Efficacy of Aflatoxin B1 and Fumonisin B1 Adsorption by Maize, Wheat, and Oat Bran
Abstract
:1. Introduction
2. Results and Discussion
2.1. Adsorption of AFB1 and FMB1 by Brans
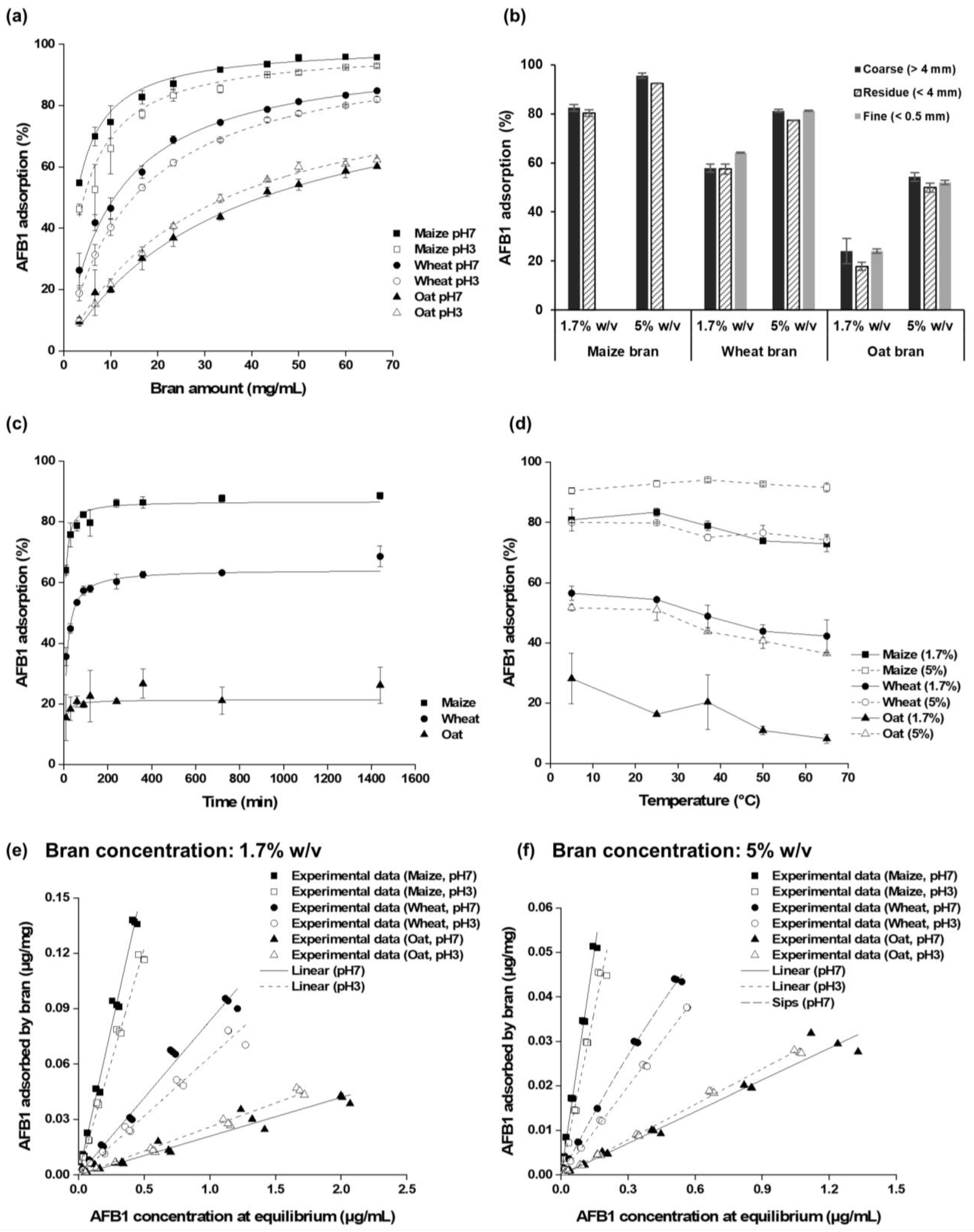
2.2. Effects of Bran Amount and pH on AFB1 Adsorption
2.3. Effect of Particle Size on AFB1 Adsorption
2.4. Kinetics and Thermodynamics of AFB1 Adsorption
2.5. Equilibrium Adsorption Isotherms
2.6. Effect of Solvent Volume on AFB1 Adsorption
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Materials
4.2. AFB1 and FMB1 Adsorption Experiments
Factors Influencing AFB1 Adsorption
4.3. Monosaccharide Analysis in Brans
4.4. AFB1 Analysis Using UPLC-FLD
4.5. FMB1 Analysis Using UPLC-MS
4.6. Adsorption Calculation and Model Fitting
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosco, F.; Mollea, C. Mycotoxins in Food. In Food Industrial Processes-Methods and Equipment; InTech: Houston TX, USA, 2012; pp. 169–200. [Google Scholar]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, Toxicology, and Exposure Assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-Occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- IARC. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume. In Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; WHO: Geneva, Switzerland, 2002; ISBN 978-92-832-1282-9. [Google Scholar]
- IARC. Aflatoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100F, pp. 225–248. ISBN 978-92-832-1323-9. [Google Scholar]
- Van Egmond, H.P.; Jonker, M.A. Worldwide Regulations for Mycotoxins in Food and Feed in 2003; FAO Food and Nutrition Paper 81; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Zingales, V.; Taroncher, M.; Martino, P.A.; Ruiz, M.-J.; Caloni, F. Climate Change and Effects on Molds and Mycotoxins. Toxins 2022, 14, 445. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Van der Fels-Klerx, H.J. Quantitative Modeling of Climate Change Impacts on Mycotoxins in Cereals: A Review. Toxins 2021, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate Change, Food Security and Mycotoxins: Do We Know Enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin Risks under a Climate Change Scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. The Fate of Mycotoxins During the Processing of Wheat for Human Consumption. Compr. Rev. Food Sci. Food Saf. 2018, 17, 556–593. [Google Scholar] [CrossRef] [PubMed]
- Sibakov, J.; Lehtinen, P.; Poutanen, K. Cereal Brans as Dietary Fibre Ingredients. In Fibre-Rich and Wholegrain Foods; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing Limited: Sawston, UK, 2013; pp. 170–192. ISBN 978-0-85709-578-7. [Google Scholar]
- Zitterman, A. DIETARY FIBER|Bran. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 1844–1850. ISBN 978-0-12-227055-0. [Google Scholar]
- Raman, M.; Doble, M. Physicochemical Characterization of Wheat Bran and Kappaphycus Alvarezii Dietary Fibres and Their Ability to Bind Mutagens, PhIP, Trp-P-2, AαC and BαP. LWT-Food Sci. Technol. 2015, 63, 169–176. [Google Scholar] [CrossRef]
- Harris, P.J.; Sasidharan, V.K.; Roberton, A.M.; Triggs, C.M.; Blakeney, A.B.; Ferguson, L.R. Adsorption of a Hydrophobic Mutagen to Cereal Brans and Cereal Bran Dietary Fibres. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1998, 412, 323. [Google Scholar] [CrossRef]
- Ryden, P.; Robertson, J.A. The Effect of Fibre Source and Fermentation on the Apparent Hydrophobic Binding Properties of Wheat Bran Preparations for the Mutagen 2-Amino-3,8-Dimethylimidazo[4,5-f]Quinoxaline (MeIQx). Carcinogenesis 1995, 16, 209–216. [Google Scholar] [CrossRef]
- Greco, D.; D’Ascanio, V.; Santovito, E.; Logrieco, A.F.; Avantaggiato, G. Comparative Efficacy of Agricultural By-Products in Sequestering Mycotoxins. J. Sci. Food Agric. 2019, 99, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Avantaggiato, G.; Greco, D.; Damascelli, A.; Solfrizzo, M.; Visconti, A. Assessment of Multi-Mycotoxin Adsorption Efficacy of Grape Pomace. J. Agric. Food Chem. 2014, 62, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.S.; Smith, T.K. Effect of Feeding Alfalfa and Refined Plant Fibers on the Toxicity and Metabolism of T-2 Toxin in Rats. J. Nutr. 1983, 113, 304–313. [Google Scholar] [CrossRef]
- Aoudia, N.; Callu, P.; Grosjean, F.; Larondelle, Y. Effectiveness of Mycotoxin Sequestration Activity of Micronized Wheat Fibres on Distribution of Ochratoxin A in Plasma, Liver and Kidney of Piglets Fed a Naturally Contaminated Diet. Food Chem. Toxicol. 2009, 47, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Frape, D.L.; Wayman, B.J.; Tuck, M.G.; Jones, E. The Effects of Gum Arabic, Wheat Offal and Various of Its Fractions on the Metabolism of 14C-Labelled Aflatoxin B1 in the Male Weanling Rat. Br. J. Nutr. 1982, 48, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Frape, D.L.; Wayman, B.J.; Tuck, M.G. The Effect of Dietary Fibre Sources on Aflatoxicosis in the Weanling Male Rat. Br. J. Nutr. 1981, 46, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Monge, M.d.P.; Magnoli, A.P.; Bergesio, M.V.; Tancredi, N.; Magnoli, C.E.; Chiacchiera, S.M. Activated Carbons as Potentially Useful Non-Nutritive Additives to Prevent the Effect of Fumonisin B1 on Sodium Bentonite Activity against Chronic Aflatoxicosis. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- FooDB. Available online: https://foodb.ca/compounds/FDB013235 (accessed on 13 May 2023).
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef]
- Sensoy, I. A Review on the Food Digestion in the Digestive Tract and the Used in Vitro Models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef]
- Adunphatcharaphon, S.; Petchkongkaew, A.; Greco, D.; D’Ascanio, V.; Visessanguan, W.; Avantaggiato, G. The Effectiveness of Durian Peel as a Multi-Mycotoxin Adsorbent. Toxins 2020, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Roye, C.; Bulckaen, K.; De Bondt, Y.; Liberloo, I.; Van De Walle, D.; Dewettinck, K.; Courtin, C.M. Side-by-Side Comparison of Composition and Structural Properties of Wheat, Rye, Oat, and Maize Bran and Their Impact on in Vitro Fermentability. Cereal Chem. 2020, 97, 20–33. [Google Scholar] [CrossRef]
- Palade, L.M.; Dore, M.I.; Marin, D.E.; Rotar, M.C.; Taranu, I. Assessment of Food By-Products’ Potential for Simultaneous Binding of Aflatoxin B1 and Zearalenone. Toxins 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383–122404. [Google Scholar] [CrossRef] [PubMed]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, e3039817. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Döll, S.; Dänicke, S.; Valenta, H.; Flachowsky, G. In Vitro Studies on the Evaluation of Mycotoxin Detoxifying Agents for Their Efficacy on Deoxynivalenol and Zearalenone. Arch. Anim. Nutr. 2004, 58, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Sundherg, A.; Sundherg, K.; Lillandt, C.; Holmhom, B. Determination of Hemicelluloses and Pectins in Wood and Pulp Fibres by Acid Methanolysis and Gas Chromatography. Nord. Pulp Pap. Res. J. 1996, 11, 216–219. [Google Scholar] [CrossRef]
- Van Craeyveld, V.; Swennen, K.; Dornez, E.; Van de Wiele, T.; Marzorati, M.; Verstraete, W.; Delaedt, Y.; Onagbesan, O.; Decuypere, E.; Buyse, J.; et al. Structurally Different Wheat-Derived Arabinoxylooligosaccharides Have Different Prebiotic and Fermentation Properties in Rats1,2. J. Nutr. 2008, 138, 2348–2355. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.H.; You, D.-J.; Kim, C.-I.; Lee, J.-Y.; Park, H.-M. Analytical Method of Multi-Mycotoxins in Table-Ready Foods for a Total Diet Study Using Stable Isotope Dilution Liquid Chromatography-Tandem Mass Spectrometry. J. AOAC Int. 2019, 102, 1657–1665. [Google Scholar] [CrossRef]

| Cereal Bran | Carbohydrate Composition (Mean ± SD, %) | ||||
|---|---|---|---|---|---|
| Arabinose | Xylose | Galactose | Glucose | Arabinoxylan † | |
| Maize | 14.1 ± 3.0 | 27.8 ± 5.4 | 4.6 ± 0.6 | 12.5 ± 0.9 | 38.6 ± 8.0 |
| Wheat | 5.9 ± 1.1 | 11.8 ± 1.4 | 0.87 ± 0.06 | 37.7 ± 2.7 | 17.1 ± 2.5 |
| Oat | 1.16 ± 0.03 | 1.44 ± 0.02 | 0.57 ± 0.08 | 71.3 ± 2.7 | 2.21 ± 0.08 |
| Model /Parameters | Bran Concentration | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.7% w/v | 5.0% w/v | |||||||||||
| Maize Bran | Wheat Bran | Oat Bran | Maize Bran | Wheat Bran | Oat Bran | |||||||
| pH 7 | pH 3 | pH 7 | pH 3 | pH 7 | pH 3 | pH 7 | pH 3 | pH 7 | pH 3 | pH 7 | pH 3 | |
| Linear | ||||||||||||
| K (mL/mg) | 0.3185 | 0.2452 | 0.0834 | 0.0638 | 0.0210 | 0.0260 | 0.3371 | 0.2467 | 0.0859 | 0.0664 | 0.0237 | 0.0265 |
| R2 | 0.9951 | 0.9963 | 0.9929 | 0.9919 | 0.9780 | 0.9949 | 0.9960 | 0.9921 | 0.9979 | 0.9994 | 0.9886 | 0.9986 |
| Langmuir | ||||||||||||
| qm (μg/mg) | 3.4216 | 1.0344 | 0.7289 | 0.8121 | 0.2371 | 59.0617 | 0.6173 | 1.0212 | 0.2970 | 4.3319 | 0.5291 | 0.4553 |
| KL (mL/μg) | 0.0963 | 0.2624 | 0.1286 | 0.0853 | 0.1044 | 0.0004 | 0.5852 | 0.2509 | 0.3309 | 0.0154 | 0.0470 | 0.0613 |
| R2 | 0.9909 | 0.9945 | 0.9888 | 0.9858 | 0.9617 | 0.9907 | 0.9934 | 0.9855 | 0.9986 | 0.9989 | 0.9788 | 0.9978 |
| Freundlich | ||||||||||||
| KF (mL1/n μg1−1/n/mg) | 0.3126 | 0.2329 | 0.0829 | 0.0637 | 0.0219 | 0.0253 | 0.3106 | 0.2490 | 0.0800 | 0.0656 | 0.0237 | 0.0264 |
| 1/n | 0.9824 | 0.9464 | 0.9424 | 0.9581 | 0.9158 | 1.0951 | 0.9623 | 1.0047 | 0.9208 | 0.9841 | 0.9832 | 0.9774 |
| R2 | 0.9908 | 0.9940 | 0.9877 | 0.9854 | 0.9604 | 0.9931 | 0.9931 | 0.9852 | 0.9982 | 0.9990 | 0.9786 | 0.9975 |
| Sips | ||||||||||||
| qms (μg/mg) | 1.2661 | 0.4603 | 0.2295 | 0.3151 | 0.1022 | 8.9526 | 0.2902 | 0.0982 | 0.2546 | 7.8490 | 0.1136 | 0.1110 |
| KS (mLns/μgns) | 0.2901 | 0.7519 | 0.5769 | 0.2560 | 0.2894 | 0.0028 | 1.5528 | 9.0377 | 0.4015 | 0.0084 | 0.2683 | 0.3107 |
| 1/ns | 1.0434 | 1.1024 | 1.2525 | 1.1072 | 1.1938 | 1.0979 | 1.0627 | 1.4011 | 1.0167 | 0.9866 | 1.1535 | 1.1373 |
| R2 | 0.9909 | 0.9948 | 0.9903 | 0.9861 | 0.9626 | 0.9931 | 0.9935 | 0.9896 | 0.9986 | 0.9990 | 0.9795 | 0.9983 |
| Initial Volume (mL) | Added Volume (mL) | Settling Time (min) | Aflatoxin B1 Adsorption † (Mean ± SD, %) | ||
|---|---|---|---|---|---|
| Maize Bran | Wheat Bran | Charcoal | |||
| 1.5 | - | 15 | 82.4 ± 2.8 a | 54.1 ± 3.7 a | 100 |
| 1.5 | 1.5 | 15 | 81.4 ± 1.4 a | 55.2 ± 6.9 a | 100 |
| 3.0 | - | 15 | 68.6 ± 1.2 b | 39.6 ± 1.2 b | 100 |
| Model | Equation | Description |
|---|---|---|
| Linear (Henry’s law) | qe is the adsorbed amount at equilibrium (μg/mg) Ce is the adsorbate concentration at equilibrium (μg/mL) K is the partition coefficient (mL/mg) | |
| Langmuir | qm is the maximum adsorption capacity (μg/mg) KL is the ratio of the adsorption rate and desorption rate (mL/μg) | |
| RL † is the separation factor C0 is the low initial adsorbate concentration (μg/mL) | ||
| Freundlich | KF (mL1/n μg1−1/n/mg) and n are the Freundlich constants | |
| Sips ‡ | qms is the maximum adsorbed amount (μg/mg) Ks (mLns/μgns) and ns are the Sips constants |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Lemmetty, J.M.; Nihtilä, H.; Koivula, H.; Samandoulougou, S.; Sawadogo-Lingani, H.; Katina, K.; Maina, N.H. Efficacy of Aflatoxin B1 and Fumonisin B1 Adsorption by Maize, Wheat, and Oat Bran. Toxins 2024, 16, 288. https://doi.org/10.3390/toxins16070288
Lee Y, Lemmetty JM, Nihtilä H, Koivula H, Samandoulougou S, Sawadogo-Lingani H, Katina K, Maina NH. Efficacy of Aflatoxin B1 and Fumonisin B1 Adsorption by Maize, Wheat, and Oat Bran. Toxins. 2024; 16(7):288. https://doi.org/10.3390/toxins16070288
Chicago/Turabian StyleLee, Youngsun, Jenna M. Lemmetty, Hanna Nihtilä, Hanna Koivula, Serge Samandoulougou, Hagretou Sawadogo-Lingani, Kati Katina, and Ndegwa H. Maina. 2024. "Efficacy of Aflatoxin B1 and Fumonisin B1 Adsorption by Maize, Wheat, and Oat Bran" Toxins 16, no. 7: 288. https://doi.org/10.3390/toxins16070288





