Application of Biosensors for the Detection of Mycotoxins for the Improvement of Food Safety
Abstract
1. Introduction
2. Current Approaches in Mycotoxins Detection
2.1. Sampling
2.2. Sample Preparation: Extraction and Purification
2.3. Methods Used for Mycotoxin Detection
3. Mycotoxin Detection Techniques Based on Biosensors
3.1. Sensing Strategy for Mycotoxin Analysis
3.2. Aptamer-Based Biosensor
3.3. Molecular Imprinting
4. Integrated Biosensor Applications in Food Safety Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pandey, A.K.; Samota, M.K.; Kumar, A.; Silva, A.S.; Dubey, N.K. Fungal mycotoxins in food commodities: Present status and future concerns. Front. Sustain. Food Syst. 2023, 7, 1162595. [Google Scholar] [CrossRef]
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Ceremuga, M.; Stela, M.; Saluk-Bijak, J.; Siadkowski, A.; Bijak, M. Molecular Aspects of Mycotoxins—A Serious Problem for Human Health. Int. J. Mol. Sci. 2020, 21, 8187. [Google Scholar] [CrossRef]
- Cazier, J.-B.; Gekas, V. Water Activity and Its Prediction: A Review. Int. J. Food Prop. 2001, 4, 35–43. [Google Scholar] [CrossRef]
- Sun, L.; Li, R.; Tai, B.; Hussain, S.; Wang, G.; Liu, X.; Xing, F. Current Status of Major Mycotoxins Contamination in Food and Feed in Asia—A Review. ACS Food Sci. Technol. 2023, 3, 231–244. [Google Scholar] [CrossRef]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef] [PubMed]
- Zamir-Nasta, T.; Pazhouhi, M.; Ghanbari, A.; Abdolmaleki, A.; Jalili, C. Expression of cyclin D1, p21, and estrogen receptor alpha in aflatoxin G1-induced disturbance in testicular tissue of albino mice. Res. Pharm. Sci. 2021, 16, 182–192. [Google Scholar] [PubMed]
- Wu, X.; Meng, W.; Duan, C.; Cao, J.; Wei, Y.; Cui, X.; Zhu, D.; Lv, P.; Shen, H.; Zhang, X. AFG1-induced TNF-α-mediated inflammation enhances gastric epithelial cell injury via CYP2E1. Food Chem. Toxicol. 2023, 176, 113756. [Google Scholar] [CrossRef]
- Zamir-Nasta, T.; Abbasi, A.; Kakebaraie, S.; Ahmadi, A.; Pazhouhi, M.; Jalili, C. Aflatoxin G1 exposure altered the expression of BDNF and GFAP, histopathological of brain tissue, and oxidative stress factors in male rats. Res. Pharm. Sci. 2022, 17, 677–685. [Google Scholar] [CrossRef]
- Güç, İ.; Yalçin, E.; Çavuşoğlu, K.; Acar, A. Toxicity mechanisms of aflatoxin M1 assisted with molecular docking and the toxicity-limiting role of trans-resveratrol. Sci. Rep. 2022, 12, 14471. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A Producing Fungi, Biosynthetic Pathway and Regulatory Mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Więckowska, M.; Szelenberger, R.; Niemcewicz, M.; Harmata, P.; Poplawski, T.; Bijak, M. Ochratoxin A-The Current Knowledge Concerning Hepatotoxicity, Mode of Action and Possible Prevention. Molecules 2023, 28, 6617. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Bognanno, M.; Galvano, F. Toxicity of ochratoxin a and its modulation by antioxidants: A review. Toxins 2013, 5, 1742–1766. [Google Scholar] [CrossRef] [PubMed]
- Koszegi, T.; Poor, M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Heussner, A.H.; Bingle, L.E. Comparative Ochratoxin Toxicity: A Review of the Available Data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef]
- Barad, S.; Sionov, E.; Prusky, D. Role of patulin in post-harvest diseases. Fungal Biol. Rev. 2016, 30, 24–32. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Gürkan, R. A novel, green and safe ultrasound-assisted emulsification liquid phase microextraction based on alcohol-based deep eutectic solvent for determination of patulin in fruit juices by spectrophotometry. J. Food Compos. Anal. 2019, 82, 103256. [Google Scholar] [CrossRef]
- Song, E.; Xia, X.; Su, C.; Dong, W.; Xian, Y.; Wang, W.; Song, Y. Hepatotoxicity and genotoxicity of patulin in mice, and its modulation by green tea polyphenols administration. Food Chem. Toxicol. 2014, 71, 122–127. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Diwald, T.T.; Metzler, M. Patulin reduces glutathione level and enzyme activities in rat liver slices. Mol. Nutr. Food Res. 2005, 49, 329–336. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Stela, M.; Bijak, M. T-2 Toxin-The Most Toxic Trichothecene Mycotoxin: Metabolism, Toxicity, and Decontamination Strategies. Molecules 2021, 26, 6868. [Google Scholar] [CrossRef]
- Somoskői, B.; Kovács, M.; Cseh, S. T-2 mycotoxin slows down the development of mouse blastocysts, decreases their blastomere number and increases chromatin damage. Acta Vet. Hung. 2016, 64, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tu, D.; Yuan, L.Y.; Yi, J.E.; Tian, Y. T-2 toxin regulates steroid hormone secretion of rat ovarian granulosa cells through cAMP-PKA pathway. Toxicol. Lett. 2015, 232, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research Progress on Fumonisin B1 Contamination and Toxicity: A Review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Luo, K.; Zhu, Q.; Peng, J.; Liu, C.; Wang, X.; Li, S.; Zhang, H. The natural occurrence, toxicity mechanisms and management strategies of Fumonisin B1: A review. Environ. Pollut. 2023, 320, 121065. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Tola, M.; Kebede, B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016, 2, 1191103. [Google Scholar] [CrossRef]
- Abassi, H.; Ayed-Boussema, I.; Shirley, S.; Abid, S.; Bacha, H.; Micheau, O. The mycotoxin zearalenone enhances cell proliferation, colony formation and promotes cell migration in the human colon carcinoma cell line HCT116. Toxicol. Lett. 2016, 254, 1–7. [Google Scholar] [CrossRef]
- Karaman, E.F.; Zeybel, M.; Ozden, S. Evaluation of the epigenetic alterations and gene expression levels of HepG2 cells exposed to zearalenone and α-zearalenol. Toxicol. Lett. 2020, 326, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Shan, A.; Jin, Y.; Fang, H.; Zhao, Y.; Shen, J.; Zhou, C.; Zhou, Y.; Fu, Y.; et al. Toxic effects of Zearalenone on intestinal microflora and intestinal mucosal immunity in mice. Food Agric. Immunol. 2018, 29, 1002–1011. [Google Scholar] [CrossRef]
- Silva, L.J.G.; Pereira, A.; Pena, A.; Lino, C.M. Citrinin in Foods and Supplements: A Review of Occurrence and Analytical Methodologies. Foods 2020, 10, 14. [Google Scholar] [CrossRef]
- Zargar, S.; Wani, T.A. Food Toxicity of Mycotoxin Citrinin and Molecular Mechanisms of Its Potential Toxicity Effects through the Implicated Targets Predicted by Computer-Aided Multidimensional Data Analysis. Life 2023, 13, 880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ahima, J.; Yang, Q.; Zhao, L.; Zhang, X.; Zheng, X. A review on citrinin: Its occurrence, risk implications, analytical techniques, biosynthesis, physiochemical properties and control. Food Res. Int. 2021, 141, 110075. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- European Parliament; Directorate-General for Internal Policies of the Union; Brouwer, F.; Rau, M.; Hoste, R.; Wagenberg, C. Comparative Analysis of EU Standards in Food Safety, Environment, Animal Welfare and Other Non-Trade Concerns with Some Selected Countries; Publications Office: Luxembourg, 2012. [Google Scholar]
- Zhang, L.; Dou, X.-W.; Zhang, C.; Logrieco, A.F.; Yang, M.-H. A review of current methods for analysis of mycotoxins in herbal medicines. Toxins 2018, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union L 2006, 70, 12–34. [Google Scholar]
- European Commission. Commission Regulation (EU) No 519/2014 of 16 May 2014 amending Regulation (EC) No 401/2006 as regards methods of sampling of large lots, spices and food supplements, performance criteria for T-2, HT-2 toxin and citrinin and screening methods of analysis. Off. J. Eur. Union 2014, 147, 29–43. [Google Scholar]
- Nakhjavan, B.; Ahmed, N.S.; Khosravifard, M. Development of an improved method of sample extraction and quantitation of multi-mycotoxin in feed by LC-MS/MS. Toxins 2020, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Zhang, Y.; Zhou, Y.; Wei, B.; Feng, X. Recent Insights into Sample Pretreatment Methods for Mycotoxins in Different Food Matrices: A Critical Review on Novel Materials. Toxins 2023, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Pérez, J.F.; Arroyo-Manzanares, N.; Hitzler, D.; Castro-Guerrero, F.G.; Gámiz-Gracia, L.; García-Campaña, A.M. Simple determination of aflatoxins in rice by ultra-high performance liquid chromatography coupled to chemical post-column derivatization and fluorescence detection. Food Chem. 2018, 245, 189–195. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Gorniak, L.; Stela, M.; Bijak, M. The Existing Methods and Novel Approaches in Mycotoxins’ Detection. Molecules 2021, 26, 3981. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Fernandes, J.; Cunha, S. Comparative assessment of three cleanup procedures after QuEChERS extraction for determination of trichothecenes (type A and type B) in processed cereal-based baby foods by GC–MS. Food Chem. 2015, 182, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.; Freitas, A.; Barbosa, J.; Ramos, F. Comprehensive assessment of different extraction methodologies for optimization and validation of an analytical multi-method for determination of emerging and regulated mycotoxins in maize by UHPLC-MS/MS. Food Chem. Adv. 2023, 2, 100145. [Google Scholar] [CrossRef]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for detection of aflatoxins in agricultural food crops. J. Appl. Chem. 2014, 2014, 706291. [Google Scholar] [CrossRef]
- Meyers, C.L.F.; Meyers, D.J. Thin-Layer Chromatography. Curr. Protoc. Nucleic Acid. Chem. 2008, 34, A.3D.1–A.3D.13. [Google Scholar] [CrossRef] [PubMed]
- Overy, D.P.; Seifert, K.A.; Savard, M.E.; Frisvad, J.C. Spoilage fungi and their mycotoxins in commercially marketed chestnuts. Int. J. Food Microbiol. 2003, 88, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Aiko, V.; Mehta, A. Prevalence of toxigenic fungi in common medicinal herbs and spices in India. 3 Biotech 2016, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Tozlovanu, M.; Tran, T.L.; Pfohl-Leszkowicz, A. Occurrence of aflatoxin B1, citrinin and ochratoxin A in rice in five provinces of the central region of Vietnam. Food Chem. 2007, 105, 42–47. [Google Scholar] [CrossRef]
- Brera, C.; Soriano, J.M.; Debegnach, F.; Miraglia, M. Exposure assessment to ochratoxin A from the consumption of Italian and Hungarian wines. Microchem. J. 2005, 79, 109–113. [Google Scholar] [CrossRef]
- Spadaro, D.; Ciavorella, A.; Frati, S.; Garibaldi, A.; Gullino, M.L. Incidence and level of patulin contamination in pure and mixed apple juices marketed in Italy. Food Control 2007, 18, 1098–1102. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Berrada, H.; Mañes, J. A survey of trichothecenes, zearalenone and patulin in milled grain-based products using GC–MS/MS. Food Chem. 2014, 146, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.S.; Haddad, M.A.; Parisi, S. Validation of HPLC and Enzyme-Linked Immunosorbent Assay (ELISA) techniques for detection and quantification of aflatoxins in different food samples. Foods 2020, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Marín, S.; Sanchis, V.; Ramos, A.J. Screening of mycotoxin multicontamination in medicinal and aromatic herbs sampled in Spain. J. Sci. Food Agric. 2009, 89, 1802–1807. [Google Scholar] [CrossRef]
- Xing, K.-Y.; Peng, J.; Shan, S.; Liu, D.-F.; Huang, Y.-N.; Lai, W.-H. Green enzyme-linked immunosorbent assay based on the single-stranded binding protein-assisted aptamer for the detection of mycotoxin. Anal. Chem. 2020, 92, 8422–8426. [Google Scholar] [CrossRef] [PubMed]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Wu, X.; Cui, G.; Liu, L.; Kuang, H.; Xu, C. Development of an ic-ELISA and Immunochromatographic Strip Assay for the Detection of Diacetoxyscirpenol in Rice. ACS Omega 2020, 5, 17876–17882. [Google Scholar] [CrossRef]
- Li, R.; Meng, C.; Wen, Y.; Fu, W.; He, P. Fluorometric lateral flow immunoassay for simultaneous determination of three mycotoxins (aflatoxin B 1, zearalenone and deoxynivalenol) using quantum dot microbeads. Microchim. Acta 2019, 186, 748. [Google Scholar] [CrossRef]
- Gaudin, V. Advances in biosensor development for the screening of antibiotic residues in food products of animal origin—A comprehensive review. Biosens. Bioelectron. 2017, 90, 363–377. [Google Scholar] [CrossRef]
- Lingerfelt, L.; Karlinsey, J.; Landers, J.; Guiseppi-Elie, A. Impedimetric detection for DNA hybridization within microfluidic biochips. Methods Mol. Biol. 2007, 385, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C. Electrochemical based biosensors. Biosensors 2012, 2, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Z.; Zhang, Q.; Li, P. Mycotoxin Determination in Foods Using Advanced Sensors Based on Antibodies or Aptamers. Toxins 2016, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, G.; Lv, Z.; Qiu, N.; Kong, W.; Gong, P.; Chen, G.; Xia, L.; Guo, X.; You, J.; et al. Facile and ultrasensitive fluorescence sensor platform for tumor invasive biomaker beta-glucuronidase detection and inhibitor evaluation with carbon quantum dots based on inner-filter effect. Biosens. Bioelectron. 2016, 85, 358–362. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.M.; Lee, S.Y. Optical Biosensors for the Detection of Pathogenic Microorganisms. Trends Biotechnol. 2016, 34, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Majdinasab, M.; Mitsubayashi, K.; Marty, J.L. Optical and Electrochemical Sensors and Biosensors for the Detection of Quinolones. Trends Biotechnol. 2019, 37, 898–915. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Gao, L.; Koo, H. Emerging Biomedical Applications of Enzyme-Like Catalytic Nanomaterials. Trends Biotechnol. 2018, 36, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.; Chen, J.; Huang, T.; Cao, H.; Liu, X. A Novel Electrochemiluminescence Immunosensor Based on Resonance Energy Transfer between g-CN and NU-1000(Zr) for Ultrasensitive Detection of Ochratoxin A in Coffee. Foods 2023, 12, 707. [Google Scholar] [CrossRef]
- Lv, X.; Tan, F.; Miao, T.; Cui, B.; Zhang, J.; Fang, Y.; Shen, Y. In situ generated PtNPs to enhance electrochemiluminescence of multifunctional nanoreactor COP T(4)VTP(6) for AFB(1) detection. Food Chem. 2023, 399, 134002. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, Z.; Miao, T.; Cui, B.; Fang, Y. Potential-Resolved Differential Electrochemiluminescence Immunosensor Based on Novel Designed Ibphf for Self-Correctable Detection of Afb1. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Tian, D.; Wang, J.; Zhuang, Q.; Wu, S.; Yu, Y.; Ding, K. An electrochemiluminescence biosensor based on Graphitic carbon nitride luminescence quenching for detection of AFB(1). Food Chem. 2023, 404, 134183. [Google Scholar] [CrossRef]
- Wang, J.; Xia, M.; Wei, J.; Jiao, T.; Chen, Q.; Chen, Q.; Chen, X. Dual-signal amplified cathodic electrochemiluminescence aptsensor based on a europium-porphyrin coordination polymer for the ultrasensitive detection of zearalenone in maize. Sens. Actuators B Chem. 2023, 382, 133532. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhao, M.; Zhuo, Y.; Chai, Y.Q.; Yuan, R. Highly Efficient Intramolecular Electrochemiluminescence Energy Transfer for Ultrasensitive Bioanalysis of Aflatoxin M1. Chemistry 2017, 23, 1853–1859. [Google Scholar] [CrossRef]
- Wei, J.; Chen, L.; Cai, X.; Lai, W.; Chen, X.; Cai, Z. 2D mesoporous silica-confined CsPbBr(3) nanocrystals and N-doped graphene quantum dot: A self-enhanced quaternary composite structures for electrochemiluminescence analysis. Biosens. Bioelectron. 2022, 216, 114664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, M.; Zhang, H.; Wen, W.; Zhang, X.; Wang, S. Enhanced electrochemiluminescence of RuSi nanoparticles for ultrasensitive detection of ochratoxin A by energy transfer with CdTe quantum dots. Biosens. Bioelectron. 2016, 79, 561–567. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, M.; Zhang, H.; Wen, W.; Zhang, X.; Wang, S. Solid-state electrochemiluminescence sensor based on RuSi nanoparticles combined with molecularly imprinted polymer for the determination of ochratoxin A. Sens. Actuators B Chem. 2016, 222, 264–269. [Google Scholar] [CrossRef]
- Li, M.; Yue, Q.; Fang, J.; Wang, C.; Cao, W.; Wei, Q. Au modified spindle-shaped cerium phosphate as an efficient co-reaction accelerator to amplify electrochemiluminescence signal of carbon quantum dots for ultrasensitive analysis of aflatoxin B1. Electrochim. Acta 2022, 407, 139912. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; He, S.; Cao, H.; Su, B.; Huang, T.; Chen, Q.; Liu, M.; Yang, D.P. Electrochemiluminescence Immunosensor Based on Nanobody and Au/CaCO3 Synthesized Using Waste Eggshells for Ultrasensitive Detection of Ochratoxin A. ACS Omega 2021, 6, 30148–30156. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Li, R.; Lin, C.; Guo, L.; Qiu, B.; Lin, Z.; Chen, G. Electrochemiluminescence biosensor for ultrasensitive determination of ochratoxin A in corn samples based on aptamer and hyperbranched rolling circle amplification. Biosens. Bioelectron. 2015, 70, 268–274. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Luo, F.; Guo, L.; Qiu, B.; Lin, Z. Highly reproducible ratiometric aptasensor based on the ratio of amplified electrochemiluminescence signal and stable internal reference electrochemical signal. Electrochim. Acta 2018, 283, 798–805. [Google Scholar] [CrossRef]
- Lu, L.; Yuan, W.; Xiong, Q.; Wang, M.; Liu, Y.; Cao, M.; Xiong, X. One-step grain pretreatment for ochratoxin A detection based on bipolar electrode-electrochemiluminescence biosensor. Anal. Chim. Acta 2021, 1141, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhuo, Z.; Chen, L.; Chen, C.; Luo, F.; Chen, Y.; Guo, L.; Qiu, B.; Lin, Z.; Chen, G. Enhanced performance of a hyperbranched rolling circle amplification based electrochemiluminescence aptasensor for ochratoxin A using an electrically heated indium tin oxide electrode. Electrochem. Commun. 2018, 88, 75–78. [Google Scholar] [CrossRef]
- Shi, X.; Zhu, X.; Chai, Y.; Zhou, Y.; Yuan, R. Non-enzymatic electrochemiluminescence biosensor for ultrasensitive detection of ochratoxin A based on efficient DNA walker. Food Chem. 2023, 407, 135113. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wei, S.; Liu, G.; Xie, S.; Chai, Y.; Yuan, R. Ultrasensitive electrochemiluminescent aptasensor for ochratoxin A detection with the loop-mediated isothermal amplification. Anal. Chim. Acta 2014, 811, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Wang, C.; Xu, E.; Chen, J.; Xu, X.; Wei, W.; Liu, S. A simple and sensitive electrochemiluminescence aptasensor for determination of ochratoxin A based on a nicking endonuclease-powered DNA walking machine. Food Chem. 2019, 282, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Jia, B.; Liao, X.; Shi, L.; Zhang, Z.; Liu, M.; Zhou, L.; Li, D.; Kong, W. A CdSe@CdS quantum dots based electrochemiluminescence aptasensor for sensitive detection of ochratoxin A. Chemosphere 2022, 287, 131994. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.L.; Lu, H.J.; Xu, J.J.; Zhou, H.; Chen, H.Y. Recent advances of ratiometric electrochemiluminescence biosensors. J. Mater. Chem. B 2019, 7, 6469–6475. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ke, Y.; Yi, H.; Dai, H.; Fang, D.; Lin, Y.; Hong, Z.; Li, X. A bifunctional reagent regulated ratiometric electrochemiluminescence biosensor constructed on surfactant-assisted synthesis of TiO2 mesocrystals for the sensing of deoxynivalenol. Talanta 2019, 196, 600–607. [Google Scholar] [CrossRef]
- Luo, L.; Liu, X.; Bi, X.; Li, L.; You, T. Dual-quenching effects of methylene blue on the luminophore and co-reactant: Application for electrochemiluminescent-electrochemical ratiometric zearalenone detection. Biosens. Bioelectron. 2023, 222, 114991. [Google Scholar] [CrossRef]
- Ge, J.; Zhao, Y.; Li, C.; Jie, G. Versatile Electrochemiluminescence and Electrochemical “On-Off” Assays of Methyltransferases and Aflatoxin B1 Based on a Novel Multifunctional DNA Nanotube. Anal. Chem. 2019, 91, 3546–3554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiong, C.; Li, J.; Deng, Q.; Zhang, X.; Wang, S.; Chen, M.M. High-performance electrochemiluminescence sensors based on ultra-stable perovskite quantum dots@ZIF-8 composites for aflatoxin B1 monitoring in corn samples. Food Chem. 2023, 410, 135325. [Google Scholar] [CrossRef] [PubMed]
- Yugender Goud, K.; Sunil Kumar, V.; Hayat, A.; Vengatajalabathy Gobi, K.; Song, H.; Kim, K.-H.; Marty, J.L. A highly sensitive electrochemical immunosensor for zearalenone using screen-printed disposable electrodes. J. Electroanal. Chem. 2019, 832, 336–342. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, H.; Chen, M.; Zhang, X.; Wang, S. Surface-enhanced molecularly imprinted electrochemiluminescence sensor based on Ru@SiO2 for ultrasensitive detection of fumonisin B1. Biosens. Bioelectron. 2017, 96, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Xiong, C.; Deng, Q.; Zhang, X.; Wang, S.; Chen, M.-M. An ultrasensitive CH3NH3PbBr3 quantum dots@SiO2-based electrochemiluminescence sensing platform using an organic electrolyte for aflatoxin B1 detection in corn oil. Food Chem. 2022, 390, 133200. [Google Scholar] [CrossRef] [PubMed]
- Sorbo, A.; Pucci, E.; Nobili, C.; Taglieri, I.; Passeri, D.; Zoani, C. Food Safety Assessment: Overview of Metrological Issues and Regulatory Aspects in the European Union. Separations 2022, 9, 53. [Google Scholar] [CrossRef]
- Adams, R. Food Safety Regulations and Consumer Confidence. Int. J. Livest. Policy 2024, 2, 15–25. [Google Scholar] [CrossRef]
- Lizakowski P, K.A. Legal regulations and administration of food safety. World Sci. News 2019, 122, 133–144. [Google Scholar]
- Vapa-Tankosić, J.; Puvača, N.; Giannenas, I.; Tufarelli, V.; Ignjatijević, S. Food Safety Policy in the European Union. J. Agron. Technol. Eng. Manag. (JATEM) 2022, 5, 712–717. [Google Scholar] [CrossRef]
- Sosnowski, M.; Osek, J. Microbiological Safety of Food of Animal Origin from Organic Farms. J. Vet. Res. 2021, 65, 87–92. [Google Scholar] [CrossRef]
- Lee, J.C.; Daraba, A.; Voidarou, C.; Rozos, G.; Enshasy, H.A.E.; Varzakas, T. Implementation of Food Safety Management Systems along with Other Management Tools (HAZOP, FMEA, Ishikawa, Pareto). The Case Study of Listeria monocytogenes and Correlation with Microbiological Criteria. Foods 2021, 10, 2169. [Google Scholar] [CrossRef]
- Muncke, J.; Backhaus, T.; Geueke, B.; Maffini Maricel, V.; Martin Olwenn, V.; Myers John, P.; Soto Ana, M.; Trasande, L.; Trier, X.; Scheringer, M. Scientific Challenges in the Risk Assessment of Food Contact Materials. Environ. Health Perspect. 2017, 125, 095001. [Google Scholar] [CrossRef] [PubMed]
- López-Santiago, J.; Md Som, A.; Asyadi Bin Md Yusof, F.; Mazarrón, F.R.; Gómez-Villarino, M.T. Exploring Sustainability in Wineries: Evaluating Food Safety and Environmental Management Aligning with the Farm to Fork Strategy. Agriculture 2024, 14, 330. [Google Scholar] [CrossRef]
- Hung, Y.T.; Liu, C.T.; Peng, I.C.; Hsu, C.; Yu, R.C.; Cheng, K.C. The implementation of a Hazard Analysis and Critical Control Point management system in a peanut butter ice cream plant. J. Food Drug Anal. 2015, 23, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Okpala, C.O.R.; Korzeniowska, M. Understanding the Relevance of Quality Management in Agro-food Product Industry: From Ethical Considerations to Assuring Food Hygiene Quality Safety Standards and Its Associated Processes. Food Rev. Int. 2023, 39, 1879–1952. [Google Scholar] [CrossRef]
- Fumagalli, F.; Ottoboni, M.; Pinotti, L.; Cheli, F. Integrated Mycotoxin Management System in the Feed Supply Chain: Innovative Approaches. Toxins 2021, 13, 572. [Google Scholar] [CrossRef]
- Nada, S.; Nikola, T.; Bozidar, U.; Ilija, D.; Andreja, R. Prevention and practical strategies to control mycotoxins in the wheat and maize chain. Food Control 2022, 136, 108855. [Google Scholar] [CrossRef]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef]
- Niewczas-Dobrowolska, M.; Sikora, T.; Prusak, A. Food Risk Analysis. Proc. Eng. Sci. 2019, 1, 261–272. [Google Scholar] [CrossRef]
- Chavez, R.A.; Cheng, X.; Stasiewicz, M.J. A Review of the Methodology of Analyzing Aflatoxin and Fumonisin in Single Corn Kernels and the Potential Impacts of These Methods on Food Security. Foods 2020, 9, 297. [Google Scholar] [CrossRef]
- Franchino, C.; Vita, V.; Iammarino, M.; De Pace, R. Monitoring of Animal Feed Contamination by Mycotoxins: Results of Five Years of Official Control by an Accredited Italian Laboratory. Microorganisms 2024, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Nešić, K.; Habschied, K.; Mastanjević, K. Modified Mycotoxins and Multitoxin Contamination of Food and Feed as Major Analytical Challenges. Toxins 2023, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Mureşan, C.C.; Marc, R.A.; Jimborean, M.; Rusu, I.; Mureşan, A.; Nistor, A.; Cozma, A.; Suharoschi, R. Food Safety System (HACCP) as Quality Checkpoints in a Spin-Off Small-Scale Yogurt Processing Plant. Sustainability 2020, 12, 9472. [Google Scholar] [CrossRef]
- Ferris, I. 10. Hazard analysis and critical control points (HACCP). In Applied Food Science; Wageningen Academic: Wageningen, The Netherlands, November 2022; pp. 187–213. [Google Scholar]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Curulli, A. Electrochemical Biosensors in Food Safety: Challenges and Perspectives. Molecules 2021, 26, 2940. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Tung, S. Development and Applications of Portable Biosensors. J. Lab. Autom. 2015, 20, 365–389. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Zhang, Y.; Wang, X.; Zhang, C.; Cheng, N. Intelligent Biosensors Promise Smarter Solutions in Food Safety 4.0. Foods 2024, 13, 235. [Google Scholar] [CrossRef]
- Ali, A.A.; Altemimi, A.B.; Alhelfi, N.; Ibrahim, S.A. Application of Biosensors for Detection of Pathogenic Food Bacteria: A Review. Biosensors 2020, 10, 58. [Google Scholar] [CrossRef]
- El-Sayed, R.; Jebur, A.; Kang, W.; El-Esawi, M.; El-Demerdash, F. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Future Foods 2020, 2, 91–102. [Google Scholar] [CrossRef]
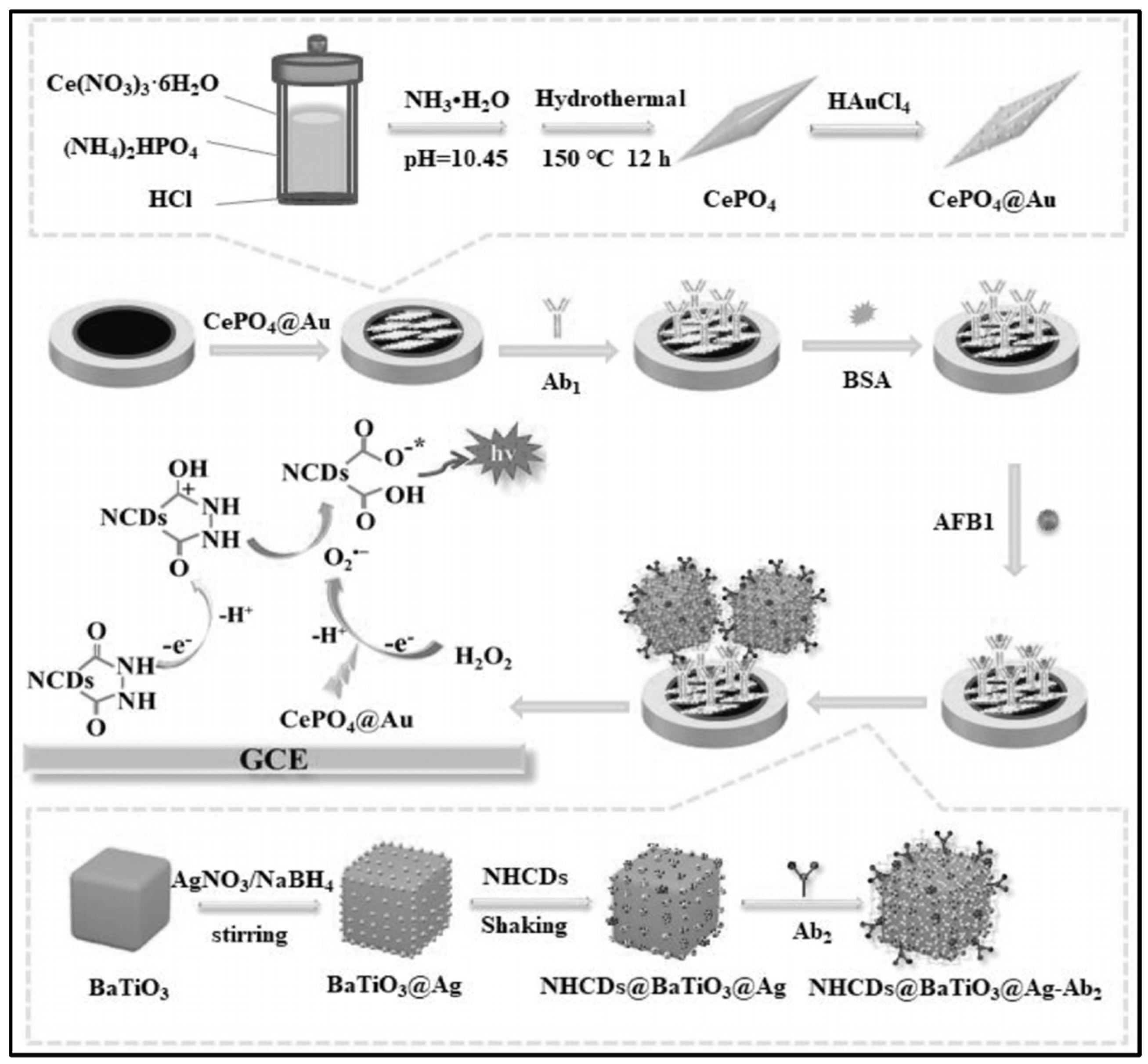
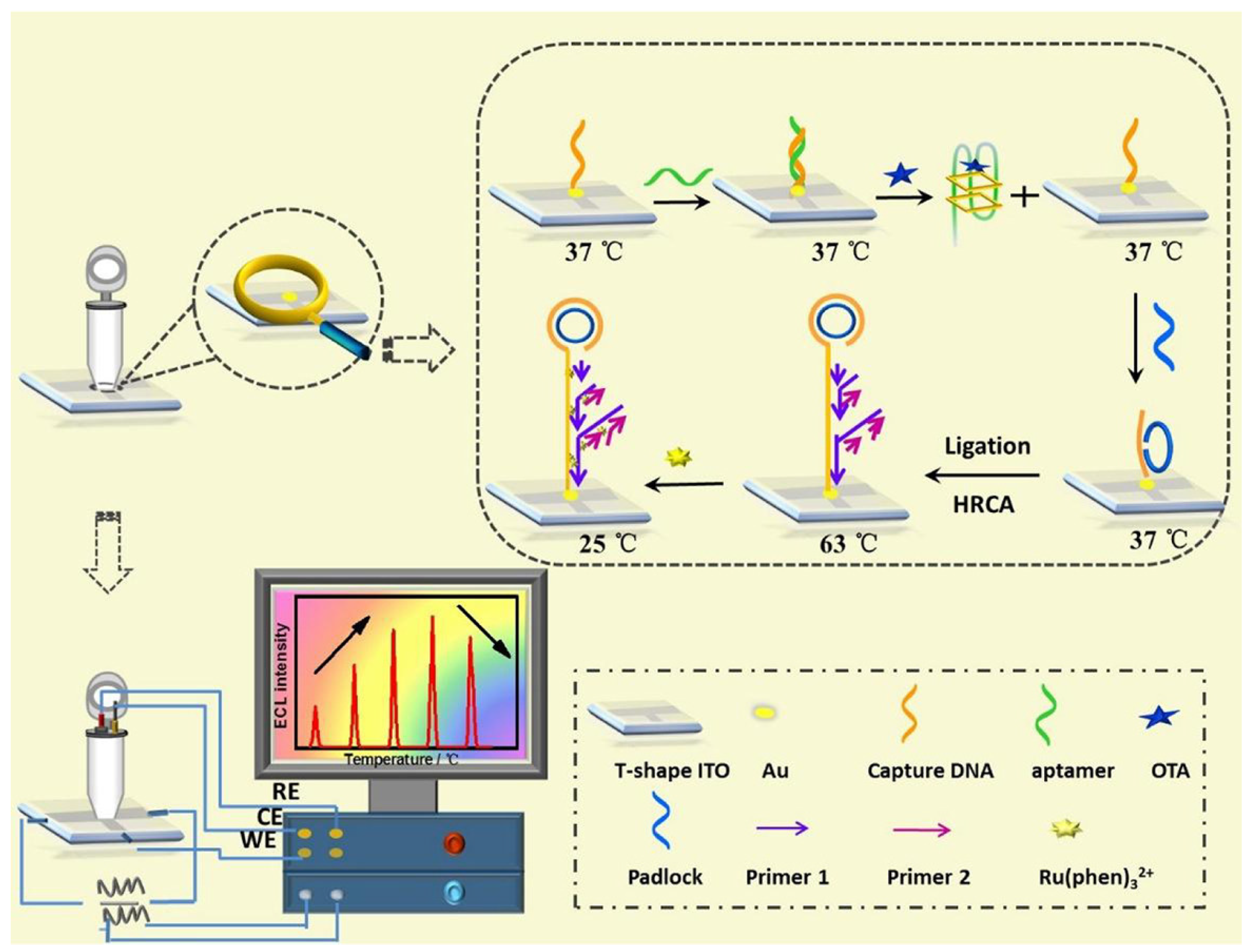
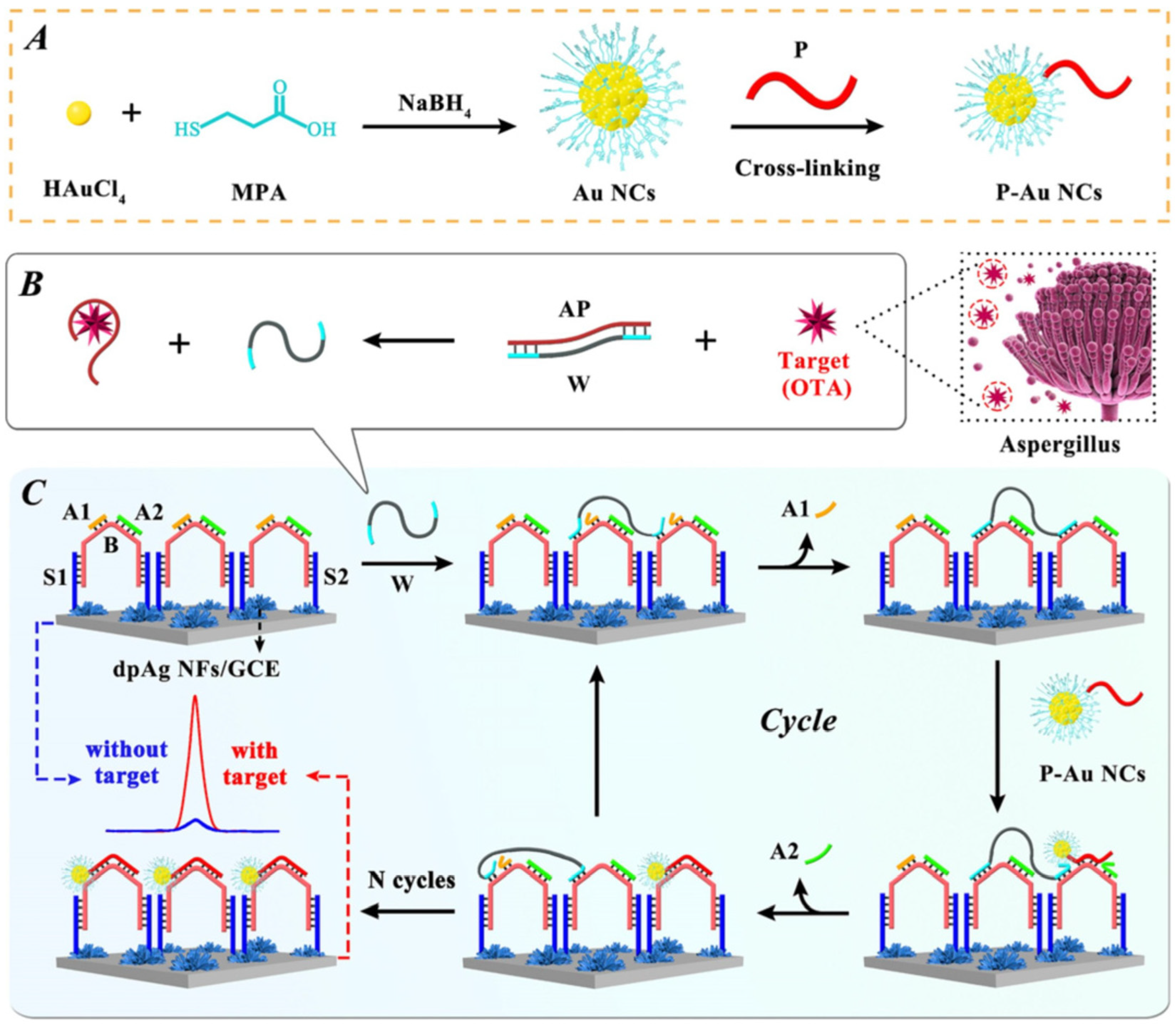
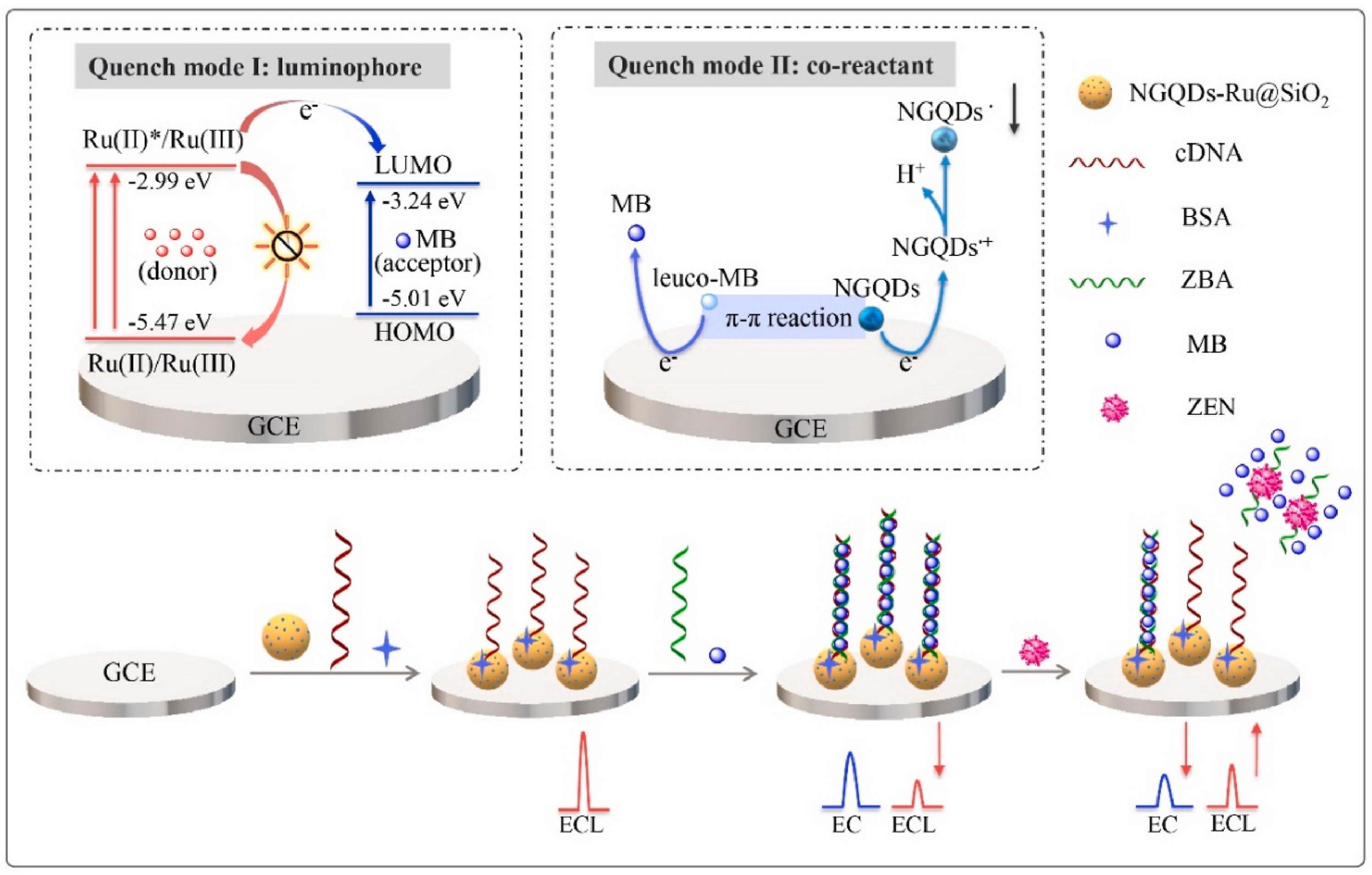
| Mycotoxin | Toxic Effect | Species | Ref |
|---|---|---|---|
| Aflatoxin B1 (AFB1) and B2 (AFB2) | Strongly hepatotoxic, immunosuppressive, and carcinogenic. Mostly linked with hepatocellular carcinoma (HCC). Its metabolite (AFBO—AFB1-exo-8,9-epoxide) possesses a high affinity for forming DNA adducts, stimulates mutations, induces oxidative stress, and leads to a loss of cell integrity. AFBO inhibits basic metabolism, nucleic acid synthesis, protein synthesis, and DNA repair, thus leading to a disruption of vital cell functions. | Aspergillus (mainly A. flavus, A. parasiticus, A. nomius) | [3,6] |
| Aflatoxin G1 (AFG1) and G2 (AFG2) | Induce atrophy of spermatogenic tubules and upregulate the expression of cyclin D1 at the mRNA and protein level in spermatocytogenic and spermatogenic cells in albino mice (20 µg/kg of AFG1). Stimulate gastric inflammation and DNA damage in mice after oral administration (100 µg/kg of AFG1). In the human GES-1 cell line, AFG1 leads to oxidative stress and DNA damage via Cytochrome P450 (particularly CYP2E1) through NF-ĸB). In Wistar Rats, the administration of AFG1 (2 mg/kg) caused the overexpression of GFAP and reduced expression of BDNF in brain tissue. Furthermore, AFG1 caused morphological changes in nerve cells and neuroglia and stimulated cell necrosis. There are currently no publications focused on the toxicity of AFG2. | [7,8,9] | |
| Aflatoxin M1 (AFM1) and M2 (AFM2) | Generated via the biotransformation of AFB1/AFB2 in the hydroxylation reaction. Derived from animal milk. In mice (16 mg/kg of AFM1), they caused a significant reduction in the weight of the liver and kidneys, increased malondialdehyde (MDA), and reduced glutathione (GSH) levels in the liver and kidneys. Furthermore, AFM1 increased alanine transaminase (ALT), aspartate transaminase (AST), creatinine, and blood urea nitrogen parameters, augmented the formation of micronuclei and chromosomal abnormalities frequencies, and induced DNA fragmentation. There are currently no publications focused on the toxicity of AFM2. | [10] | |
| Ochratoxin A (OTA) and Ochratoxin B (OTB) | OTA was shown to be strongly nephrotoxic, hepatotoxic, teratogenic, genotoxic, immunotoxic, and neurotoxic. OTA stimulates apoptosis, necrosis, and the generation of reactive oxygen species (ROS), nitric oxide, and lipid peroxides. Furthermore, OTA inhibits glutathione, cell proliferation processes, and glutamate absorption, which are crucial in the stimulation of neurodegeneration. OTB exhibits significantly lower toxicity compared to OTA. The mechanism of action is very similar; however, the biological effect is diminished. | Aspergillus (mainly A. ochraceus, A. carbonarius, A. niger) Penicillium (mainly P. verrucosum, P. nordicum, P. viridicatum) | [11,12,13,14,15] |
| Patulin | Strongly hepatotoxic, nephrotoxic, neurotoxic, and immunosuppressive. Highly toxic for the gastrointestinal tract. Stimulates the activity of serum aspartate (AST) and alanine (ALT) transaminase, the generation of ROS, and lipid peroxidation in mice. What is more, patulin induces chromosomal and micronucleus abnormalities. Reduces the GSH level in rat hepatocytes. | Penicillium (mainly P. expansum, P. coprobium, P. carneum, P. clavigerum, P. dipodomyicola, P. glandicola, P. concentricum, P. gladioli, P. griseofulvum, P. marinum, P. paneum, P. sclerotigenum, P. vulpinum) Aspergillus (mainly A. giganteus, A. longivesica, A. clavatus) Byssochlamys (B. nivea) Paecilomyces (P. saturatus) | [16,17,18,19] |
| T-2 | Belongs to the Trichothecenes group. Possesses neurotoxic, nephrotoxic, hepatotoxic, and immunotoxic properties. Affects skin, vascular, and reproductive system disorders in humans. Stimulates apoptosis, autophagy, and necrosis in human and animal cells. In mice, the administration of T-2 causes abnormal development of blastocysts, a lower number of blastomeres, and induces chromatin damage. T-2 induces the production of ROS and activation of inflammatory mediators in rat hepatocytes. | Fusarium (mainly F. sporotrichoides, F. poae, F. acuminatum, F. equiseti) | [20,21,22] |
| Fumonisin | Immunotoxic, nephrotoxic, hepatotoxic, genotoxic, and neurotoxic. Highly toxic to the heart, lungs, and gastrointestinal tract in humans. Possesses the ability to compete with sphingolipids in ceramide synthase, affecting cell–cell interactions and recognition in humans and animals. Induce soxidative stress by augmenting the production of ROS in animals and humans. Induces DNA damage and epigenetic modifications, i.e., gene methylation. Induces apoptosis and autophagy, and increases the activity of TNF-α and IL-1β. | Fusarium (mainly F. verticilliodes, F. proliferatum) | [23,24] |
| Zearalenone | Belongs to the Trichothecenes group. Causes hepatotoxicity, genotoxicity, and immunotoxicity. Induces epigenetic alterations, including DNA methylation and histone modifications. Affects crucial pathways associated with metabolism like IGF1, PXR, H2K, and PPARγ in human cells. Inhibits the growth of beneficial microbiota, thus disrupting intestinal balance in mice. | Fusarium (mainly F. graminearum, F. cerealis, F. culmorum, F. equiseti, F. oxysporum, F. nivale) | [25,26,27,28,29] |
| Citrinin | Hepatotoxic, nephrotoxic, immunosuppressive, and carcinogenic in human and animal cell lines. Induces renal mitochondrial dysfunction, and stimulates the production of ROS, causing Balkan nephropathy. May present a synergistic effect in combination with OTA. | Aspergillus (mainly A. terreus, A. niveus, oryaze, flavus) Penicillium (mainly P. citrinum, P. expansum, P. radicicola, P. verrucosum, cameberti, notatum) Monascus (mainly M. ruber, M. purpureus | [30,31,32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szelenberger, R.; Cichoń, N.; Zajaczkowski, W.; Bijak, M. Application of Biosensors for the Detection of Mycotoxins for the Improvement of Food Safety. Toxins 2024, 16, 249. https://doi.org/10.3390/toxins16060249
Szelenberger R, Cichoń N, Zajaczkowski W, Bijak M. Application of Biosensors for the Detection of Mycotoxins for the Improvement of Food Safety. Toxins. 2024; 16(6):249. https://doi.org/10.3390/toxins16060249
Chicago/Turabian StyleSzelenberger, Rafał, Natalia Cichoń, Wojciech Zajaczkowski, and Michal Bijak. 2024. "Application of Biosensors for the Detection of Mycotoxins for the Improvement of Food Safety" Toxins 16, no. 6: 249. https://doi.org/10.3390/toxins16060249
APA StyleSzelenberger, R., Cichoń, N., Zajaczkowski, W., & Bijak, M. (2024). Application of Biosensors for the Detection of Mycotoxins for the Improvement of Food Safety. Toxins, 16(6), 249. https://doi.org/10.3390/toxins16060249





