Antifungal Activity of Ribosome-Inactivating Proteins
Abstract
:1. Introduction
2. Ribosome-Inactivating Proteins
| RIP | IC50 * (nM) | References | ||||||
|---|---|---|---|---|---|---|---|---|
| Cucurbitaceae | Brassicacea | Euphorbiaceae | Fabaceae | Phytolaccaceae | Poaceae | Solanaceae | ||
| AMARANTHACEAE | ||||||||
| Beta vulgaris L. | ||||||||
| BE27 | Yes ** (VS) | [30,34] | ||||||
| ASPARAGACEAE | ||||||||
| Agave tequilana F.A.C.Weber | ||||||||
| Mayahuelin | 10.43 (TA) | [35] | ||||||
| Asparagus officinalis L. | ||||||||
| Asparin 1 | 1333 (CM) | No * (VS) | [36,37] | |||||
| Muscari armeniacum H.J.Veitch | ||||||||
| Musarmins 1-2-3 | No * (VS) | No * (TA) | [38] | |||||
| CARYOPHYLLACEAE | ||||||||
| Dianthus caryophyllus L. | ||||||||
| Dianthin 30 | No * (CM) | [37] | ||||||
| Dianthin 32 | Yes ** (NT) | [39] | ||||||
| Saponaria officinalis L. | ||||||||
| Saporin-L1 | 26.7 (CM) 17.3 (CS) | 0.99 (VS) | 40 (TA) | [37,40] | ||||
| Saporin-L2 | 31 (CS) | 20.91 (VS) | 23.7 (TA) | [40] | ||||
| Saporin-R1 | 1105 (CS) | 0.22 (VS) | 582 (TA) | [40] | ||||
| Saporin-R2 | 55.7 (CS) | 0.97 (VS) | 3.1 (TA) | [40] | ||||
| Saporin-R3 | 959 (CS) | 0.02 (VS) | 176 (TA) | [40] | ||||
| Saporin-S5 | 10 (CM) 0.03 (CS) | 0.34–0.48 (VS) | 772 (TA) | [31,37,40,41] | ||||
| Saporin-S6 | 3606 (CS) | 0.31 (VS) | 32 (TA) | [40] | ||||
| Silene glaucifolia Lag. (=Petrocoptis glaucifolia Boiss.) | ||||||||
| Petroglaucin 1 | 219 (CS) | 49 (VS) | 30 (TA) | [42] | ||||
| Petroglaucin 2 | 27–29 (CS) | 0.2–6 (VS) 127 (VL) | 30–73 (TA) | [36,42,43] | ||||
| Silene laxipruinosa Mayol and Rosselló (=Petrocoptis grandiflora Rothm.) | ||||||||
| Petrograndin | 186 (CS) | 5 (VS) | 100(TA) | [42] | ||||
| CUCURBITACEAE | ||||||||
| Bryonia dioica Sessé and Moc. | ||||||||
| Bryodin | No * (CS) | [41] | ||||||
| EUPHORBIACEAE | ||||||||
| Ricinus communis L. | ||||||||
| Ricin | 1473 (CL) | 1470 (RC) | 923 (PS) | 1700 (PA) | 1313 (TA) 980 (HV) | Yes ** (NT) | [33,39,44] | |
| RCA | No * (CM) 3767 (CL) | 8167 (RC) | No * (VS) 7500 (PS) | 19,333 (TA) 7567 (HV) | [44,45] | |||
| FABACEAE | ||||||||
| Abrus precatorius L. | ||||||||
| APA | No * (CM) | No * (VS) | No * (TA) | [45] | ||||
| PHYTOLACCACEAE | ||||||||
| Phytolacca americana L | ||||||||
| PAP (PAP I) | 1.1–2.9 (PA) | 0.3 (TA) | Yes ** (NT) | [33,39] | ||||
| PAP II | 3.9 (PA) | [33] | ||||||
| PAP-S | 54–500 (CS) | 0.26–0.38 (VS) | 6.7 (PA) | 4.5 (TA) | [31,33,36,41,46] | |||
| Phytolacca dioica L. | ||||||||
| PD L4-S2, Dioicin 2 | Yes ** (VS) | [30] | ||||||
| Phytolacca dodecandra L’Hér. | ||||||||
| Dodecandrin | 0.8–3.1 (PD) | 0.2 (TA) | [33] | |||||
| POACEAE | ||||||||
| Hordeum vulgare L. | ||||||||
| Barley RIP 30 | No ** (NT) | [39] | ||||||
| Oryza sativa L. | ||||||||
| OsRIP1 | 1500 (TA) | [47] | ||||||
| Triticum aestivum L. | ||||||||
| Tritin (Tritin-S) | No ** (AT) | No ** (LJ) | No ** (TA) | No ** (NT) | [39,48] | |||
| Tritin-L | Yes ** (AT) | Yes ** (LJ) | Yes ** (TA) | Yes ** (NT) | [48] | |||
| Zea mays L. | ||||||||
| pro-RIP, αβ RIP | No ** (ZM) | [49] | ||||||
| SANTALACEAE | ||||||||
| Viscum album L. | ||||||||
| VAA | No * (CM) | No * (VS) | No * (TA) | [45] | ||||
| VIBURNACEAE | ||||||||
| Sambucus ebulus L. | ||||||||
| Ebulin f | No * (CS) | No * (VS) | No * (TA) | [50] | ||||
| Ebulin r1–r2 | No * (CM) | No * (VS) | No * (TA) | [50] | ||||
| α-β-γ-Ebulitin | No * (CM) | No * (VS) | No * (TA) | [50] | ||||
| Sambucus nigra L. | ||||||||
| Nigrin b | No * (CS) | No * (VS) | No * (TA) | [50] | ||||
| Nigrin f | No * (CS) | No * (VS) | No * (TA) | [50] | ||||
| basic Nigrin b | No * (TA) | [50] | ||||||
| Nigritin f1–f2 | No * (CS) | No * (VS) | No * (TA) | [50] | ||||
3. Mechanism of Ribosome Inactivation by RIPs
4. RIP-like Proteins and Ribotoxin-like Proteins
5. Endocytosis of Ribosome-Inactivating Proteins
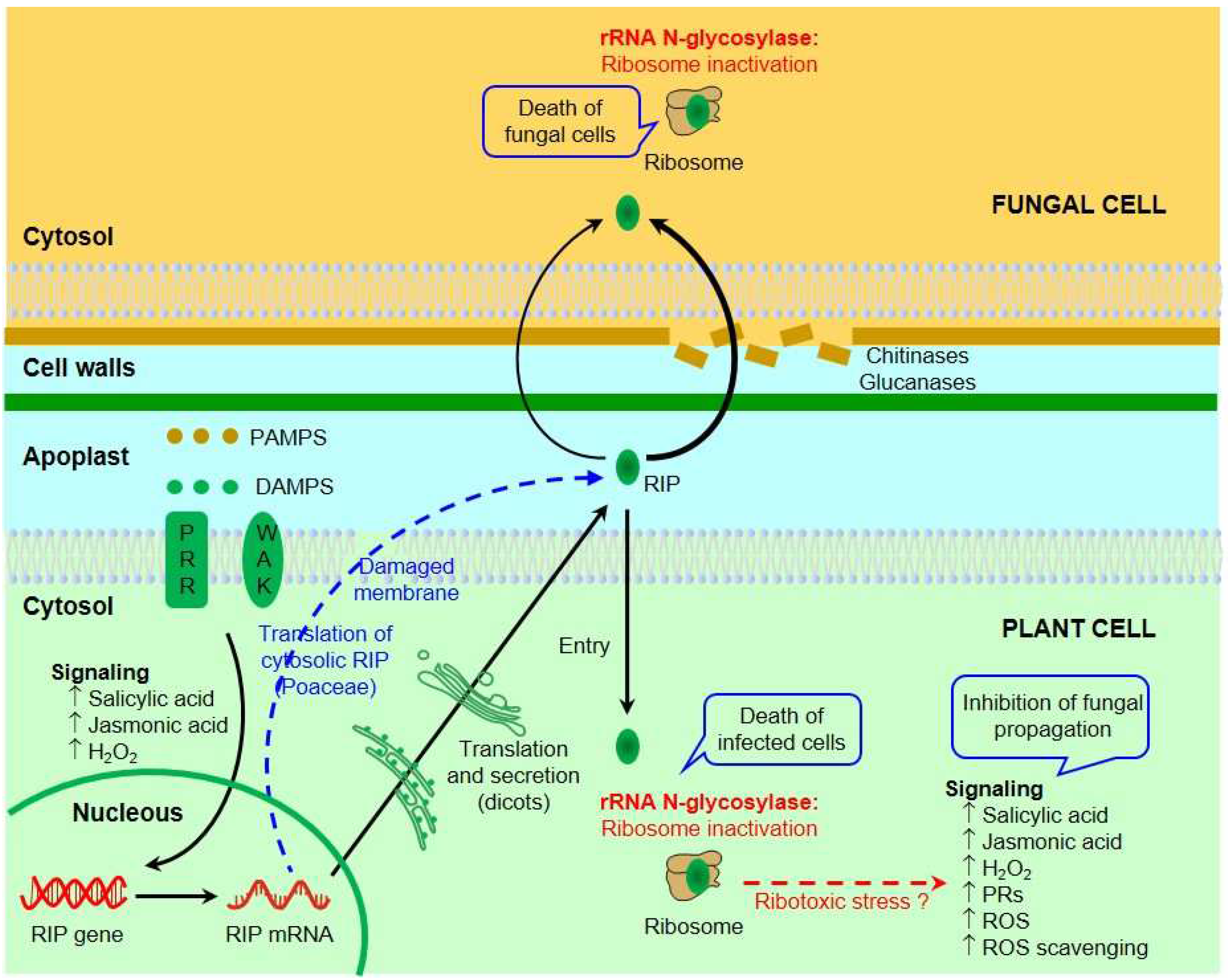
6. Inhibition of Fungal Growth by RIPs
| Order | Family | Species | References |
|---|---|---|---|
| Division Basidiomycota | |||
| CLASS AGARICOMYCETES | |||
| Cantharellales | Ceratobasidiaceae | Rhizoctonia solani J.G. Kühn | [28,76,78,79,80,81,82,83,84,85,86,87] |
| Polyporales | Polyporaceae | Ganoderma boninense Pat. | [88] |
| Agaricales | Agaricaceae | Coprinus comatus (O.F. Müll.) Pers. | [89,90] |
| Division Ascomycota | |||
| CLASS LEOTIOMYCETES | |||
| Helotiales | Erysiphaceae | Blumeria graminis (DC.) Speer | [91] |
| Sclerotiniaceae | Botrytis cinerea Pers. | [76,87,92] | |
| Clarireedia homoeocarpa (F.T. Benn.) L.A. Beirn, B.B. Clarke, C. Salgado and J.A. Crouch | [93] | ||
| Sclerotinia sclerotiorum (Lib.) de Bary | [94] | ||
| CLASS SORDARIOMYCETES | |||
| Amphisphaeriales | Pestalotiopsidaceae | Pestalotia sp. | [95] |
| Diaporthales | Cryphonectriaceae | Cryphonectria parasitica (Murrill) M.E. Barr | [96] |
| Valsaceae | Cytospora sp. * | [95] | |
| Glomerellales | Plectosphaerellaceae | Verticillium dahliae Kleb. | [27] |
| Hypocreales | Hypocreaceae | Trichoderma reesei E.G. Simmons | [27,95] |
| Trichoderma harzianum Rifai | [27] | ||
| Nectriaceae | Fusarium culmorum (Wm.G. Sm.) Sacc. | [97] | |
| Fusarium fujikuroi Nirenberg | [98,99] | ||
| Fusarium graminearum Schwabe | [94] | ||
| Fusarium oxysporum Schltdl. | [27,90,95,100,101,102] | ||
| F. proliferatum (Matsush.) Nirenberg ex Gerlach and Nirenberg | [27] | ||
| Fusarium sporotrichioides Sherb. | [76] | ||
| Magnaporthales | Pyriculariaceae | Pyricularia grisea Cooke ex Sacc. | [103] |
| Pyricularia oryzae Cavara | [104,105] | ||
| Sordariales | Sordariaceae | Neurospora crassa Shear and B.O. Dodge ** | [77] |
| Xylariales | Hyponectriaceae | Physalospora pyricola Nose | [89,90] |
| CLASS EUROTIOMYCETES | |||
| Eurotiales | Aspergillaceae | Aspergillus flavus Link | [94,106] |
| Aspergillus nidulans (Eidam) G. Winter | [106] | ||
| Aspergillus niger Tiegh. | [94,107] | ||
| Penicillium digitatum (Pers.) Sacc. | [29,30,108,109] | ||
| CLASS DOTHIDEOMYCETES | |||
| Pleosporales | Corynesporascaceae | Corynespora cassiicola (Berk. and M.A. Curtis) C.T. Wei | [110] |
| Didymellaceae | Didymella arachidicola (Khokhr.) Tomilin | [90,101] | |
| Phoma sp. | [95] | ||
| Pleosporaceae | Alternaria alternata (Fr.) Keissl. | [92] | |
| Alternaria brassicae (Berk.) Sacc. | [111] | ||
| Alternaria solani Sorauer | [27,102] | ||
| Cochliobolus heterostrophus (Drechsler) Drechsler | [94] | ||
| Species and RIP | Fungi | Ref. |
|---|---|---|
| POACEAE | ||
| Hordeum vulgare L. | ||
| Barley RIP30 | Botrytis cinerea, Fusarium sporotrichioides, Neurospora crassa *, Rhizoctonia solani, Trichoderma reesei | [76,77] |
| Zea mays L. | ||
| Maize b-32 (MOD1) | Aspergillus flavus, A. nidulans, R. solani | [80,106] |
| AMARANTHACEAE | ||
| Salsola soda L. | ||
| Sodin 5 | Penicillium digitatum | [109] |
| Chenopodium quinoa Willd. | ||
| Quinoin | Cryphonectria parasitica, P. digitatum | [96,109] |
| Beta vulgaris L. | ||
| BE27 | P. digitatum | [29,108] |
| PHYTOLACCACEAE | ||
| Phytolacca dioica L. | ||
| Dioicin 2 | P. digitatum | [30] |
| PD-S2 | P. digitatum | [30] |
| Phytolacca heterotepala H.Walter | ||
| PhRIP I | B. cinerea | [92] |
| NYCTAGINACEAE | ||
| Mirabilis expansa (Ruiz and Pav.) Standl. | ||
| ME1 and ME2 | Alternaria solani, Fusarium oxysporum, F. proliferatum, Globisporangium irregulare, Phytophthora drechsleri, R. solani, Trichoderma harzianum, T. reesei, Verticillium dahliae | [27,28] |
| CUCURBITACEAE | ||
| Momordica charantia L. | ||
| Alpha-momorcharin (α-MMC) | A. flavus, A. niger, Cochliobolus heterostrophus, Fusarium graminearum, F. oxysporum, F. solani, Pyricularia oryzae, Sclerotinia sclerotiorum | [94,100,105] |
| Momordica balsamina L. | ||
| MbRIP-1 | Aspergillus niger | [107] |
| Benincasa hispida Cogn. | ||
| Hispin | Coprinus comatus, Didymella arachidicola, F. oxysporum, Physalospora pyricola | [90] |
| SOLANACEAE | ||
| Nicotiana tabacum L. | ||
| TRIP | C. heterostrophus, Cytospora sp., F. oxysporum, Pestalotia sp., Phoma sp., T. reesei | [95] |
| ARECACEAE | ||
| Elaeis guineensis Jacq. | ||
| EgRIP-1a and EgRIP-1b | Ganoderma boninense | [88] |
| VIBURNACEAE | ||
| Sambucus ebulus L. | ||
| Pebulin | A. solani, F. oxysporum | [102] |
| RIP | Host | Pathogen | Ref. |
|---|---|---|---|
| Barley RIP30 | Nicotiana tabacum L. | Rhizoctonia solani | [78,79] |
| Triticum aestivum L. | Blumeria graminis | [91] | |
| Solanum tuberosum L. | R. solani | [86,112] | |
| Brassica juncea (L.) Czern. | Alternaria brassicae | [111] | |
| Vigna mungo (L.) Hepper | Corynespora cassiicola | [110] | |
| Maize b-32 | N. tabacum | R. solani | [80] |
| T. aestivum L | Fusarium culmorum | [97] | |
| Zea mays L. | Fusarium fujikuroi | [98] | |
| MOD1 | Oryza sativa L. | R. solani | [84] |
| Z. mays | F. fujikuroi | [99] | |
| PAP (PAP I) | N. tabacum | R. solani | [81,83] |
| PAPII | N. tabacum | R. solani | [82] |
| Agrostis stolonifera L. | Clarireedia homoeocarpa | [93] | |
| PhRIP I | N. tabacum | Botrytis cinerea, Alternaria alternata | [92] |
| S. tuberosum | B. cinerea, R. solani | [87] | |
| TCS | O. sativa | Pyricularia oryzae | [104] |
| α-MMC | O. sativa | Pyricularia grisea | [103] |
| Curcin 2 | N. tabacum | R. solani | [85] |
7. Mechanisms of Antifungal Activity
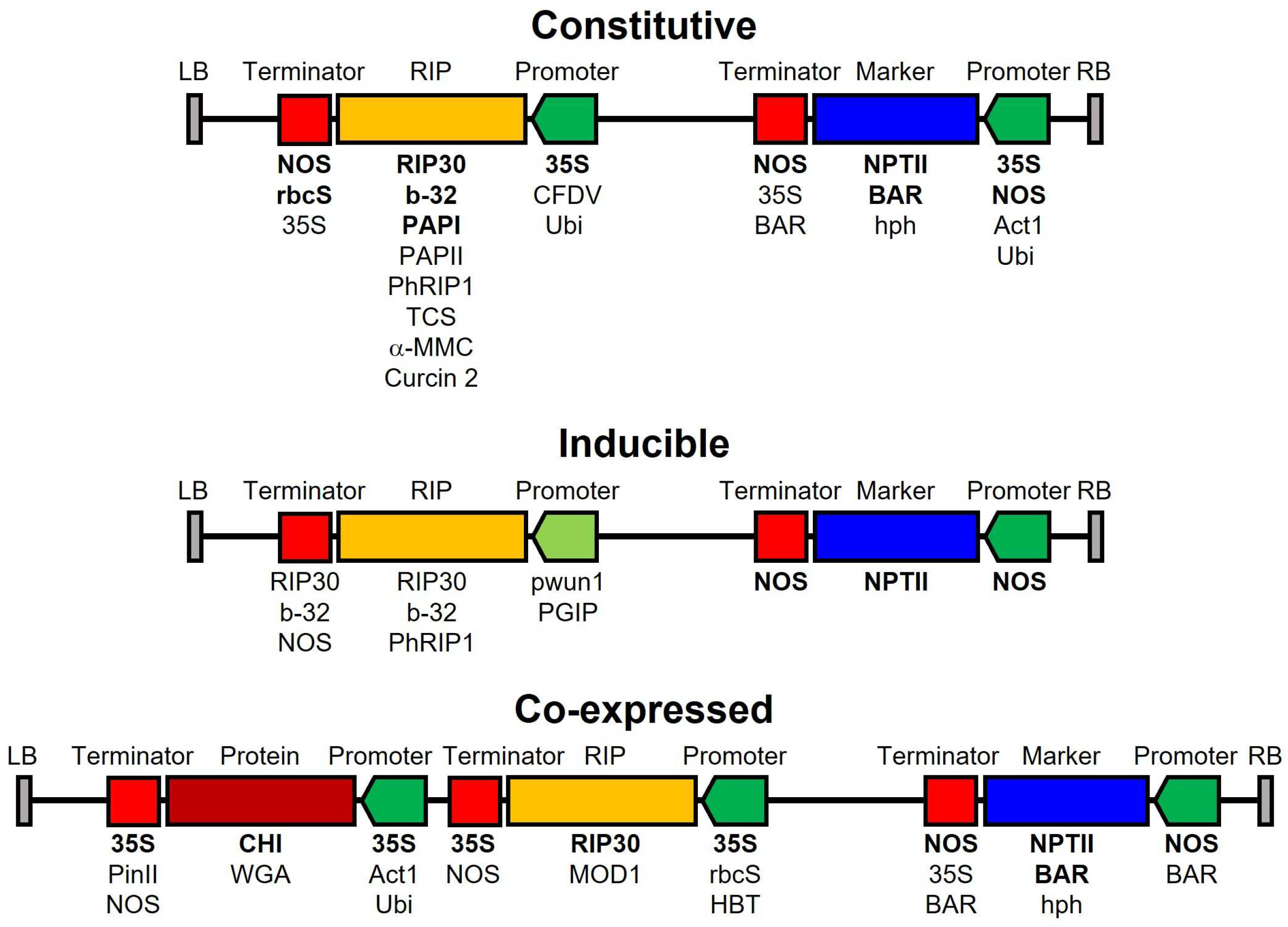
8. Transgenic Plants Resistant to Fungal Infection
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nazarov, P.; Baleev, D.; Ivanova, M.; Sokolova, L.; Karakozova, M. Infectious Plant Diseases: Etiology, Current Status, Problems and Prospects in Plant Protection. Acta Nat. 2020, 12, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant Pathogenic Fungi. Microbiol. Spectr. 2017, 5, 701–726. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Cha, B.; Kim, J. Recent Trends in Studies on Botanical Fungicides in Agriculture. Plant Pathol. J. 2013, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.; Poucet, T.; Li, Y. The potential of plant proteins as antifungal agents for agricultural applications. Synth. Syst. Biotechnol. 2022, 7, 1075–1083. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, X.; Han, X.; Fan, B. Application and Development of Biocontrol Agents in China. Pathogens 2022, 11, 1120. [Google Scholar] [CrossRef]
- Ng, T. Antifungal proteins and peptides of leguminous and non-leguminous origins. Peptides 2004, 25, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Ng, T.; Cheung, R.; Ye, X.; Wang, H.; Lam, S.; Lin, P.; Chan, Y.; Fang, E.; Ngai, P.; et al. Proteins with antifungal properties and other medicinal applications from plants and mushrooms. Appl. Microbiol. Biotechnol. 2010, 87, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhou, Y.K.; Ji, Z.L.; Chen, X.R. The Plant Ribosome-Inactivating Proteins Play Important Roles in Defense against Pathogens and Insect Pest Attacks. Front. Plant Sci. 2018, 9, 146. [Google Scholar] [CrossRef]
- Stirpe, F. Ribosome-inactivating proteins. Toxicon 2004, 44, 371–383. [Google Scholar] [CrossRef]
- Bertholdo-Vargas, L.; Martins, J.; Bordin, D.; Salvador, M.; Schafer, A.; de Barros, N.; Barbieri, L.; Stirpe, F.; Carlini, C. Type 1 ribosome-inactivating proteins-Entomotoxic, oxidative and genotoxic action on Anticarsia gemmatalis (Hubner) and Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). J. Insect Physiol. 2009, 55, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Citores, L.; Iglesias, R.; Ferreras, J.M. Antiviral Activity of Ribosome-Inactivating Proteins. Toxins 2021, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F. Ribosome-inactivating proteins: From toxins to useful proteins. Toxicon 2013, 67, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Battelli, M.G.; Stirpe, F. Ribosome-Inactivating Proteins from Plants. Biochim. Biophys. Acta 1993, 1154, 237–282. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Polito, L. Ribosome-Inactivating Proteins from Plants: A Historical Overview. Molecules 2016, 21, 19. [Google Scholar] [CrossRef]
- Stirpe, F.; Battelli, M. Ribosome-inactivating proteins: Progress and problems. Cell. Mol. Life Sci. 2006, 63, 1850–1866. [Google Scholar] [CrossRef]
- Peumans, W.; Hao, Q.; Van Damme, E. Ribosome-inactivating proteins from plants: More than RNA N-glycosidases? FASEB J. 2001, 15, 1493–1506. [Google Scholar] [CrossRef]
- Wang, P.; Tumer, N.; Maramorosch, K.; Murphy, F.; Shatkin, A. Virus resistance mediated by ribosome inactivating proteins. Adv. Virus Res. 2000, 55, 325–355. [Google Scholar] [CrossRef]
- Bolognesi, A.; Polito, L. Immunotoxins and other conjugates: Pre-clinical studies. Mini-Rev. Med. Chem. 2004, 4, 563–583. [Google Scholar] [CrossRef]
- Pastan, I.; Hassan, R.; FitzGerald, D.; Kreitman, R. Immunotoxin treatment of cancer. Annu. Rev. Med. 2007, 58, 221–237. [Google Scholar] [CrossRef]
- Becker, N.; Benhar, I. Antibody Based Immunotoxins for the Treatment of Cancer. Antibodies 2012, 1, 39–69. [Google Scholar] [CrossRef]
- Polito, L.; Djemil, A.; Bortolotti, M. Plant Toxin-Based Immunotoxins for Cancer Therapy: A Short Overview. Biomedicines 2016, 4, 12. [Google Scholar] [CrossRef]
- Dougherty, K.; Hudak, K. Phylogeny and domain architecture of plant ribosome inactivating proteins. Phytochemistry 2022, 202, 113337. [Google Scholar] [CrossRef]
- Liu, J.; Wen, D.; Song, X.; Su, P.; Lou, J.; Yao, D.; Zhang, C. Evolution and natural selection of ribosome-inactivating proteins in bacteria, fungi, and plants. Int. J. Biol. Macromol. 2023, 248, 125929. [Google Scholar] [CrossRef] [PubMed]
- Sandvig, K.; Bergan, J.; Dyve, A.; Skotland, T.; Torgersen, M. Endocytosis and retrograde transport of Shiga toxin. Toxicon 2010, 56, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Cenini, P.; Carnicelli, D.; Stirpe, F. Effect of Plant Ribosome-Inactivating Proteins on Ribosomes from Various Metazoan Species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1990, 96, 581–584. [Google Scholar] [CrossRef]
- Vivanco, J.M.; Savary, B.J.; Flores, H.E. Characterization of two novel type I ribosome-inactivating proteins from the storage roots of the Andean crop Mirabilis expansa. Plant Physiol. 1999, 119, 1447–1456. [Google Scholar] [CrossRef]
- Park, S.W.; Stevens, N.M.; Vivanco, J.M. Enzymatic specificity of three ribosome-inactivating proteins against fungal ribosomes, and correlation with antifungal activity. Planta 2002, 216, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Citores, L.; Iglesias, R.; Gay, C.; Miguel Ferreras, J. Antifungal activity of the ribosome-inactivating protein BE27 from sugar beet (Beta vulgaris L.) against the green mould Penicillium digitatum. Mol. Plant Pathol. 2016, 17, 261–271. [Google Scholar] [CrossRef]
- Iglesias, R.; Citores, L.; Ragucci, S.; Russo, R.; Di Maro, A.; Ferreras, J.M. Biological and antipathogenic activities of ribosome-inactivating proteins from Phytolacca dioica L. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 1256–1264. [Google Scholar] [CrossRef]
- Iglesias, R.; Arias, F.; Rojo, M.; Escarmis, C.; Ferreras, J.; Girbes, T. Molecular Action of the Type 1 Ribosome-Inactivating Protein Saporin 5 on Vicia Sativa Ribosomes. FEBS Lett. 1993, 325, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Vepachedu, R.; Sharma, N.; Vivanco, J.M. Ribosome-inactivating proteins in plant biology. Planta 2004, 219, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Bonness, M.S.; Ready, M.P.; Irvin, J.D.; Mabry, T.J. Pokeweed Antiviral Protein Inactivates Pokeweed Ribosomes-Implications for the Antiviral Mechanism. Plant J. 1994, 5, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, R.; Perez, Y.; de Torre, C.; Ferreras, J.; Antolin, P.; Jimenez, P.; Rojo, M.; Mendez, E.; Girbes, T. Molecular characterization and systemic induction of single-chain ribosome-inactivating proteins (RIPs) in sugar beet (Beta vulgaris) leaves. J. Exp. Bot. 2005, 56, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Lledías, F.; Gutiérrez, J.; Martínez-Hernández, A.; García-Mendoza, A.; Sosa, E.; Hernández-Bermúdez, F.; Dinkova, T.; Reyes, S.; Cassab, G.; Nieto-Sotelo, J. Mayahuelin, a Type I Ribosome Inactivating Protein: Characterization, Evolution, and Utilization in Phylogenetic Analyses of Agave. Front. Plant Sci. 2020, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Arias, F.; Rojo, M.; Iglesias, R.; Ferreras, J.; Girbes, T. Vicia sativa L. “Run-off” and Purified Ribosomes: Polyphenylalanine Synthesis and Molecular Action of Ribosome-Inactivating Proteins. J. Exp. Bot. 1993, 44, 1297–1304. [Google Scholar] [CrossRef]
- Rojo, M.; Arias, F.; Ferreras, J.; Iglesias, R.; Munoz, R.; Girbes, T. Development of a Cell-free Translation System from Cucumis melo: Preparation, Optimization and Evaluation of Sensitivity to some Translational Inhibitors. Plant Sci. 1993, 90, 127–134. [Google Scholar] [CrossRef]
- Arias, F.; Antolin, P.; de Torre, C.; Barriuso, B.; Iglesias, R.; Rojo, M.; Ferreras, J.; Benvenuto, E.; Mendez, E.; Girbes, T. Musarmins: Three single-chain ribosome-inactivating protein isoforms from bulbs of Muscari armeniacum L. and Miller. Int. J. Biochem. Cell Biol. 2003, 35, 61–78. [Google Scholar] [CrossRef]
- Taylor, S.; Massiah, A.; Lomonossoff, G.; Roberts, L.M.; Lord, J.M.; Hartley, M. Correlation between the Activities of 5 Ribosome-Inactivating Proteins in Depurination of Tobacco Ribosomes and Inhibition of Tobacco Mosaic Virus Infection. Plant J. 1994, 5, 827–835. [Google Scholar] [CrossRef]
- Ferreras, J.; Barbieri, L.; Girbes, T.; Battelli, M.; Rojo, M.; Arias, F.; Rocher, M.; Soriano, F.; Mendez, E.; Stirpe, F. Distribution and Properties of Major Ribosome-Inactivating Proteins (28 S rRNA N-Glycosidases) of the Plant Saponaria officinalis L. (Caryophyllaceae). Biochim. Biophys. Acta 1993, 1216, 31–42. [Google Scholar] [CrossRef]
- Rojo, M.; Arias, F.; Iglesias, R.; Ferreras, J.; Munoz, R.; Girbes, T. A Cucumis Sativus Cell-free Translation System: Preparation, Optimization and Sensitivity to some Antibiotics and Ribosome-Inactivating Proteins. Physiol. Plant. 1993, 88, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Arias, F.; Rojo, M.; Ferreras, J.; Iglesias, R.; Munoz, R.; Soriano, F.; Mendez, E.; Barbieri, L.; Girbes, T. Isolation and Characterization of Two New N-Glycosidase Type 1 Ribosome-Inactivating Proteins, Unrelated in Amino Acid Sequence, from Petrocoptis Species. Planta 1994, 194, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Arias, F.; Rojo, M.; Ferreras, J.; Iglesias, R.; Munoz, R.; Rocher, A.; Mendez, E.; Barbieri, L.; Girbes, T. Isolation and Partial Characterization of a New Ribosome-Inactivating Protein from Petrocoptis glaucifolia (Lag.) Boiss. Planta 1992, 186, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Harley, S.M.; Beevers, H. Ricin Inhibition of In Vitro Protein Synthesis by Plant Ribosomes. Proc. Natl. Acad. Sci. USA 1982, 79, 5935–5938. [Google Scholar] [CrossRef] [PubMed]
- Citores, L.; Ferreras, J.; Iglesias, R.; Carbajales, M.; Arias, F.; Jimenez, P.; Rojo, M.; Girbes, T. Molecular mechanism of inhibition of mammalian protein synthesis by some four-chain agglutinins. Proposal of an extended classification of plant ribosome-inactivating proteins (rRNA N-glycosidases). FEBS Lett. 1993, 329, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Arias, F.; Rojo, M.; Ferreras, J.; Iglesias, R.; Munoz, R.; Girbes, T. Preparation and Optimization of a Cell-free Translation System from Vicia sativa Germ Lacking Ribosome-Inactivating Protein Activity. J. Exp. Bot. 1992, 43, 729–737. [Google Scholar] [CrossRef]
- De Zaeytijd, J.; Rouge, P.; Smagghe, G.; Van Damme, E.J.M. Structure and Activity of a Cytosolic Ribosome-Inactivating Protein from Rice. Toxins 2019, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Massiah, A.J.; Hartley, M.R. Wheat Ribosome-Inactivating Proteins—Seed and Leaf Forms with Different Specificities and Cofactor Requirements. Planta 1995, 197, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Hey, T.; Hartley, M.; Walsh, T. Maize ribosome-inactivating protein (b-32)-homologs in related species, effects on maize ribosomes, and modulation of activity by pro-peptide deletions. Plant Physiol. 1995, 107, 1323–1332. [Google Scholar] [CrossRef]
- Ferreras, J.; Citores, L.; Martinez de Benito, F.; Arias, F.; Rojo, M.; Munoz, R.; Iglesias, R.; Girbes, T. Ribosome-inactivating proteins and lectins from Sambucus. Curr. Top. Phytochem. 2000, 3, 113–128. [Google Scholar]
- Endo, Y.; Mitsui, K.; Motizuki, M.; Tsurugi, K. The Mechanism of Action of Ricin and Related Toxic Lectins on Eukaryotic Ribosomes—The Site and the Characteristics of the Modification in 28-S Ribosomal-RNA Caused by the Toxins. J. Biol. Chem. 1987, 262, 5908–5912. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Tsurugi, K. The RNA N-glycosidase activity of ricin A-chain. The characteristics of the enzymatic activity of ricin A-chain with ribosomes and with rRNA. J. Biol. Chem. 1988, 263, 8735–8739. [Google Scholar] [CrossRef]
- Maracci, C.; Rodnina, M. Translational GTPases. Biopolymers 2016, 105, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Grela, P.; Szajwaj, M.; Horbowicz-Drozdzal, P.; Tchorzewski, M. How Ricin Damages the Ribosome. Toxins 2019, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Wong, E.; Lee, K.; Wong, K. Structures of Eukaryotic Ribosomal Stalk Proteins and Its Complex with Trichosanthin, and Their Implications in Recruiting Ribosome-Inactivating Proteins to the Ribosomes. Toxins 2015, 7, 638–647. [Google Scholar] [CrossRef]
- Lacadena, J.; Alvarez-García, E.; Carreras-Sangrà, N.; Herrero-Galán, E.; Alegre-Cebollada, J.; García-Ortega, L.; Oñaderra, M.; Gavilanes, J.; del Pozo, A. Fungal ribotoxins: Molecular dissection of a family of natural killers. FEMS Microbiol. Rev. 2007, 31, 212–237. [Google Scholar] [CrossRef]
- Olombrada, M.; Lazaro-Gorines, R.; Lopez-Rodriguez, J.; Martinez-del-Pozo, A.; Onaderra, M.; Maestro-Lopez, M.; Lacadena, J.; Gavilanes, J.; Garcia-Ortega, L. Fungal Ribotoxins: A Review of Potential Biotechnological Applications. Toxins 2017, 9, 71. [Google Scholar] [CrossRef]
- Endo, Y.; Wool, I.G. The site of action of alpha-sarcin on eukaryotic ribosomes—The sequence at the alpha-sarcin cleavage site in 28 S-ribosomal ribonucleic-acid. J. Biol. Chem. 1982, 257, 9054–9060. [Google Scholar] [CrossRef] [PubMed]
- García-Ortega, L.; Alvarez-García, E.; Gavilanes, J.; Martínez-del-Pozo, A.; Joseph, S. Cleavage of the sarcin-ricin loop of 23S rRNA differentially affects EF-G and EF-Tu binding. Nucleic Acids Res. 2010, 38, 4108–4119. [Google Scholar] [CrossRef]
- Barbieri, L.; Valbonesi, P.; Bonora, E.; Gorini, P.; Bolognesi, A.; Stirpe, F. Polynucleotide:adenosine glycosidase activity of ribosome-inactivating proteins: Effect on DNA, RNA and poly(A). Nucleic Acids Res. 1997, 25, 518–522. [Google Scholar] [CrossRef]
- Schrot, J.; Weng, A.; Melzig, M. Ribosome-Inactivating and Related Proteins. Toxins 2015, 7, 1556–1615. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Hussain, H.Z.F.; Pedone, P.V.; Ragucci, S.; Di Maro, A. Ribotoxic Proteins, Known as Inhibitors of Protein Synthesis, from Mushrooms and Other Fungi According to Endo’s Fragment Detection. Toxins 2022, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Citores, L.; Ragucci, S.; Russo, R.; Gay, C.; Chambery, A.; Di Maro, A.; Iglesias, R.; Ferreras, J. Structural and functional characterization of the cytotoxic protein ledodin, an atypical ribosome-inactivating protein from shiitake mushroom (Lentinula edodes). Protein Sci. 2023, 32, e4621. [Google Scholar] [CrossRef] [PubMed]
- Ragucci, S.; Landi, N.; Russo, R.; Valletta, M.; Pedone, P.; Chambery, A.; Di Maro, A. Ageritin from Pioppino Mushroom: The Prototype of Ribotoxin-Like Proteins, a Novel Family of Specific Ribonucleases in Edible Mushrooms. Toxins 2021, 13, 263. [Google Scholar] [CrossRef] [PubMed]
- Spooner, R.; Lord, J. Ricin Trafficking in Cells. Toxins 2015, 7, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, M.; Katayama, M.; Nakase, I.; Vago, R. Plant Ribosome-Inactivating Proteins: Progesses, Challenges and Biotechnological Applications (and a Few Digressions). Toxins 2017, 9, 314. [Google Scholar] [CrossRef]
- Qin, S.; Wang, X.; Han, P.; Lai, Z.; Ren, Y.; Ma, R.; Cheng, C.; Wang, T.; Xu, Y. LRP1-Mediated Endocytosis May Be the Main Reason for the Difference in Cytotoxicity of Curcin and Curcin C on U2OS Osteosarcoma Cells. Toxins 2022, 14, 771. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Polito, L.; Scicchitano, V.; Orrico, C.; Pasquinelli, G.; Musiani, S.; Santi, S.; Riccio, M.; Bortolotti, M.; Battelli, M. Endocytosis and intracellular localisation of type 1 ribosome-inactivating protein saporin-S6. J. Biol. Regul. Homeost. Agents 2012, 26, 97–109. [Google Scholar] [PubMed]
- Walsh, M.; Dodd, J.; Hautbergue, G. Ribosome-inactivating proteins Potent poisons and molecular tools. Virulence 2013, 4, 774–784. [Google Scholar] [CrossRef]
- Goode, B.; Eskin, J.; Wendland, B. Actin and Endocytosis in Budding Yeast. Genetics 2015, 199, 315–358. [Google Scholar] [CrossRef]
- Commer, B.; Shaw, B.D. Current views on endocytosis in filamentous fungi. Mycology 2020, 12, 1–9. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Siavoshi, F.; Jazayeri, M.; Sarrafnejad, A.; Saniee, P.; Mobini, M. Physicochemical properties of intact fungal cell wall determine vesicles release and nanoparticles internalization. Heliyon 2023, 9, e13834. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.; Latge, J.; Munro, C. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, L.; Polito, L.; Bolognesi, A.; Ciani, M.; Pelosi, E.; Farini, V.; Jha, A.; Sharma, N.; Vivanco, J.; Chambery, A.; et al. Ribosome-inactivating proteins in edible plants and purification and characterization of a new ribosome-inactivating protein from Cucurbita moschata. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Hohl, T.; Feldmesser, M. Aspergillus fumigatus: Principles of pathogenesis and host defense. Eukaryot. Cell 2007, 6, 1953–1963. [Google Scholar] [CrossRef] [PubMed]
- Leah, R.; Tommerup, H.; Svendsen, I.; Mundy, J. Biochemical and molecular characterization of 3 barley seed proteins with antifungal properties. J. Biol. Chem. 1991, 266, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.; Selitrennikoff, C. Isolation and partial characterization of 2 antifungal proteins from barley. Biochim. Biophys. Acta 1986, 880, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Logemann, J.; Jach, G.; Tommerup, H.; Mundy, J.; Schell, J. Expression of a barley ribosome-inactivating protein leads to increased fungal protection in transgenic tobacco plants. Bio-Technology 1992, 10, 305–308. [Google Scholar] [CrossRef]
- Jach, G.; Gornhardt, B.; Mundy, J.; Logemann, J.; Pinsdorf, P.; Leah, R.; Schell, J.; Maas, C. Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J. 1995, 8, 97–109. [Google Scholar] [CrossRef]
- Maddaloni, M.; Forlani, F.; Balmas, V.; Donini, G.; Stasse, L.; Corazza, L.; Motto, M. Tolerance to the fungal pathogen Rhizoctonia solani AG4 of transgenic tobacco expressing the maize ribosome-inactivating protein b-32. Transgenic Res. 1997, 6, 393–402. [Google Scholar] [CrossRef]
- Zoubenko, O.; Uckun, F.; Hur, Y.; Chet, I.; Tumer, N. Plant resistance to fungal infection induced by nontoxic pokeweed antiviral protein mutants. Nat. Biotechnol. 1997, 15, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.G.; Zoubenko, O.; Tumer, N.E. Reduced toxicity and broad spectrum resistance to viral and fungal infection in transgenic plants expressing pokeweed antiviral protein II. Plant Mol. Biol. 1998, 38, 957–964. [Google Scholar] [CrossRef]
- Zoubenko, O.; Hudak, K.; Tumer, N.E. A non-toxic pokeweed antiviral protein mutant inhibits pathogen infection via a novel salicylic acid-independent pathway. Plant Mol. Biol. 2000, 44, 219–229. [Google Scholar] [CrossRef]
- Kim, J.; Jang, I.; Wu, R.; Zu, W.; Boston, R.; Lee, Y.; Alm, P.; Nahm, B. Co-expression of a modified maize ribosome-inactivating protein and a rice basic chitinase gene in transgenic rice plants confers enhanced resistance to sheath blight. Transgenic Res. 2003, 12, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.-X.; Hou, P.; Wei, Q.; Xu, Y.; Chen, F. A ribosome-inactivating protein (curcin 2) induced from Jatropha curcas can reduce viral and fungal infection in transgenic tobacco. Plant Growth Regul. 2008, 54, 115–123. [Google Scholar] [CrossRef]
- M’hamdi, M.; Chikh-Rouhou, H.; Boughalleb, N.; de Galarreta, J. Enhanced resistance to Rhizoctonia solani by combined expression of chitinase and Ribosome Inactivating Protein in transgenic potatoes (Solanum tuberosum L.). Span. J. Agric. Res. 2012, 10, 778–785. [Google Scholar] [CrossRef]
- Gonzales-Salazar, R.; Cecere, B.; Ruocco, M.; Rao, R.; Corrado, G. A comparison between constitutive and inducible transgenic expression of the PhRIP I gene for broad-spectrum resistance against phytopathogens in potato. Biotechnol. Lett. 2017, 39, 1049–1058. [Google Scholar] [CrossRef]
- Sargolzaei, M.; Ho, C.; Wong, M. Characterization of novel type I ribosome-inactivating proteins isolated from oil palm (Elaeis guineensis) inoculated with Ganoderma boninense, the causal agent of basal stem rot. Physiol. Mol. Plant Pathol. 2016, 94, 53–61. [Google Scholar] [CrossRef]
- Ng, T.; Parkash, A.; Tso, W. Purification and characterization of α- and β-benincasins, arginine/glutamate-rich peptides with translation-inhibiting activity from wax gourd seeds. Peptides 2003, 24, 11–16. [Google Scholar] [CrossRef]
- Ng, T.; Parkash, A. Hispin, a novel ribosome inactivating protein with antifungal activity from hairy melon seeds. Protein Expr. Purif. 2002, 26, 211–217. [Google Scholar] [CrossRef]
- Bieri, S.; Potrykus, I.; Fütterer, J. Expression of active barley seed ribosome-inactivating protein in transgenic wheat. Theor. Appl. Genet. 2000, 100, 755–763. [Google Scholar] [CrossRef]
- Corrado, G.; Bovi, P.; Ciliento, R.; Gaudio, L.; Di Maro, A.; Aceto, S.; Lorito, M.; Rao, R. Inducible expression of a Phytolacca heterotepala ribosome-inactivating protein leads to enhanced resistance against major fungal pathogens in tobacco. Phytopathology 2005, 95, 206–215. [Google Scholar] [CrossRef]
- Dai, W.; Bonos, S.; Guo, Z.; Meyer, W.; Day, P.; Belanger, F. Expression of pokeweed antiviral proteins in creeping bentgrass. Plant Cell Rep. 2003, 21, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhang, P.; Meng, Y.F.; Xu, F.; Zhang, D.W.; Cheng, J.; Lin, H.H.; Xi, D.H. Alpha-momorcharin, a RIP produced by bitter melon, enhances defense response in tobacco plants against diverse plant viruses and shows antifungal activity in vitro. Planta 2013, 237, 77–88. [Google Scholar] [CrossRef]
- Sharma, N.; Park, S.; Vepachedu, R.; Barbieri, L.; Ciani, M.; Stirpe, F.; Savary, B.; Vivanco, J. Isolation and characterization of an RIP (ribosome-inactivating protein)-like protein from tobacco with dual enzymatic activity. Plant Physiol. 2004, 134, 171–181. [Google Scholar] [CrossRef]
- Ragucci, S.; Bulgari, D.; Landi, N.; Russo, R.; Clemente, A.; Valletta, M.; Chambery, A.; Gobbi, E.; Faoro, F.; Di Maro, A. The Structural Characterization and Antipathogenic Activities of Quinoin, a Type 1 Ribosome-Inactivating Protein from Quinoa Seeds. Int. J. Mol. Sci. 2021, 22, 8964. [Google Scholar] [CrossRef] [PubMed]
- Balconi, C.; Lanzanova, C.; Conti, E.; Triulzi, T.; Forlani, F.; Cattaneo, M.; Lupotto, E. Fusarium head blight evaluation in wheat transgenic plants expressing the maize b-32 antifungal gene. Eur. J. Plant Pathol. 2007, 117, 129–140. [Google Scholar] [CrossRef]
- Lanzanova, C.; Giuffrida, M.; Motto, M.; Baro, C.; Donn, G.; Hartings, H.; Lupotto, E.; Careri, M.; Elviri, L.; Balconi, C. The Zea mays b-32 ribosome-inactivating protein efficiently inhibits growth of Fusarium verticillioides on leaf pieces in vitro. Eur. J. Plant Pathol. 2009, 124, 471–482. [Google Scholar] [CrossRef]
- Dowd, P.; Johnson, E.; Price, N. Enhanced Pest Resistance of Maize Leaves Expressing Monocot Crop Plant-Derived Ribosome-Inactivating Protein and Agglutinin. J. Agric. Food Chem. 2012, 60, 10768–10775. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Liu, H.; He, Y.; Yan, J.; Wu, Z.; Ding, Y. Molecular cloning and functional analysis of a recombinant ribosome-inactivating protein (alpha-momorcharin) from Momordica charantia. Appl. Microbiol. Biotechnol. 2012, 96, 939–950. [Google Scholar] [CrossRef]
- Parkash, A.; Ng, T.; Tso, W. Isolation and characterization of luffacylin, a ribosome inactivating peptide with anti-fungal activity from sponge gourd (Luffa cylindrica) seeds. Peptides 2002, 23, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Rezaei-Moshaei, M.; Dehestani, A.; Bandehagh, A.; Pakdin-Parizi, A.; Golkar, M.; Heidari-Japelaghi, R. Recombinant pebulin protein, a type 2 ribosome-inactivating protein isolated from dwarf elder (Sambucus ebulus L.) shows anticancer and antifungal activities in vitro. Int. J. Biol. Macromol. 2021, 174, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Huang, L.; Yi, R.; Wang, S.; Ding, Y. Enhanced resistance to blast fungus in rice (Oryza sativa L.) by expressing the ribosome-inactivating protein alpha-momorcharin. Plant Sci. 2014, 217, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ming, X.; Wang, L.; Hu, P.; An, C.; Chen, Z. Expression of a gene encoding trichosanthin in transgenic rice plants enhances resistance to fungus blast disease. Plant Cell Rep. 2002, 20, 992–998. [Google Scholar] [CrossRef]
- Nguyen, T.; Dang, D. Molecular cloning and isolation of a recombinant alpha-Momorcharin in E. coli against Pyricularia oryzae. Sci. Technol. Dev. J. 2023, 26, 2665–2671. [Google Scholar] [CrossRef]
- Nielsen, K.; Payne, G.; Boston, R. Maize ribosome-inactivating protein inhibits normal development of Aspergillus nidulans and Aspergillus flavus. Mol. Plant Microbe Interact. 2001, 14, 164–172. [Google Scholar] [CrossRef]
- Kushwaha, G.; Pandey, N.; Sinha, M.; Singh, S.; Kaur, P.; Sharma, S.; Singh, T. Crystal structures of a type-1 ribosome inactivating protein from Momordica balsamina in the bound and unbound states. Biochim. Biophys. Acta Proteins Proteom. 2012, 1824, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Citores, L.; Valletta, M.; Singh, V.; Pedone, P.; Iglesias, R.; Ferreras, J.; Chambery, A.; Russo, R. Deciphering Molecular Determinants Underlying Penicillium digitatum’s Response to Biological and Chemical Antifungal Agents by Tandem Mass Tag (TMT)-Based High-Resolution LC-MS/MS. Int. J. Mol. Sci. 2022, 23, 680. [Google Scholar] [CrossRef]
- Landi, N.; Ragucci, S.; Citores, L.; Clemente, A.; Hussain, H.; Iglesias, R.; Ferreras, J.; Di Maro, A. Isolation, Characterization and Biological Action of Type-1 Ribosome-Inactivating Proteins from Tissues of Salsola soda L. Toxins 2022, 14, 566. [Google Scholar] [CrossRef]
- Chopra, R.; Saini, R. Transformation of Blackgram (Vigna mungo (L.) Hepper) by Barley Chitinase and Ribosome-Inactivating Protein Genes Towards Improving Resistance to Corynespora Leaf Spot Fungal Disease. Appl. Biochem. Biotechnol. 2014, 174, 2791–2800. [Google Scholar] [CrossRef]
- Chhikara, S.; Chaudhury, D.; Dhankher, O.; Jaiwal, P. Combined expression of a barley class II chitinase and type I ribosome inactivating protein in transgenic Brassica juncea provides protection against Alternaria brassicae. Plant Cell Tissue Organ Cult. 2012, 108, 83–89. [Google Scholar] [CrossRef]
- M’hamdi, M.; Chikh-Rouhou, H.; Boughalleb, N.; de Galarreta, J. Ribosome Inactivating Protein of barley enhanced resistance to Rhizoctonia solani in transgenic potato cultivar ‘Desiree’ in greenhouse conditions. Biotechnol. Agron. Soc. Environ. 2013, 17, 20–26. [Google Scholar]
- Parente, A.; Chambery, A.; Di Maro, A.; Russo, R.; Severino, V. Ribosome-inactivating Proteins from Phytolaccaceae. In Ribosome-Inactivating Proteins: Ricin and Related Proteins; Stirpe, F., Lappi, D.A., Eds.; Wiley: Ames, IA, USA, 2014; pp. 28–43. [Google Scholar] [CrossRef]
- Citores, L.; Ragucci, S.; Ferreras, J.M.; Di Maro, A.; Iglesias, R. Ageritin, a Ribotoxin from Poplar Mushroom (Agrocybe aegerita) with Defensive and Antiproliferative Activities. ACS Chem. Biol. 2019, 14, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Ragucci, S.; Castaldi, S.; Landi, N.; Isticato, R.; Di Maro, A. Antifungal Activity of Ageritin, a Ribotoxin-like Protein from Cyclocybe aegerita Edible Mushroom, against Phytopathogenic Fungi. Toxins 2023, 15, 578. [Google Scholar] [CrossRef] [PubMed]
- Ragucci, S.; Landi, N.; Citores, L.; Iglesias, R.; Russo, R.; Clemente, A.; Saviano, M.; Pedone, P.; Chambery, A.; Ferreras, J.; et al. The Biological Action and Structural Characterization of Eryngitin 3 and 4, Ribotoxin-like Proteins from Pleurotus eryngii Fruiting Bodies. Int. J. Mol. Sci. 2023, 24, 14435. [Google Scholar] [CrossRef] [PubMed]
- Ready, M.; Brown, D.; Robertus, J. Extracellular localization of Pokeweed Antiviral Protein. Proc. Natl. Acad. Sci. USA 1986, 83, 5053–5056. [Google Scholar] [CrossRef] [PubMed]
- De Zaeytijd, J.; Van Damme, E. Extensive Evolution of Cereal Ribosome-Inactivating Proteins Translates into Unique Structural Features, Activation Mechanisms, and Physiological Roles. Toxins 2017, 9, 123. [Google Scholar] [CrossRef]
- Marshall, R.; D’Avila, F.; Di Cola, A.; Traini, R.; Spanò, L.; Fabbrini, M.; Ceriotti, A. Signal peptide-regulated toxicity of a plant ribosome-inactivating protein during cell stress. Plant J. 2011, 65, 218–229. [Google Scholar] [CrossRef]
- Kaur, S.; Samota, M.; Choudhary, M.; Pandey, A.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Yang, T.; Meng, Y.; Chen, L.J.; Lin, H.-H.; Xi, D.-H. The Roles of Alpha-Momorcharinand Jasmonic Acid in Modulating the Response of Momordica charantia to Cucumber Mosaic Virus. Front. Microbiol. 2016, 7, 1796. [Google Scholar] [CrossRef]
- Qin, X.; Shao, C.; Hou, P.; Gao, J.; Lei, N.; Jiang, L.; Ye, S.; Gou, C.; Luo, S.; Zheng, X.; et al. Different functions and expression profiles of curcin and curcin-L in Jatropha curcas L. Z. Naturforsch. C J. Biosci. 2010, 65, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.; Schekman, R. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhu, P.X.; Xu, F.; Che, Y.P.; Ma, Y.M.; Ji, Z.L. Alpha-momorcharin enhances Nicotiana benthamiana resistance to tobacco mosaic virus infection through modulation of reactive oxygen species. Mol. Plant Pathol. 2020, 21, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhu, L.-S.; Meng, Y.; Lv, R.; Zhou, Z.; Zhu, L.; Lin, H.-h.; Xi, D.-h. Alpha-momorcharin enhances Tobacco mosaic virus resistance in tobacco(NN) by manipulating jasmonic acid-salicylic acid crosstalk. J. Plant Physiol. 2018, 223, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, S.; Shulaev, V.; Tumer, N.E. Expression of pokeweed antiviral protein in transgenic plants induces virus resistance in grafted wild-type plants independently of salicylic acid accumulation and pathogenesis-related protein synthesis. Plant Physiol. 1997, 114, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Vind, A.C.; Genzor, A.V.; Bekker-Jensen, S. Ribosomal stress-surveillance: Three pathways is a magic number. Nucleic Acids Res. 2020, 48, 10648–10661. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, C.; Doohan, F.M. Trichothecene toxicity in eukaryotes: Cellular and molecular mechanisms in plants and animals. Toxicol. Lett. 2013, 217, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Moosa, A.; Farzand, A.; Sahi, S.; Khan, S. Transgenic expression of antifungal pathogenesis-related proteins against phytopathogenic fungi-15 years of success. Isr. J. Plant Sci. 2018, 65, 38–54. [Google Scholar] [CrossRef]
- Collinge, D.; Sarrocco, S. Transgenic approaches for plant disease control: Status and prospects 2021. Plant Pathol. 2022, 71, 207–225. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, R.; Citores, L.; Gay, C.C.; Ferreras, J.M. Antifungal Activity of Ribosome-Inactivating Proteins. Toxins 2024, 16, 192. https://doi.org/10.3390/toxins16040192
Iglesias R, Citores L, Gay CC, Ferreras JM. Antifungal Activity of Ribosome-Inactivating Proteins. Toxins. 2024; 16(4):192. https://doi.org/10.3390/toxins16040192
Chicago/Turabian StyleIglesias, Rosario, Lucía Citores, Claudia C. Gay, and José M. Ferreras. 2024. "Antifungal Activity of Ribosome-Inactivating Proteins" Toxins 16, no. 4: 192. https://doi.org/10.3390/toxins16040192
APA StyleIglesias, R., Citores, L., Gay, C. C., & Ferreras, J. M. (2024). Antifungal Activity of Ribosome-Inactivating Proteins. Toxins, 16(4), 192. https://doi.org/10.3390/toxins16040192







