In Vitro Digestion and Intestinal Absorption of Mycotoxins Due to Exposure from Breakfast Cereals: Implications for Children’s Health
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Performance
2.2. Mycotoxins Bioaccessibility
2.3. Cell Monolayer Integrity Control
2.4. Intestinal In Vitro Absorption Assays
3. Conclusions
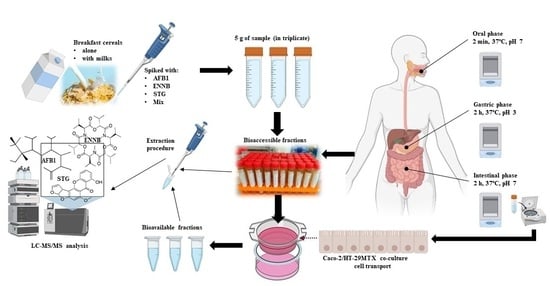
4. Materials and Methods
4.1. Reagents and Materials
4.2. Cell Culture
4.3. Spiking Samples
4.4. In Vitro Digestion
4.5. Cytotoxic Assay
4.6. Transport Assay
4.7. Mycotoxins Extraction and Cleanup
4.8. Instrument and Analytical Conditions
4.9. Method Validation and Quality Control
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diana Di Mavungu, J.; Monbaliu, S.; Scippo, M.-L.; Maghuin-Rogister, G.; Schneider, Y.-J.; Larondelle, Y.; Callebaut, A.; Robbens, J.; Van Peteghem, C.; De Saeger, S. LC-MS/MS multi-analyte method for mycotoxin determination in food supplements. Food Addit. Contam. 2009, 26, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Sá, S.V.d.; Monteiro, C.; Fernandes, J.O.; Pinto, E.; Faria, M.A.; Cunha, S.C. Emerging mycotoxins in infant and children foods: A review. Crit. Rev. Food Sci. Nutr. 2021, 63, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, e06040. [Google Scholar]
- International Agency for Research on Cancer, IARC. 2006. Available online: http://www.iarc.fr (accessed on 11 March 2024).
- HMDB. Human Metabolome Database. Available online: https://hmdb.ca/metabolites (accessed on 11 March 2024).
- Vaclavikova, M.; Malachova, A.; Veprikova, Z.; Dzuman, Z.; Zachariasova, M.; Hajslova, J. ‘Emerging’mycotoxins in cereals processing chains: Changes of enniatins during beer and bread making. Food Chem. 2013, 136, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, O.A.; De Boevre, M.; Dellafiora, L.; De Saeger, S.; Moretti, A.; Pinson-Gadais, L.; Ponts, N.; Richard-Forget, F.; Susca, A.; Dall’Asta, C. The Occurrence of Non-Regulated Mycotoxins in Foods: A Systematic Review. Toxins 2023, 15, 583. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Tenore, G.C.; Manyes, L.; Meca, G.; Ritieni, A. Risk analysis of main mycotoxins occurring in food for children: An overview. Food Chem. Toxicol. 2015, 84, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Rebellato, A.P.; dos Santos Caramês, E.T.; Pallone, J.A.L.; de Oliveira Rocha, L. Mycotoxin bioaccessibility in baby food through in vitro digestion: An overview focusing on risk assessment. Curr. Opin. Food Sci. 2021, 41, 107–115. [Google Scholar] [CrossRef]
- Raiola, A.; Meca, G.; Mañes, J.; Ritieni, A. Bioaccessibility of deoxynivalenol and its natural co-occurrence with ochratoxin A and aflatoxin B1 in Italian commercial pasta. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Palumbo, R.; Giorni, P.; Dall’Asta, C.; Dellafiora, L.; Gkrillas, A.; Toscano, P.; Crisci, A.; Brera, C.; De Santis, B. Mycotoxin mixtures in food and feed: Holistic, innovative, flexible risk assessment modelling approach: MYCHIF. EFSA Support. Publ. 2020, 17, 1757E. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA J. 2013, 11, 3254. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Castella, G.; Kostecki, M.; Golinski, P.; Ritieni, A.; Chelkowski, J. Beauvericin Production by FusariumSpecies. Appl. Environ. Microbiol. 1998, 64, 3084–3088. [Google Scholar] [CrossRef]
- Thrane, U. Development in the taxonomy of Fusarium species based on secondary metabolites. In Fusarium: Paul E. Nelson Memorial Symposium; APS Press: Eagan, MN, USA, 2001; pp. 29–49. [Google Scholar]
- Santini, A.; Raiola, A.; Meca, G.; Ritieni, A. Aflatoxins, ochratoxins, trichotecenes, patulin, fumonisins and beauvericin in finished products for human consumption. J. Clin. Toxicol 2015, 5, 265–276. [Google Scholar]
- Wätjen, W.; Debbab, A.; Hohlfeld, A.; Chovolou, Y.; Kampkötter, A.; Edrada, R.A.; Ebel, R.; Hakiki, A.; Mosaddak, M.; Totzke, F. Enniatins A1, B and B1 from an endophytic strain of Fusarium tricinctum induce apoptotic cell death in H4IIE hepatoma cells accompanied by inhibition of ERK phosphorylation. Mol. Nutr. Food Res. 2009, 53, 431–440. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Font, G.; Ruiz, M.J. Interaction effects of enniatin B, deoxinivalenol and alternariol in Caco-2 cells. Toxicol. Lett. 2016, 241, 38–48. [Google Scholar] [CrossRef]
- Prosperini, A.; Juan-García, A.; Font, G.; Ruiz, M.J. Reactive oxygen species involvement in apoptosis and mitochondrial damage in Caco-2 cells induced by enniatins A, A1, B and B1. Toxicol. Lett. 2013, 222, 36–44. [Google Scholar] [CrossRef]
- Versantvoort, C.H.; Oomen, A.G.; Van de Kamp, E.; Rompelberg, C.J.; Sips, A.J. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez-Alvarez, J.A.; Fernández-López, J. In vitro digestion models suitable for foods: Opportunities for new fields of application and challenges. Food Res. Int. 2018, 107, 423–436. [Google Scholar] [CrossRef]
- Fedi, A.; Vitale, C.; Ponschin, G.; Ayehunie, S.; Fato, M.; Scaglione, S. In vitro models replicating the human intestinal epithelium for absorption and metabolism studies: A systematic review. J. Control. Release 2021, 335, 247–268. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Assunção, R.; Martins, C.; Dupont, D.; Alvito, P. Patulin and ochratoxin A co-occurrence and their bioaccessibility in processed cereal-based foods: A contribution for Portuguese children risk assessment. Food Chem. Toxicol. 2016, 96, 205–214. [Google Scholar] [CrossRef]
- Prosperini, A.; Meca, G.; Font, G.; Ruiz, M.J. Bioaccessibility of enniatins A, A1, B, and B1 in different commercial breakfast cereals, cookies, and breads of Spain. J. Agric. Food Chem. 2013, 61, 456–461. [Google Scholar] [CrossRef]
- González-Arias, C.A.; Marín, S.; Sanchis, V.; Ramos, A. Mycotoxin bioaccessibility/absorption assessment using in vitro digestion models: A review. World Mycotoxin J. 2013, 6, 167–184. [Google Scholar] [CrossRef]
- Romero-Sánchez, I.; Alonso-Núñez, I.; Gracia Lor, E.; Madrid, Y. Analysis and Evaluation of in Vitro Bioaccessibility of Aflatoxins B1, B2, G1 and G2 in Plant-Based Milks. SSRN, 2024; preprint. [Google Scholar]
- Wu, C.; Gao, Y.; Li, S.; Huang, X.; Bao, X.; Wang, J.; Zheng, N. Modulation of intestinal epithelial permeability and mucin mRNA (MUC2, MUC5AC, and MUC5B) expression and protein secretion in Caco-2/HT29-MTX co-cultures exposed to aflatoxin M1, ochratoxin A, and zearalenone individually or collectively. Toxicol. Lett. 2019, 309, 1–9. [Google Scholar] [CrossRef]
- Pereira, C.; Cunha, S.C.; Fernandes, J.O. Mycotoxins of Concern in Children and Infant Cereal Food at European Level: Incidence and Bioaccessibility. Toxins 2022, 14, 488. [Google Scholar] [CrossRef] [PubMed]
- IARC. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2002; p. 36. [Google Scholar]
- Sobral, M.M.C.; Cunha, S.C.; Faria, M.A.; Martins, Z.E.; Ferreira, I.M. Influence of oven and microwave cooking with the addition of herbs on the exposure to multi-mycotoxins from chicken breast muscle. Food Chem. 2019, 276, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Aoudia, N.; Callu, P.; Grosjean, F.; Larondelle, Y. Effectiveness of mycotoxin sequestration activity of micronized wheat fibres on distribution of ochratoxin A in plasma, liver and kidney of piglets fed a naturally contaminated diet. Food Chem. Toxicol. 2009, 47, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Aoudia, N.; Tangni, E.; Larondelle, Y. Distribution of ochratoxin A in plasma and tissues of rats fed a naturally contaminated diet amended with micronized wheat fibres: Effectiveness of mycotoxin sequestering activity. Food Chem. Toxicol. 2008, 46, 871–878. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.; Var, I.l. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef]
- Feudjio, F.T.; Dornetshuber, R.; Lemmens, M.; Hoffmann, O.; Lemmens-Gruber, R.; Berger, W. Beauvericin and enniatin: Emerging toxins and/or remedies? World Mycotoxin J. 2010, 3, 415–430. [Google Scholar] [CrossRef]
- Hurni, M.A.; Noach, A.; Blom-Roosemalen, M.; de Boer, A.G.; Nagelkerke, J.F.; Breimer, D.D. Permeability enhancement in Caco-2 cell monolayers by sodium salicylate and sodium taurodihydrofusidate: Assessment of effect-reversibility and imaging of transepithelial transport routes by confocal laser scanning microscopy. J. Pharmacol. Exp. Ther. 1993, 267, 942–950. [Google Scholar] [PubMed]
- Kumar, P.; Gupta, A.; Mahato, D.K.; Pandhi, S.; Pandey, A.K.; Kargwal, R.; Mishra, S.; Suhag, R.; Sharma, N.; Saurabh, V. Aflatoxins in Cereals and Cereal-Based Products: Occurrence, Toxicity, Impact on Human Health, and Their Detoxification and Management Strategies. Toxins 2022, 14, 687. [Google Scholar] [CrossRef] [PubMed]
- EC. Commission Regulation (EC) No 1881_2006 (02006R1881—EN—01.01.2023—033.001)—Setting Maximum Levels for Certain Contaminants in Foodstuffs; Official Journal of the European Union: Geneva, Switzerland, 2006. [Google Scholar]
- Tran, V.N.; Viktorová, J.; Ruml, T. Mycotoxins: Biotransformation and bioavailability assessment using caco-2 cell monolayer. Toxins 2020, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Tavelin, S.; Gråsjö, J.; Taipalensuu, J.; Ocklind, G.; Artursson, P. Applications of epithelial cell culture in studies of drug transport. Epithel. Cell Cult. Protoc. 2002, 233–272. [Google Scholar]
- Hubatsch, I.; Ragnarsson, E.G.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2001, 46, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Skolnik, S.; Lin, X.; Wang, J.; Chen, X.-H.; He, T.; Zhang, B. Towards prediction of in vivo intestinal absorption using a 96-well Caco-2 assay. J. Pharm. Sci. 2010, 99, 3246–3265. [Google Scholar] [CrossRef] [PubMed]
- Tavelin, S.; Taipalensuu, J.; Söderberg, L.; Morrison, R.; Chong, S.; Artursson, P. Prediction of the oral absorption of low-permeability drugs using small intestine-like 2/4/A1 cell monolayers. Pharm. Res. 2003, 20, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Tomková, I.; Ševčíková, Z.; Levkut, M.; Revajová, V.; Čonková, E.; Laciaková, A.; Lenhardt, L. Effect of aflatoxin B 1 on CD3 T cells andalkaline phosphatase in the intestine of mice. Mycopathologia 2002, 154, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Hernandez, E. In situ absorption of aflatoxins in rat small intestine. Mycopathologia 1996, 134, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.M.C.; Faria, M.A.; Cunha, S.C.; Miladinovic, B.; Ferreira, I.M. Transport of mycotoxins across human gastric NCI-N87 and intestinal Caco-2 cell models. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 131, 110595. [Google Scholar] [CrossRef] [PubMed]
- Avantaggiato, G.; Havenaar, R.; Visconti, A. Evaluation of the intestinal absorption of deoxynivalenol and nivalenol by an in vitro gastrointestinal model, and the binding efficacy of activated carbon and other adsorbent materials. Food Chem. Toxicol. 2004, 42, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Bony, S.; Carcelen, M.; Olivier, L.; Devaux, A. Genotoxicity assessment of deoxynivalenol in the Caco-2 cell line model using the Comet assay. Toxicol. Lett. 2006, 166, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bakker, W.; Zheng, W.; de Haan, L.; Rietjens, I.M.; Bouwmeester, H. Exposure to the mycotoxin deoxynivalenol reduces the transport of conjugated bile acids by intestinal Caco-2 cells. Arch. Toxicol. 2022, 96, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Kondrashina, A.; Arranz, E.; Cilla, A.; Faria, M.A.; Santos-Hernández, M.; Miralles, B.; Hashemi, N.; Rasmussen, M.K.; Young, J.F.; Barberá, R. Coupling in vitro food digestion with in vitro epithelial absorption; Recommendations for biocompatibility. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]




| Mycotoxin | In Vitro Digestion | Bioaccessibility (%) | |||||
|---|---|---|---|---|---|---|---|
| BC | BCSSM | BCSSMLF | BCSB |  | |||
| AFB1 | Gastric | Isolated | <LOD | <LOD | <LOD | <LOD | |
| Mixture | <LOD | <LOD | <LOD | <LOD | |||
| Intestinal | Isolated | 57.6 ± 0.1 * ↗ | 24.2 ± 0.3 * ↗ | 20.3 ± 1.0 * ↗ | 3.13 ± 0.17 * ↗ | ||
| Mixture | 86.2 ± 1.6 * ↗ | 27.5 ± 10.3 * ↗ | 18.1 ± 6.8 * ↗ | 9.06 ± 4.32 * ↗ | |||
| ENNB | Gastric | Isolated | 49.2 ± 1.6 a, b | 47.2 ± 21.6 b | 18.1 ± 4.2 b | 26.3 ± 6.3 a, b | |
| Mixture | 52.4 ± 4.5 a, b | 40.5 ± 0.3 b | 21.6 ± 4.0 b | 26.7 ± 8.4 a, b | |||
| Intestinal | Isolated | 91.2 ± 6.4 a, b ↗ | 18.5 ± 9.5 b | 4.54 ± 1.42 b | 21.9 ± 2.2 b | ||
| Mixture | 98.2 ± 7.3 a, b ↗ | 10.7 ± 4.2 b | 0.62 ± 0.17 b | 10.8 ± 0.5 b | |||
| STG | Gastric | Isolated | <LOD | <LOD | <LOD | <LOD | |
| Mixture | <LOD | <LOD | <LOD | <LOD | |||
| Intestinal | Isolated | 53.6 ± 1.7 a, b ↗ | 8.17 ± 0.69 b, * ↗ | 19.7 ± 1.7 b ↗ | 1.45 ± 0.06 a, b, * ↗ | ||
| Mixture | 59.3 ± 3.7 a, b ↗ | 9.52 ± 2.80 b, * ↗ | 8.22 ± 1.49 b ↗ | 1.97 ± 0.79 a, b, * ↗ | |||
| Papp (×10−6 cm/s) | MB (%) | FA (%) | |||
|---|---|---|---|---|---|
| Breakfast cereal | AFB1 | Isolated | 94.4 ± 0.1 | 156 ± 16 | 98.8 * |
| Mix | 3.31 ± 1.10 | 28.3 ± 2.0 | 66.1 * | ||
| ENNB | Isolated | 83.2 ± 0.0 | 145 ± 20 | 98.6 | |
| Mix | 78.6 ± 0.0 | 99.6 ± 2.0 | 98.5 | ||
| STG | Isolated | 37.6 ± 0.0 | 72.6 ± 1.9 | 96.7 | |
| Mix | 25.4 ± 0.0 | 58.7 ± 3.6 | 94.9 | ||
| Breakfast cereal w/semi-skimmed milk | AFB1 | Isolated | 7.35 ± 0.00 | 29.2 ± 1.4 | 82.6 |
| Mix | 8.18 ± 0.00 | 36.7 ± 2.6 | 84.2 | ||
| ENNB | Isolated | 91.6 ± 0.0 | 131 ± 12 | 98.7 | |
| Mix | 72.5 ± 0.0 | 115 ± 0.3 | 98.4 | ||
| STG | Isolated | 81.2 ± 0.2 | 172 ± 11 | 98.6 | |
| Mix | 100.0 ± 0.2 | 167 ± 18 | 98.9 | ||
| Breakfast Cereal Sample | Fat | Fiber | Protein | Carbohydrates |
|---|---|---|---|---|
| (g/100 g) | (g/100 g) | (g/100 g) | (g/100 g) | |
| Breakfast cereal with corn balls with organic honey | 0.6 | 3.0 | 5.5 | 82.0 |
| Beverages samples | (g/100 mL) | (g/100 mL) | (g/100 mL) | (g/100 mL) |
| Semi-skimmed milk | 1.6 | - | 3.4 | 4.9 |
| Semi-skimmed lactose-free milk | 1.6 | - | 3.3 | 4.9 |
| Soy beverage | 1.8 | 0.5 | 3.0 | 2.5 |
| Mycotoxins | Retention Time (min) | MRM Transition (m/z) | CV (V) | CE (eV) | |||
|---|---|---|---|---|---|---|---|
| QIT (m/z) | CIT (m/z) | QIT | CIT | QIT | CIT | ||
| AFB1 | 15.61 | 313 > 241.4 | 313 > 285.3 | 47 | 47 | 36 | 23 |
| OTA-d5 (IS1) | 19.14 | 409 > 239 | 409 > 363 | 32 | 32 | 22 | 22 |
| STG | 20.19 | 325 > 254 | 325 > 310 | 35 | 40 | 35 | 25 |
| 13C18-STG (IS2) | 20.19 | 342.7 > 297.4 | 342.7 > 326.7 | 24 | 24 | 28 | 28 |
| ENNB | 21.63 | 663 > 218 | 663 > 336 | 60 | 60 | 70 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sá, S.V.M.d.; Faria, M.A.; Fernandes, J.O.; Cunha, S.C. In Vitro Digestion and Intestinal Absorption of Mycotoxins Due to Exposure from Breakfast Cereals: Implications for Children’s Health. Toxins 2024, 16, 205. https://doi.org/10.3390/toxins16050205
Sá SVMd, Faria MA, Fernandes JO, Cunha SC. In Vitro Digestion and Intestinal Absorption of Mycotoxins Due to Exposure from Breakfast Cereals: Implications for Children’s Health. Toxins. 2024; 16(5):205. https://doi.org/10.3390/toxins16050205
Chicago/Turabian StyleSá, Soraia V. M. de, Miguel A. Faria, José O. Fernandes, and Sara C. Cunha. 2024. "In Vitro Digestion and Intestinal Absorption of Mycotoxins Due to Exposure from Breakfast Cereals: Implications for Children’s Health" Toxins 16, no. 5: 205. https://doi.org/10.3390/toxins16050205
APA StyleSá, S. V. M. d., Faria, M. A., Fernandes, J. O., & Cunha, S. C. (2024). In Vitro Digestion and Intestinal Absorption of Mycotoxins Due to Exposure from Breakfast Cereals: Implications for Children’s Health. Toxins, 16(5), 205. https://doi.org/10.3390/toxins16050205