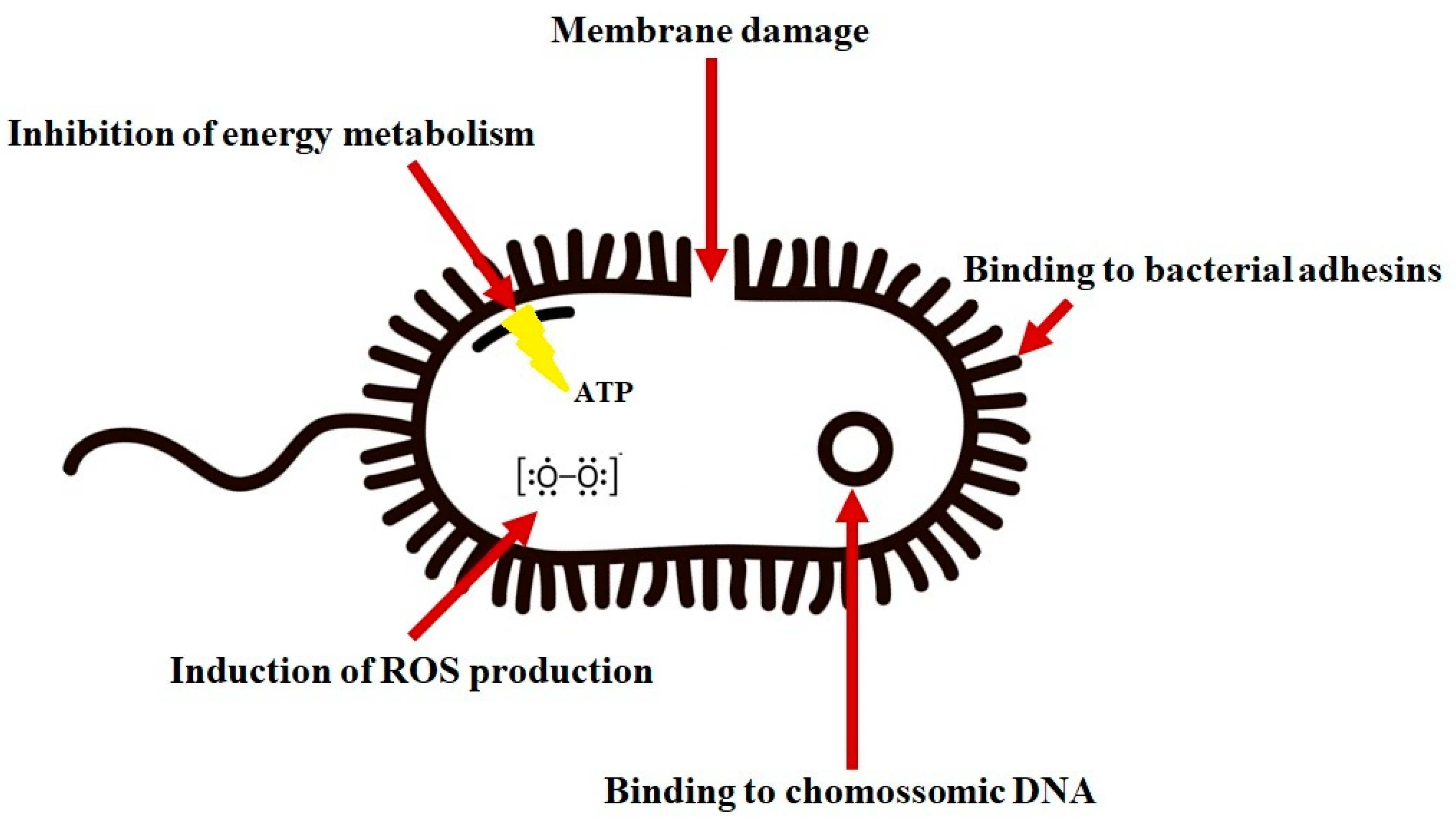
4.1. Antibacterial Activity
4.2. Antifungal Activity
4.3. Antiparasitic Activity
4.4. Anti-Inflammatory Effect
4.5. Healing and Hemostatic Effect
4.6. Antitumoral Activity
5. Toxicity
6. Stability
7. Conclusions Remakes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Bee Products as a Source of Promising Therapeutic and Chemoprophylaxis Strategies against COVID-19 (SARS-CoV-2). Phytother. Res. 2020, 35, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Fratellone, P.M.; Tsimis, F.; Fratellone, G. Apitherapy Products for Medicinal Use. J. Altern. Complement. Med. 2016, 22, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Moreira Brito, J.C.; Stephanie da Cruz Nizer, W.; Sampaio de Assis, D.C. Antifungal, Antibiofilm and Anti-Resistance Activities of Brazilian Monofloral Honeys against Candida spp. Biocatal. Agric. Biotechnol. 2022, 42, 102335. [Google Scholar] [CrossRef]
- Lima, W.G.; Batista Filho, F.L.; Lima, I.P.; Simião, D.C.; Brito, J.C.M.; da Cruz Nizer, W.S.; Cardoso, V.N.; Fernandes, S.O.A. Antibacterial, Anti-Biofilm, and Anti-Adhesive Activities of Melittin, a Honeybee Venom-Derived Peptide, against Quinolone-Resistant Uropathogenic Escherichia coli (UPEC). Nat. Prod. Res. 2022, 36, 6381–6388. [Google Scholar] [CrossRef]
- Lima, W.G.; de Brito, J.C.M.; Cardoso, V.N.; Fernandes, S.O.A. In-Depth Characterization of Antibacterial Activity of Melittin against Staphylococcus Aureus and Use in a Model of Non-Surgical MRSA-Infected Skin Wounds. Eur. J. Pharm. Sci. 2021, 156, 105592. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.A.A.A. Anti-Inflammatory and Protective Effects of Royal Jelly against Hepatic and Renal Damage Induced by Valproic Acid in Rats. Genet. Mol. Res. 2023, 22, GMR19063. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, M.; Wang, L.; Lin, T.; Wang, G.; Peng, J.; Su, S. The In Vitro and In Vivo Wound-Healing Effects of Royal Jelly Derived from Apis mellifera L. during Blossom Seasons of Castanea mollissima Bl. and Brassica napus L. in South China Exhibited Distinct Patterns. BMC Complement. Med. Ther. 2020, 20, 357. [Google Scholar] [CrossRef] [PubMed]
- Botezan, S.; Baci, G.M.; Bagameri, L.; Pașca, C.; Dezmirean, D.S. Current Status of the Bioactive Properties of Royal Jelly: A Comprehensive Review with a Focus on Its Anticancer, Anti-Inflammatory, and Antioxidant Effects. Molecules 2023, 28, 1510. [Google Scholar] [CrossRef]
- Kunugi, H.; Ali, A.M. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef]
- Miyata, Y.; Sakai, H. Anti-Cancer and Protective Effects of Royal Jelly for Therapy-Induced Toxicities in Malignancies. Int. J. Mol. Sci. 2018, 19, 3270. [Google Scholar] [CrossRef]
- Harwood, G.; Salmela, H.; Freitak, D.; Amdam, G. Social Immunity in Honey Bees: Royal Jelly as a Vehicle in Transferring Bacterial Pathogen Fragments between Nestmates. J. Exp. Biol. 2021, 224, jeb231076. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive Compounds and Health-Promoting Properties of Royal Jelly: A Review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Fontana, R.; Mendes, M.A.; De Souza, B.M.; Konno, K.; César, L.M.M.; Malaspina, O.; Palma, M.S. Jelleines: A Family of Antimicrobial Peptides from the Royal Jelly of Honeybees (Apis mellifera). Peptides 2004, 25, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Bagameri, L.; Baci, G.M.; Dezmirean, D.S. Royal Jelly as a Nutraceutical Natural Product with a Focus on Its Antibacterial Activity. Pharmaceutics 2022, 14, 1142. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G. Peptide Antibiotics and Their Role in Innate Immunity. Annu. Rev. Immunol. 1995, 13, 61–92. [Google Scholar] [CrossRef]
- Bulet, P.; Hetru, C.; Dimarcq, J.L.; Hoffmann, D. Antimicrobial Peptides in Insects; Structure and Function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Romanelli, A.; Moggio, L.; Montella, R.C.; Campiglia, P.; Iannaccone, M.; Capuano, F.; Pedone, C.; Capparelli, R. Peptides from Royal Jelly: Studies on the Antimicrobial Activity of Jelleins, Jelleins Analogs and Synergy with Temporins. J. Pept. Sci. 2011, 17, 348–352. [Google Scholar] [CrossRef]
- Lim, K.; Chua, R.R.Y.; Saravanan, R.; Basu, A.; Mishra, B.; Tambyah, P.A.; Ho, B.; Leong, S.S.J. Immobilization Studies of an Engineered Arginine-Tryptophan-Rich Peptide on a Silicone Surface with Antimicrobial and Antibiofilm Activity. ACS Appl. Mater. Interfaces 2013, 5, 6412–6422. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J.; Peng, J.; Zhao, P.; Kong, Z.; Wang, K.; Yan, W.; Wang, R. The in vitro, in vivo Antifungal Activity and the Action Mode of Jelleine-I against Candida Species. Amino Acids 2018, 50, 229–239. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J.; Zhang, L.; Zhou, J.; He, Y.; Lu, Y.; Liu, K.; Yan, W.; Wang, K. Multiple Action Mechanism and In Vivo Antimicrobial Efficacy of Antimicrobial Peptide Jelleine-I. J. Pept. Sci. 2021, 27, e3294. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Yu, Q.; Wang, R.; Zhao, L.; Yuan, F.; Guo, H.; Shen, Y.; He, F. Optimized Antimicrobial Peptide Jelleine-I Derivative Br-J-I Inhibits Fusobacterium Nucleatum to Suppress Colorectal Cancer Progression. Int. J. Mol. Sci. 2023, 24, 1469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, L.; He, Y.; Liu, K.; Zhang, F.; Zhang, H.; Lu, Y.; Yang, C.; Wang, Z.; Fareed, M.S.; et al. An Optimized Analog of Antimicrobial Peptide Jelleine-1 Shows Enhanced Antimicrobial Activity against Multidrug Resistant P. aeruginosa and Negligible Toxicity in vitro and in vivo. Eur. J. Med. Chem. 2021, 219, 113433. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Ding, K.; Wang, L.; Tian, J.; Huang, X.; Zhang, M.; Dang, X. In Vitro and in vivo Antimicrobial Activity of Antimicrobial Peptide Jelleine-I against Foodborne Pathogen Listeria Monocytogenes. Int. J. Food Microbiol. 2023, 387, 110050. [Google Scholar] [CrossRef]
- Kim, S.R.; Choi, K.H.; Kim, K.Y.; Kwon, H.Y.; Park, S.W. Development of a Novel Short Synthetic Antibacterial Peptide Derived from the Swallowtail Butterfly Papilio Xuthus Larvae. J. Microbiol. Biotechnol. 2020, 30, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.B.; Pacca, C.C.; da Silva, A.M.B.; de Souza, B.M.; de Almeida, M.T.G.; Palma, M.S.; Arcisio-Miranda, M.; dos Santos Cabrera, M.P. Comparing Activity, Toxicity and Model Membrane Interactions of Jelleine-I and Trp/Arg Analogs: Analysis of Peptide Aggregation. Amino Acids 2020, 52, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Petrin, T.H.C.; Fadel, V.; Martins, D.B.; Dias, S.A.; Cruz, A.; Sergio, L.M.; Arcisio-Miranda, M.; Castanho, M.A.R.B.; Dos Santos Cabrera, M.P. Synthesis and Characterization of Peptide-Chitosan Conjugates (PepChis) with Lipid Bilayer Affinity and Antibacterial Activity. Biomacromolecules 2019, 20, 2743–2753. [Google Scholar] [CrossRef]
- Jia, F.; Zhang, Y.; Wang, J.; Peng, J.; Zhao, P.; Zhang, L.; Yao, H.; Ni, J.; Wang, K. The Effect of Halogenation on the Antimicrobial Activity, Antibiofilm Activity, Cytotoxicity and Proteolytic Stability of the Antimicrobial Peptide Jelleine-I. Peptides 2019, 112, 56–66. [Google Scholar] [CrossRef]
- Capparelli, R.; De Chiara, F.; Nocerino, N.; Montella, R.C.; Iannaccone, M.; Fulgione, A.; Romanelli, A.; Avitabile, C.; Blaiotta, G.; Capuano, F. New Perspectives for Natural Antimicrobial Peptides: Application as Antinflammatory Drugs in a Murine Model. BMC Immunol. 2012, 13, 61. [Google Scholar] [CrossRef]
- Zahedifard, F.; Lee, H.; No, J.H.; Salimi, M.; Seyed, N.; Asoodeh, A.; Rafati, S. Comparative Study of Different Forms of Jellein Antimicrobial Peptide on Leishmania Parasite. Exp. Parasitol. 2020, 209, 107823. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Z.; Yang, C.; Zhang, H.; Fareed, M.S.; He, Y.; Su, J.; Wang, P.; Shen, Z.; Yan, W.; et al. A Carrier-Free, Dual-Functional Hydrogel Constructed of Antimicrobial Peptide Jelleine-1 and 8Br-CAMP for MRSA Infected Diabetic Wound Healing. Acta Biomater. 2022, 151, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, H.; Fareed, M.S.; He, Y.; Lu, Y.; Yang, C.; Wang, Z.; Su, J.; Wang, P.; Yan, W.; et al. An Injectable Peptide Hydrogel Constructed of Natural Antimicrobial Peptide J-1 and ADP Shows Anti-Infection, Hemostasis, and Antiadhesion Efficacy. ACS Nano 2022, 16, 7636–7650. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, M.D.; Albert, S.; Kucharski, R.; Prusko, C.; Maleszka, R. Evolution of the Yellow/Major Royal Jelly Protein Family and the Emergence of Social Behavior in Honey Bees. Genome Res. 2006, 16, 1385. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A Review on Royal Jelly Proteins and Peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Mureşan, C.I.; Dezmirean, D.S.; Marc, B.D.; Suharoschi, R.; Pop, O.L.; Buttstedt, A. Biological Properties and Activities of Major Royal Jelly Proteins and Their Derived Peptides. J. Funct. Foods 2022, 98, 105286. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, B.Y.; Deng, Y.; Park, H.G.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Antioxidant Capacity of Major Royal Jelly Proteins of Honeybee (Apis mellifera) Royal Jelly. J. Asia Pac. Entomol. 2020, 23, 445–448. [Google Scholar] [CrossRef]
- Tamura, S.; Amano, S.; Kono, T.; Kondoh, J.; Yamaguchi, K.; Kobayashi, S.; Ayabe, T.; Moriyama, T. Molecular Characteristics and Physiological Functions of Major Royal Jelly Protein 1 Oligomer. Proteomics 2009, 9, 5534–5543. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Sjaarda, C.; Lannigan, R. MRJP1-Containing Glycoproteins Isolated from Honey, a Novel Antibacterial Drug Candidate with Broad Spectrum Activity against Multi-Drug Resistant Clinical Isolates. Front. Microbiol. 2015, 6, 711. [Google Scholar] [CrossRef]
- Fan, P.; Han, B.; Feng, M.; Fang, Y.; Zhang, L.; Hu, H.; Hao, Y.; Qi, Y.; Zhang, X.; Li, J. Functional and Proteomic Investigations Reveal Major Royal Jelly Protein 1 Associated with Anti-Hypertension Activity in Mouse Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 30230. [Google Scholar] [CrossRef]
- Hayashi, T.; Takamatsu, N.; Nakashima, T.; Arita, T. Immunological Characterization of Honey Proteins and Identification of MRJP 1 as an IgE-Binding Protein. OUP 2014, 75, 556–560. [Google Scholar] [CrossRef]
- Kashima, Y.; Kanematsu, S.; Asai, S.; Kusada, M.; Watanabe, S.; Kawashima, T.; Nakamura, T.; Shimada, M.; Goto, T.; Nagaoka, S. Identification of a Novel Hypocholesterolemic Protein, Major Royal Jelly Protein 1, Derived from Royal Jelly. PLoS ONE 2014, 9, e105073. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, F.; Wan, J.B.; Lai, C.Q.; Shen, L.R. Effect of Major Royal Jelly Proteins on Spatial Memory in Aged Rats: Metabolomics Analysis in Urine. J. Agric. Food Chem. 2017, 65, 3151–3159. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, C.; Chen, Y.; Shi, F.; Lai, C.; Shen, L. Major Royal Jelly Proteins Accelerate Onset of Puberty and Promote Ovarian Follicular Development in Immature Female Mice. Food Sci. Hum. Wellness 2020, 9, 338–345. [Google Scholar] [CrossRef]
- Brudzynski, K.; Sjaarda, C. Honey Glycoproteins Containing Antimicrobial Peptides, Jelleins of the Major Royal Jelly Protein 1, Are Responsible for the Cell Wall Lytic and Bactericidal Activities of Honey. PLoS ONE 2015, 10, e0120238. [Google Scholar] [CrossRef]
- Leiva-Sabadini, C.; Alvarez, S.; Barrera, N.P.; Schuh, C.M.A.P.; Aguayo, S. Antibacterial Effect of Honey-Derived Exosomes Containing Antimicrobial Peptides Against Oral Streptococci. Int. J. Nanomed. 2021, 16, 4891–4900. [Google Scholar] [CrossRef]
- Tian, W.; Li, M.; Guo, H.; Peng, W.; Xue, X.; Hu, Y.; Liu, Y.; Zhao, Y.; Fang, X.; Wang, K.; et al. Architecture of the Native Major Royal Jelly Protein 1 Oligomer. Nat. Commun. 2018, 9, 3373. [Google Scholar] [CrossRef]
- Chen, H.C.; Brown, J.H.; Morell, J.L.; Huang, C.M. Synthetic Magainin Analogues with Improved Antimicrobial Activity. FEBS Lett. 1988, 236, 462–466. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Merrifield, R.; Boman, I.; Boman, H.G. Effects on Electrophoretic Mobility and Antibacterial Spectrum of Removal of Two Residues from Synthetic Sarcotoxin IA and Addition of the Same Residues to Cecropin B. FEBS Lett. 1988, 231, 299–302. [Google Scholar] [CrossRef]
- Park, N.G.; Yamato, Y.; Lee, S.; Sugihara, G. Interaction of Mastoparan-B from Venom of a Hornet in Taiwan with Phospholipid Bilayers and Its Antimicrobial Activity. Biopolymers 1995, 36, 793–801. [Google Scholar] [CrossRef]
- Cabrera, M.P.S.; Baldissera, G.; Silva-Gonçalves, L.D.C.; De Souza, B.M.; Riske, K.A.; Palma, M.S.; Ruggiero, J.R.; Arcisio-Miranda, M. Combining Experimental Evidence and Molecular Dynamic Simulations to Understand the Mechanism of Action of the Antimicrobial Octapeptide Jelleine-I. Biochemistry 2014, 53, 4857–4868. [Google Scholar] [CrossRef]
- Galzitskaya, O.V. Creation of New Antimicrobial Peptides. Int. J. Mol. Sci. 2023, 24, 9451. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.; Review on Antibiotic Resisitance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 4 March 2018).
- Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Ventilator-Associated Pneumonia (VAP) Caused by Carbapenem-Resistant Acinetobacter Baumannii in Patients with COVID-19: Two Problems, One Solution? Med. Hypotheses 2020, 144, 110139. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Poudel, A.N.; Aihusein, N.; Wang, H.; Yao, G.; Lambert, H. Antimicrobial Use in COVID-19 Patients in the First Phase of the SARS-CoV-2 Pandemic: A Scoping Review. Antibiotics 2021, 10, 745. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Brito, J.C.M.; de Lima, M.E.; Pizarro, A.C.S.T.; Vianna, M.A.M.D.M.; de Paiva, M.C.; de Assis, D.C.S.; Cardoso, V.N.; Fernandes, S.O.A. A Short Synthetic Peptide, Based on LyeTx I from Lycosa Erythrognatha Venom, Shows Potential to Treat Pneumonia Caused by Carbapenem-Resistant Acinetobacter baumannii without Detectable Resistance. J. Antibiot. 2021, 74, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Aminov, R.; Franco, O.L.; de la Fuente-Nunez, C.; Wang, J. Editorial: Community Series in Antimicrobial Peptides: Molecular Design, Structure Function Relationship and Biosynthesis Optimization. Front. Microbiol. 2023, 14, 1125426. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Chapple, D.S. Peptide Antibiotics. Antimicrob. Agents Chemother. 1999, 43, 1317–1323. [Google Scholar] [CrossRef]
- Lima, W.G.; de Lima, M.E. Therapeutic Prospection of Animal Venoms-Derived Antimicrobial Peptides against Infections by Multidrug-Resistant Acinetobacter Baumannii: A Systematic Review of Pre-Clinical Studies. Toxins 2023, 15, 268. [Google Scholar] [CrossRef]
- Mwangi, J.; Hao, X.; Lai, R.; Zhang, Z.Y. Antimicrobial Peptides: New Hope in the War against Multidrug Resistance. Zool. Res. 2019, 40, 488–505. [Google Scholar] [CrossRef]
- Arpornsuwan, T.; Paveenkittiporn, W.; Jaresitthikunchai, J.; Roytrakul, S. BAMP-28 Antimicrobial Peptide against Different MALDI Biotype of Carbapenam Resistant Enterobacteriaceae. Int. J. Pept. Res. Ther. 2019, 25, 951–960. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI, Ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Recommendations for Colistin (Polymyxin E) MIC Testing-Joint EUCAST and CLSI Recommendation. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_for_MIC_determination_of_colistin_March_2016.pdf (accessed on 16 November 2019).
- Fu, Q.; Cao, D.; Sun, J.; Liu, X.; Li, H.; Shu, C.; Liu, R. Prediction and Bioactivity of Small-Molecule Antimicrobial Peptides from Protaetia Brevitarsis Lewis Larvae. Front. Microbiol. 2023, 14, 1124672. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Lohner, K. Detergent-like Actions of Linear Amphipathic Cationic Antimicrobial Peptides. Biochim. Biophys. Acta 2006, 1758, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.A.C.; Machini, M.T. Hemocidinas Derivadas da Hemoglobina: Estruturas, Propriedades e Perspectivas. Quim. Nova 2013, 36, 1021. [Google Scholar] [CrossRef]
- Wu, M.; Maier, E.; Benz, R.; Hancock, R.E.W. Mechanism of Interaction of Different Classes of Cationic Antimicrobial Peptides with Planar Bilayers and with the Cytoplasmic Membrane of Escherichia coli. Biochemistry 1999, 38, 7235–7242. [Google Scholar] [CrossRef]
- Pletz, M.W.; Hagel, S.; Forstner, C. Who Benefits from Antimicrobial Combination Therapy? Lancet Infect. Dis. 2017, 17, 677–678. [Google Scholar] [CrossRef]
- Ahmed, A.; Azim, A.; Gurjar, M.; Baronia, A.K. Current Concepts in Combination Antibiotic Therapy for Critically Ill Patients. Indian. J. Crit. Care Med. 2014, 18, 310–314. [Google Scholar] [CrossRef]
- Rybak, M.J.; McGrath, B.J. Combination Antimicrobial Therapy for Bacterial Infections. Guidelines for the Clinician. Drugs 1996, 52, 390–405. [Google Scholar] [CrossRef]
- Tängdén, T. Combination Antibiotic Therapy for Multidrug-Resistant Gram-Negative Bacteria. Ups. J. Med. Sci. 2014, 119, 149–153. [Google Scholar] [CrossRef]
- Reis, P.V.M.; Boff, D.; Verly, R.M.; Melo-Braga, M.N.; Cortés, M.E.; Santos, D.M.; Pimenta, A.M.D.C.; Amaral, F.A.; Resende, J.M.; De Lima, M.E. LyeTxI-b, a Synthetic Peptide Derived from Lycosa Erythrognatha Spider Venom, Shows Potent Antibiotic Activity In Vitro and In Vivo. Front. Microbiol. 2018, 9, 667. [Google Scholar] [CrossRef]
- Perfect, J.R. The Antifungal Pipeline: A Reality Check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef]
- Rodrigues, M.L.; Nosanchuk, J.D. Fungal Diseases as Neglected Pathogens: A Wake-up Call to Public Health Officials. PLoS Negl. Trop. Dis. 2020, 14, e0007964. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.T.; Santos, F.R.S.; Lima, W.G.; Sousa, C.D.F.; Oliveira, L.S.F.M.; Ribeiro, R.I.M.A.; Gomes, A.J.P.S.; Araújo, M.G.F.; Villar, J.A.F.P.; Ferreira, J.M.S. Design, Synthesis, Biological Activity and Structure-Activity Relationship Studies of Chalcone Derivatives as Potential Anti-Candida Agents. J. Antibiot. 2018, 71, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Alves-Nascimento, L.A.; Andrade, J.T.; Vieira, L.; de Azambuja Ribeiro, R.I.M.; Thomé, R.G.; dos Santos, H.B.; Ferreira, J.M.S.; Soares, A.C. Are the Statins Promising Antifungal Agents against Invasive Candidiasis? Biomed. Pharmacother. 2019, 111, 270–281. [Google Scholar] [CrossRef]
- Andrade, J.T.; Lima, W.G.; Sousa, J.F.; Saldanha, A.A.; De Sá, N.P.; Morais, F.B.; Silva, M.K.P.; Viana, G.H.R.; Johann, S.; Soares, A.C.; et al. Design, Synthesis, and Biodistribution Studies of New Analogues of Marine Alkaloids: Potent in vitro and in vivo Fungicidal Agents against Candida Spp. Eur. J. Med. Chem. 2021, 210, 113048. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal Therapy: New Advances in the Understanding and Treatment of Mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef]
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell Infect. Microbiol. 2020, 10, 105. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- de Vries, H.J.C.; Reedijk, S.H.; Schallig, H.D.F.H. Cutaneous Leishmaniasis: Recent Developments in Diagnosis and Management. Am. J. Clin. Dermatol. 2015, 16, 99–109. [Google Scholar] [CrossRef]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.-C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug Resistance and Treatment Failure in Leishmaniasis: A 21st Century Challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Alcântara, L.M.; Ferreira, T.C.S.; Gadelha, F.R.; Miguel, D.C. Challenges in Drug Discovery Targeting TriTryp Diseases with an Emphasis on Leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Weiss, U. Inflammation. Nature 2002, 420, 845. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and Physiology of Wound Healing. Facial Plast. Surg. Clin. N. Am. 2011, 19, 441–453. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound Healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- de Souza, G.S.; de Jesus Sonego, L.; Santos Mundim, A.C.; de Miranda Moraes, J.; Sales-Campos, H.; Lorenzón, E.N. Antimicrobial-Wound Healing Peptides: Dual-Function Molecules for the Treatment of Skin Injuries. Peptides 2022, 148, 170707. [Google Scholar] [CrossRef]
- Cree, I.A. Cancer Biology. In Cancer Cell Culture. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 731, pp. 1–11. [Google Scholar] [CrossRef]
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 24 July 2023).
- Wheeler, H.E.; Maitland, M.L.; Dolan, M.E.; Cox, N.J.; Ratain, M.J. Cancer Pharmacogenomics: Strategies and Challenges. Nat. Rev. Genet. 2013, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.M.; Lima, W.G.; Vital, K.D.; Cardoso, V.N. Repurposing the Thalidomide to the Treatment of Irinotecan-Induced Intestinal Mucositis: An Old Drug to a New Use. Glob. J. Pharm. Pharm. Sci. 2018, 6, 8–10. [Google Scholar] [CrossRef]
- Sonis, S.T. The Pathobiology of Mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, G.; Tallima, H.; Dabbish, E.; Badr ElDin, N.; Abd El-Rahman, M.K.; Ibrahim, M.A.A.; Shoeib, T. Anti-Cancer Peptides: Status and Future Prospects. Molecules 2023, 28, 1148. [Google Scholar] [CrossRef]
- Lath, A.; Santal, A.R.; Kaur, N.; Kumari, P.; Singh, N.P. Anti-Cancer Peptides: Their Current Trends in the Development of Peptide-Based Therapy and Anti-Tumor Drugs. Biotechnol. Genet. Eng. Rev. 2023, 39, 45–84. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between Hemolytic Activity, Cytotoxicity and Systemic in vivo Toxicity of Synthetic Antimicrobial Peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef]
- Kang, S.J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial Peptides: Therapeutic Potentials. Expert. Rev. Anti-Infect. Ther. 2014, 12, 1477–1486. [Google Scholar] [CrossRef]
- Lima, W.G.; Pessoa, R.M.; Vital, K.D.; Takenaka, I.K.T.M.; Cardoso, V.N.; Fernandes, S.O.A. Effect of Probiotics on the Maintenance of Intestinal Homeostasis after Chemotherapy: Systematic Review and Meta-Analysis of Pre-Clinical Studies. Benef. Microbes 2020, 11, 305–318. [Google Scholar] [CrossRef]
- Brito, J.C.M.; Lima, W.G.; Resende, J.M.; de Assis, D.C.S.; Boff, D.; Cardoso, V.N.; Amaral, F.A.; Souza-Fagundes, E.M.; Fernandes, S.O.A.; Lima, M.E. Pegylated LyeTx I-b Peptide Is Effective against Carbapenem-Resistant Acinetobacter Baumannii in an in vivo Model of Pneumonia and Shows Reduced Toxicity. Int. J. Pharm. 2021, 609, 121156. [Google Scholar] [CrossRef]
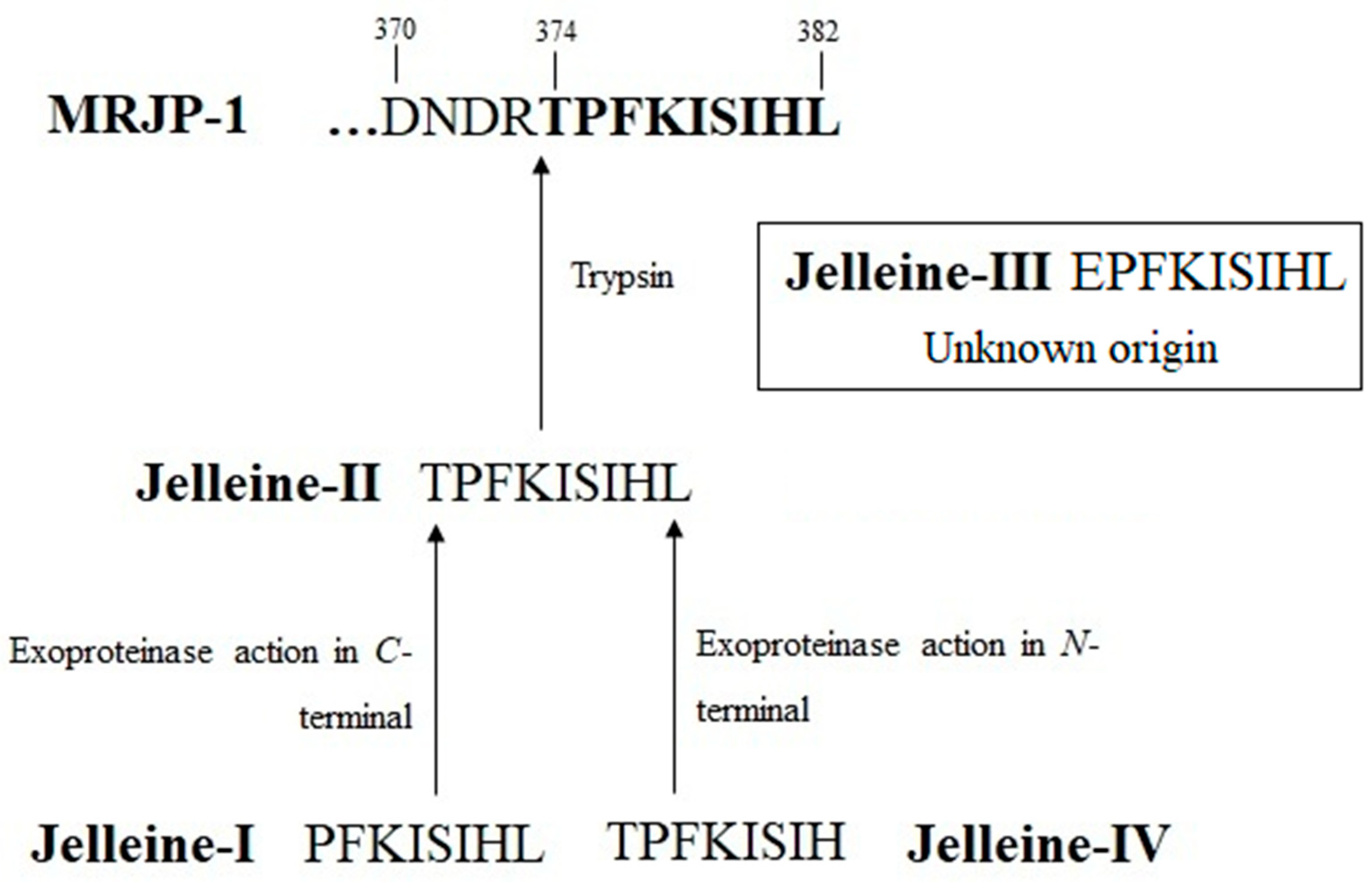
| Name | Sequence | Residues Number | Molecular Masses (Da) | Net Charge at pH 7 | Basic Residues | Acid Residues | Aromatic Residue | Hydrophobic Residues (%) | References |
|---|---|---|---|---|---|---|---|---|---|
| Jelleine-I | PFKISIHL-NH2 | 8 | 953.24 | 2 | 2 | 0 | 1 | 50 | [13] |
| Jelleine-II | TPFKISIHL-NH2 | 9 | 1054.30 | 2 | 2 | 0 | 1 | 44 | [13] |
| Jelleine-III | EPFKISIHL-NH2 | 9 | 1082.32 | 1 | 2 | 1 | 1 | 44 | [13] |
| Jelleine-IV | TPFKISIH-NH2 | 8 | 942.13 | 2 | 2 | 0 | 1 | 38 | [13] |
| RJ IC | PFKISIHLGGY-NH2 | 11 | 1230.46 | 2 | 2 | 0 | 2 | 55 | [18] |
| RJ IIC | TPFKISIHLGGY-NH2 | 12 | 1331.56 | 2 | 2 | 0 | 2 | 50 | [18] |
| RJ IIIC | EPFKISIHLGGY-NH2 | 12 | 1359.57 | 1 | 2 | 1 | 2 | 50 | [18] |
| RJ IN | YGGPFKISIHL-NH2 | 11 | 1230.46 | 2 | 2 | 0 | 2 | 55 | [18] |
| RJ IIN | YGGTPFKISIHL-NH2 | 12 | 1331.56 | 2 | 2 | 0 | 2 | 50 | [18] |
| RJ IIIN | YGGEPFKISIHL-NH2 | 12 | 1359.57 | 1 | 2 | 1 | 2 | 50 | [18] |
| F-J-I A | PFFKISIHL-NH2 | 8 | 972.99 | 2 | 2 | 0 | 1 | 50 | [28] |
| Cl-J-I A | PFClKISIHL-NH2 | 8 | 989.63 | 2 | 2 | 0 | 1 | 50 | [28] |
| Br-J-I A | PFBrKISIHL-NH2 | 8 | 1033.98 | 2 | 2 | 0 | 1 | 50 | [28] |
| I-J-I A | PFIKISIHL-NH2 | 8 | 1080.95 | 2 | 2 | 0 | 1 | 50 | [28] |
| Peptide | Gram-Positive (MIC in µg/mL) | Gram-Negative (MIC in µg/mL) | Reference | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | S. saprophyticus | S. epidermidis | B. subtilis | B. cereus | L. monocytogenes | E. faecalis | S. pneumoniae | E. coli | E. cloacae | K. pneumoniae | P. mirabilis | P. aeruginosa | S. Paratyphi | C. sakazakii | F. nucleatum | ||
| Jelleine-I | 8–128 | 15 | 64 | 4–32 | I | 12.5 | 16 | 6 | 2.5–32 | 10 | 8–64 | I | 8–62 | 200 | 64 | 160 | [13,18,19,20,21,22,23,24,25,26,27,28] |
| Jelleine-II | 15-I | 10 | - | 30 | I | I | - | - | 15 | 15 | 15 | I | 15 | I | - | - | [13,18] |
| Jelleine-III | 30-I | 30 | - | I | I | I | - | - | 15 | I | I | I | 30 | I | - | - | [13,18] |
| Jelleine-IV | I | I | - | I | I | - | - | - | I | I | I | I | I | - | - | - | [13] |
| RJ IC | 100 | - | 30 | - | - | I | - | - | 30 | - | - | - | - | 80 | - | - | [18] |
| RJ IIC | I | - | 200 | - | - | I | - | - | I | - | - | - | - | I | - | - | [18] |
| RJ IIIC | I | - | 300 | - | - | I | - | - | I | - | - | - | - | I | - | - | [18] |
| RJ IN | NR A | - | - | - | - | NR A | - | - | NR A | - | - | - | - | NR A | - | - | [18] |
| RJ IIN | NR A | - | - | - | - | NR A | - | - | NR A | - | - | - | - | NR A | - | - | [18] |
| RJ IIIN | NR A | - | - | - | - | NR A | - | - | NR A | - | - | - | - | NR A | - | - | [18] |
| F-J-I | 64 | - | 16 | 16 | - | - | - | - | 32 | - | 64 | - | 32 | - | 32 | - | [28] |
| Cl-J-I | 32 | - | 16 | 8 | - | - | - | - | 16 | - | 16 | - | 16 | - | 16 | 10 | [22,28] |
| Br-J-I | 32 | - | 16 | 8 | - | - | - | - | 16 | - | 16 | - | 16 | - | 16 | 5 | [22,28] |
| I-J-I | 32 | - | 8 | 8 | - | - | - | - | 16 | - | 16 | - | 16 | - | 8 | 10 | [22,28] |
| Peptide | Yeast | References | ||||
|---|---|---|---|---|---|---|
| C. albicans | C. glabrata | C. tropicalis | C. krusei | C. parapsilosis | ||
| Jelleine-I | 2.5–61 | 30 | 15 | 30 | 61 | [13,20] |
| Jelleine-II | 2.5 | - | - | - | - | [20] |
| Jelleine-III | I | - | - | - | - | [20] |
| Jelleine-IV | I | - | - | - | - | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, W.G.; Brito, J.C.M.; Verly, R.M.; Lima, M.E.d. Jelleine, a Family of Peptides Isolated from the Royal Jelly of the Honey Bees (Apis mellifera), as a Promising Prototype for New Medicines: A Narrative Review. Toxins 2024, 16, 24. https://doi.org/10.3390/toxins16010024
Lima WG, Brito JCM, Verly RM, Lima MEd. Jelleine, a Family of Peptides Isolated from the Royal Jelly of the Honey Bees (Apis mellifera), as a Promising Prototype for New Medicines: A Narrative Review. Toxins. 2024; 16(1):24. https://doi.org/10.3390/toxins16010024
Chicago/Turabian StyleLima, William Gustavo, Julio Cesar Moreira Brito, Rodrigo Moreira Verly, and Maria Elena de Lima. 2024. "Jelleine, a Family of Peptides Isolated from the Royal Jelly of the Honey Bees (Apis mellifera), as a Promising Prototype for New Medicines: A Narrative Review" Toxins 16, no. 1: 24. https://doi.org/10.3390/toxins16010024
APA StyleLima, W. G., Brito, J. C. M., Verly, R. M., & Lima, M. E. d. (2024). Jelleine, a Family of Peptides Isolated from the Royal Jelly of the Honey Bees (Apis mellifera), as a Promising Prototype for New Medicines: A Narrative Review. Toxins, 16(1), 24. https://doi.org/10.3390/toxins16010024






