Three Ecological Models to Evaluate the Effectiveness of Trichoderma spp. for Suppressing Aflatoxigenic Aspergillus flavus and Aspergillus parasiticus
Abstract
1. Introduction
2. Results
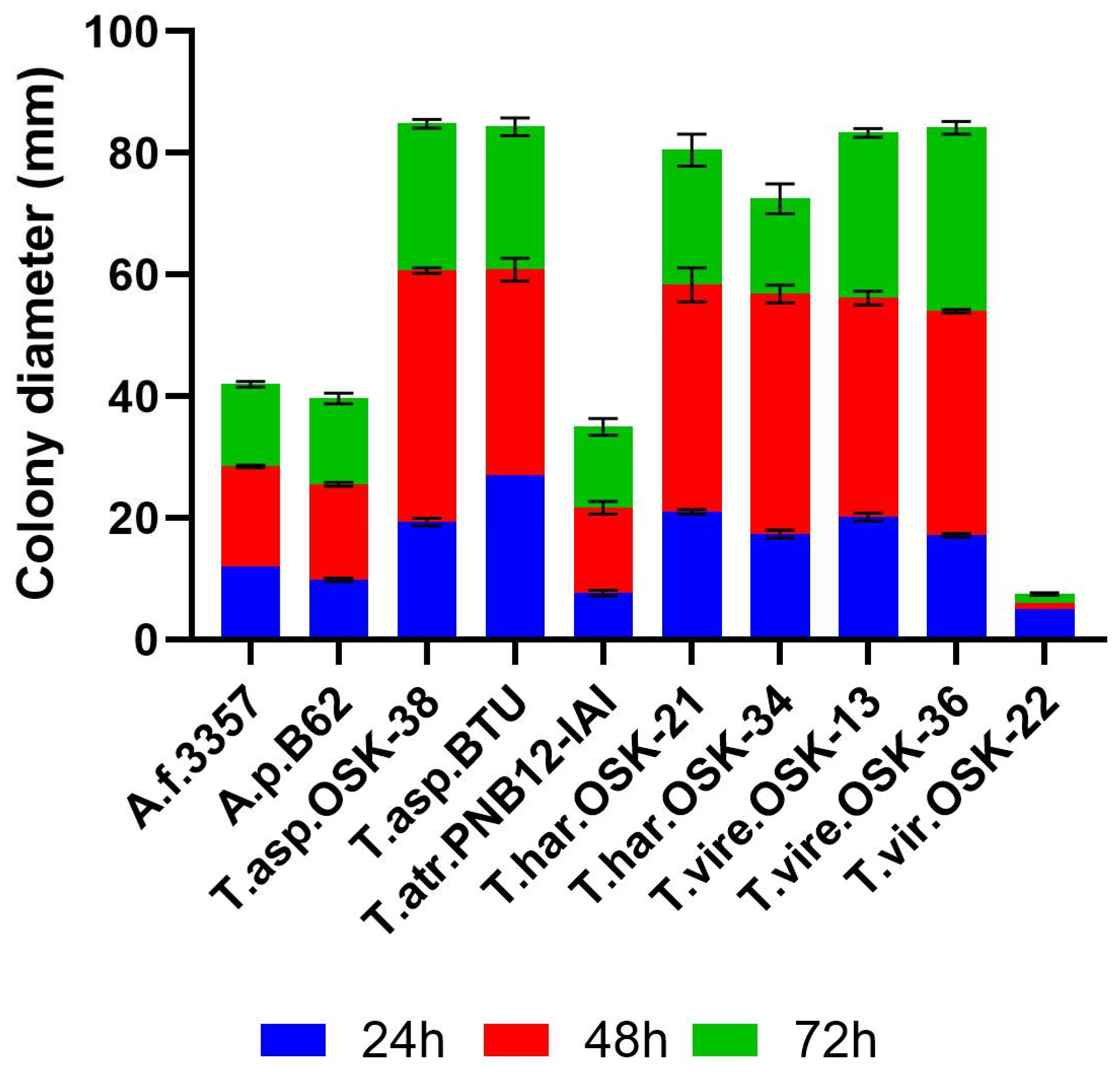
2.1. Growth Rate as a Pre-Requisite of Strain Fitness during Interaction Studies
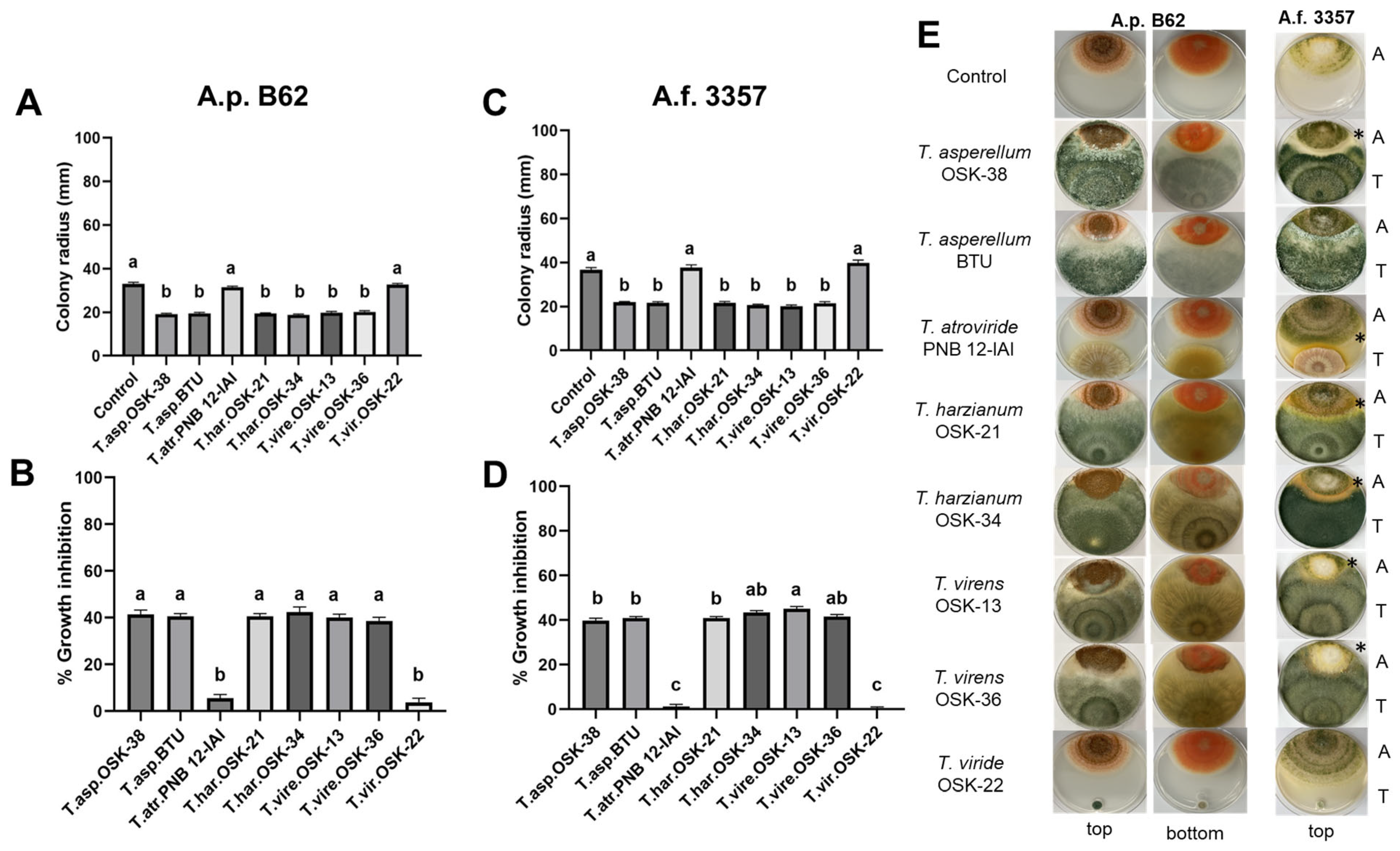
2.2. Dual Culture Interaction Assay between Trichoderma and Aspergillus
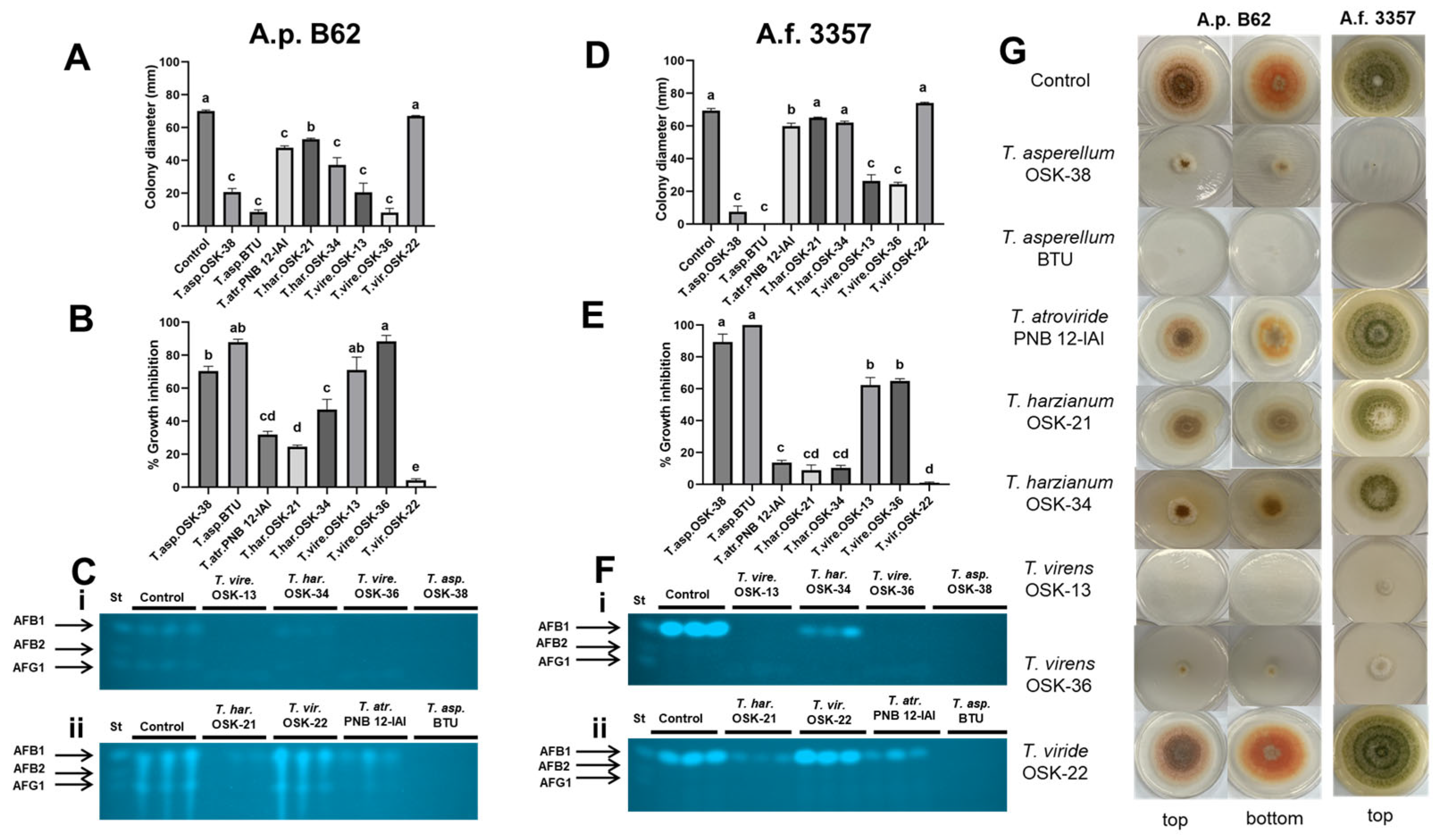
2.3. Sandwiched Plate Interaction Assay to Assess the Effect of Volatile Organic Compounds between Trichoderma and Aspergillus
2.4. Cellophane Membrane-Based Interaction Assay between Trichoderma and Aspergillus
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fungal Cultures and Growth Conditions
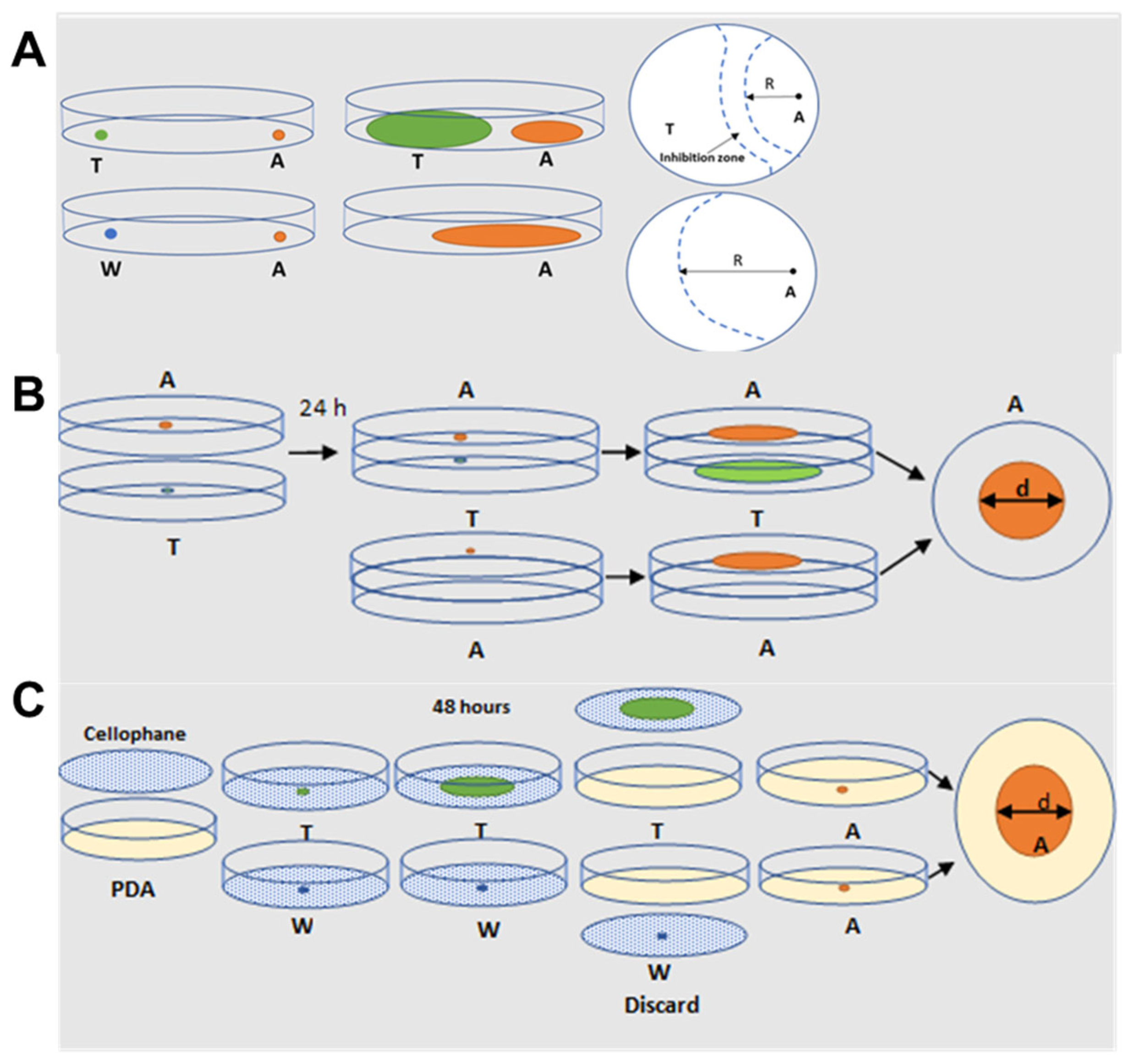
5.2. Dual Culture Plate Interaction Assay
5.3. Sandwiched Plate Assay for VOC-Mediated Interaction
5.4. Cellophane Assay for Non-Volatile Compounds Mediated Interaction
5.5. Examination of AF Levels in A. parasiticus and A. flavus by TLC
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Peles, F.; Sipos, P.; Győri, Z.; Pfliegler, W.P.; Giacometti, F.; Serraino, A.; Pagliuca, G.; Gazzotti, T.; Pócsi, I. Adverse effects, transformation and channeling of aflatoxins into food raw materials in livestock. Front. Microbiol. 2019, 10, 2861. [Google Scholar] [CrossRef] [PubMed]
- Norlia, M.; Jinap, S.; Nor-Khaizura, M.A.R.; Radu, S.; Samsudin, N.I.P.; Azri, F.A. Aspergillus section Flavi and aflatoxins: Occurrence, detection, and identification in raw peanuts and peanut-based products along the supply chain. Front. Microbiol. 2019, 10, 2602. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Rahman, M.A.H.; Selamat, J.; Samsudin, N.I.P.; Shaari, K.; Mahror, N.; John, J.M. Antagonism of nonaflatoxigenic Aspergillus flavus isolated from peanuts against aflatoxigenic A. flavus growth and aflatoxin B1 production in vitro. Food Sci. Nutr. 2022, 10, 3993–4002. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in food and feed: An overview on prevalence, detection and control strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef] [PubMed]
- Pfliegler, W.P.; Pócsi, I.; Győri, Z.; Pusztahelyi, T. The Aspergilli and their mycotoxins: Metabolic interactions with plants and the soil biota. Front. Microbiol. 2020, 10, 2921. [Google Scholar] [CrossRef]
- Katati, B.; Kovács, S.; Njapau, H.; Kachapulula, P.W.; Zwaan, B.J.; Van Diepeningen, A.D.; Schoustra, S.E. Maize Aspergillus section Flavi isolate diversity may be distinct from that of soil and subsequently the source of aflatoxin contamination. Mycotoxin Res. 2024, 1–17. [Google Scholar] [CrossRef]
- Alaniz Zanon, M.S.; Clemente, M.P.; Chulze, S.N. Characterization and competitive ability of non-aflatoxigenic Aspergillus flavus isolated from the maize agro-ecosystem in Argentina as potential aflatoxin biocontrol agents. Int. J. Food Microbiol. 2018, 277, 58–63. [Google Scholar] [CrossRef]
- Alaniz Zanon, M.S.A.; Pena, G.; Yerkovich, N.; Bossa, M.; Chiotta, M.L.; Chulze, S.N. Aflatoxins and fumonisins in maize under a climate change scenario. Biocontrol strategies at the pre-harvest stage. Eur. J. Plant Pathol. 2023, 167, 551–567. [Google Scholar] [CrossRef]
- Yu, J.; Hennessy, D.A.; Tack, J.; Wu, F. Climate change will increase aflatoxin presence in US corn. Environ. Res. Lett. 2022, 17, 054017. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [PubMed]
- Ouadhene, M.A.; Callicott, K.A.; Ortega-Beltran, A.; Mehl, H.L.; Cotty, P.J.; Battilani, P. Structure of Aspergillus flavus populations associated with maize in Greece, Spain, and Serbia: Implications for aflatoxin biocontrol on a regional scale. Environ. Microbiol. Rep. 2024, 16, e13249. [Google Scholar] [CrossRef]
- Hruska, Z.; Rajasekaran, K.; Yao, H.; Kincaid, R.; Darlington, D.; Brown, R.L.; Bhatnagar, D.; Cleveland, T.E. Co-inoculation of aflatoxigenic and non-aflatoxigenic strains of Aspergillus flavus to study fungal invasion, colonization, and competition in maize kernels. Front. Microbiol. 2014, 5, 122. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Beltran, A.; Moral, J.; Picot, A.; Puckett, R.D.; Cotty, P.J.; Michailides, T.J. Atoxigenic Aspergillus flavus isolates endemic to almond, fig, and pistachio orchards in California with potential to reduce aflatoxin contamination in these crops. Plant Dis. 2019, 103, 905–912. [Google Scholar] [CrossRef]
- Dorner, J.W.; Cole, R.J.; Blankenship, P.D. Effect of inoculum rate of biological control agents on preharvest aflatoxin contamination of peanuts. Biol. Control 1998, 12, 171–176. [Google Scholar] [CrossRef]
- Dorner, J.W.; Cole, R.J. Effect of application of nontoxigenic strains of Aspergillus flavus and A. parasiticus on subsequent aflatoxin contamination of peanuts in storage. J. Stored Prod. Res. 2002, 38, 329–339. [Google Scholar] [CrossRef]
- Dorner, J.W.; Lamb, M.C. Development and commercial use of Afla-Guard®, an aflatoxin biocontrol agent. Mycotox. Res. 2006, 22, 33–38. [Google Scholar] [CrossRef]
- Olowe, O.M.; Nicola, L.; Asemoloye, M.D.; Akanmu, A.O.; Sobowale, A.A.; Babalola, O.O. Characterization and antagonistic potentials of selected rhizosphere Trichoderma species against some Fusarium species. Front. Microbiol. 2022, 13, 985874. [Google Scholar] [CrossRef]
- Sridharan, A.P.; Thankappan, S.; Karthikeyan, G.; Uthandi, S. Comprehensive profiling of the VOCs of Trichoderma longibrachiatum EF5 while interacting with Sclerotium rolfsii and Macrophomina phaseolina. Microbiol. Res. 2020, 236, 126436. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R.S. Antagonistic assessment of Trichoderma spp. by producing volatile and non-volatile compounds against different fungal pathogens. Arch. Phytopathol. Plant Prot. 2017, 50, 629–648. [Google Scholar] [CrossRef]
- Win, T.T.; Bo, B.; Malec, P.; Khan, S.; Fu, P. Newly isolated strain of Trichoderma asperellum from disease suppressive soil is a potential bio-control agent to suppress Fusarium soil borne fungal phytopathogens. J. Plant Pathol. 2021, 103, 549–561. [Google Scholar] [CrossRef]
- Dutta, P.; Deb, L.; Pandey, A.K. Trichoderma- from lab bench to field application: Looking back over 50 years. Front. Agron. 2022, 4, 932839. [Google Scholar] [CrossRef]
- Lee, S.; Yap, M.; Behringer, G.; Hung, R.; Bennett, J.W. Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol. 2016, 3, 7. [Google Scholar] [CrossRef]
- Álvarez-García, S.; Mayo-Prieto, S.; Carro-Huerga, G.; Rodríguez-González, Á.; González-López, Ó.; Gutiérrez, S.; Casquero, P.A. Volatile organic compound chamber: A novel technology for microbiological volatile interaction assays. J. Fungi 2021, 7, 248. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Rai, P.; Srivastava, A.K.; Kumar, S. Trichoderma for climate resilient agriculture. World J. Microbiol. Biotechnol. 2017, 33, 155. [Google Scholar] [CrossRef]
- Li, N.; Alfiky, A.; Wang, W.; Islam, M.; Nourollahi, K.; Liu, X.; Kang, S. Volatile compound-mediated recognition and inhibition between Trichoderma biocontrol agents and Fusarium oxysporum. Front. Microbiol. 2018, 9, 2614. [Google Scholar] [CrossRef] [PubMed]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Ramírez-Valdespino, C.A.; Casas-Flores, S.; Olmedo-Monfil, V. Trichoderma as a model to study effector-like molecules. Front. Microbiol. 2019, 10, 1030. [Google Scholar] [CrossRef]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Seed biopriming with drought tolerant isolates of Trichoderma harzianum promote growth and drought tolerance in Triticum aestivum. Ann. Appl. Biol. 2015, 166, 171–182. [Google Scholar] [CrossRef]
- Senizza, B.; Araniti, F.; Lewin, S.; Wende, S.; Kolb, S.; Lucini, L. Trichoderma spp.-mediated mitigation of heat, drought, and their combination on the Arabidopsis thaliana holobiont: A metabolomics and metabarcoding approach. Front. Plant Sci. 2023, 14, 1190304. [Google Scholar] [CrossRef]
- Zehra, A.; Dubey, M.; Meena, M.; Upadhyay, R. Effect of different environmental conditions on growth and sporulation of some Trichoderma species. J. Environ. Biol. 2017, 38, 197–203. [Google Scholar] [CrossRef]
- Calistru, C.; McLean, M.; Berjak, P. In vitro studies on the potential for biological control of Aspergillus flavus and Fusarium moniliforme by Trichoderma species. A study of the production of extracellular metabolites by Trichoderma species. Mycopathologia 1997, 137, 115–124. [Google Scholar] [CrossRef]
- Braun, H.; Woitsch, L.; Hetzer, B.; Geisen, R.; Zange, B.; Schmidt-Heydt, M. Trichoderma harzianum: Inhibition of mycotoxin producing fungi and toxin biosynthesis. Int. J. Food Microbiol. 2018, 280, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Branà, M.T.; Haidukowski, M.; Gallo, A.; Zhang, Q.; Logrieco, A.F.; Li, P.; Zhao, S.; Altomare, C. Potential of Trichoderma spp. for biocontrol of aflatoxin-producing Aspergillus flavus. Toxins 2022, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Boukaew, S.; Kumla, J.; Prasertsan, P.; Cheirsilp, B.; Petlamul, W.; Srinuanpan, S. In vitro and in situ antifungal properties of a Trichoderma asperelloides SKRU-01 against aflatoxigenic Aspergillus species. Food Control 2023, 154, 110025. [Google Scholar] [CrossRef]
- Chiuraise, N.; Yobo, K.S.; Laing, M.D. Seed treatment with Trichoderma harzianum strain kd formulation reduced aflatoxin contamination in groundnuts. J. Plant Dis. Prot. 2015, 122, 74–80. [Google Scholar] [CrossRef]
- Lagogianni, C.S.; Tsitsigiannis, D.I. Effective biopesticides and biostimulants to reduce aflatoxins in maize fields. Front. Microbiol. 2019, 10, 2645. [Google Scholar] [CrossRef]
- Sivparsad, B.J.; Laing, M.D. Pre-harvest silk treatment with Trichoderma harzianum reduces aflatoxin contamination in sweetcorn. J. Plant Dis. Prot. 2016, 123, 285–293. [Google Scholar] [CrossRef]
- Boukaew, S.; Petlamul, W.; Yossan, S.; Srinuanpan, S.; Nooprom, K.; Zhang, Z. Biofumigant potential and inhibition mechanism of Trichoderma asperelloides SKRU-01 volatile organic compounds for controlling aflatoxigenic Aspergillus parasiticus and Aspergillus flavus in stored peanuts. Food Control 2024, 157, 110194. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of action of microbial biological control agents against plant diseases: Relevance beyond efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.M. Effect of media on growth and interactions between a range of soil-borne glasshouse pathogens and antagonistic fungi. New Phytol. 1987, 107, 127–142. [Google Scholar] [CrossRef]
- Roze, L.V.; Beaudry, R.M.; Arthur, A.E.; Calvo, A.M.; Linz, J.E. Aspergillus volatiles regulate aflatoxin synthesis and asexual sporulation in Aspergillus parasiticus. Appl. Environ. Microbiol. 2007, 73, 7268–7276. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.; Day, D.; Linz, J. Effects of Zinc chelators on aflatoxin production in Aspergillus parasiticus. Toxins 2016, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, N.; Prameeladevi, T.; Sathiyabama, M.; Kamil, D. Multiplex PCR for detection and differentiation of diverse Trichoderma species. Ann. Microbiol. 2015, 65, 1591–1595. [Google Scholar] [CrossRef]
- Abarca, M.L.; Bragulat, M.R.; Bruguera, M.T.; Cabañes, F.J. Comparison of some screening methods for aflatoxigenic moulds. Mycopathologia 1988, 104, 75–79. [Google Scholar] [CrossRef]

| Trichoderma | A.p. B62 | A.f. 3357 | ||
|---|---|---|---|---|
| % I a | Interaction Type b | % I a | Interaction Type b | |
| T. asperellum OSK-38 | 41.5 ± 1.8 | 1 | 39.8 ± 1.1 | 4 |
| T. asperellum BTU | 40.7 ± 1.1 | 1 | 40.9 ± 0.8 | 1/2 |
| T. atroviride PNB 12-IAI | 5.5 ± 1.6 | 0 | 1.2 ± 0.9 | 3 |
| T. harzianum OSK-21 | 40.6 ± 1.2 | 1 | 40.9 ± 0.8 | 3 |
| T. harzianum OSK-34 | 42.4 ± 2.2 | 1/2 | 43.5 ± 0.8 | 4 |
| T. virens OSK-13 | 40.1 ± 1.5 | 1/2 | 45.1 ± 0.9 | 3 |
| T. virens OSK-36 | 38.6 ±1.5 | 1/2 | 41.6 ± 0.9 | 3 |
| T. viride OSK-22 | 3.8 ± 1.7 | 0 | 0.6 ± 0.4 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voloshchuk, N.; Irakoze, Z.; Kang, S.; Kellogg, J.J.; Wee, J. Three Ecological Models to Evaluate the Effectiveness of Trichoderma spp. for Suppressing Aflatoxigenic Aspergillus flavus and Aspergillus parasiticus. Toxins 2024, 16, 314. https://doi.org/10.3390/toxins16070314
Voloshchuk N, Irakoze Z, Kang S, Kellogg JJ, Wee J. Three Ecological Models to Evaluate the Effectiveness of Trichoderma spp. for Suppressing Aflatoxigenic Aspergillus flavus and Aspergillus parasiticus. Toxins. 2024; 16(7):314. https://doi.org/10.3390/toxins16070314
Chicago/Turabian StyleVoloshchuk, Nataliia, Zilfa Irakoze, Seogchan Kang, Joshua J. Kellogg, and Josephine Wee. 2024. "Three Ecological Models to Evaluate the Effectiveness of Trichoderma spp. for Suppressing Aflatoxigenic Aspergillus flavus and Aspergillus parasiticus" Toxins 16, no. 7: 314. https://doi.org/10.3390/toxins16070314
APA StyleVoloshchuk, N., Irakoze, Z., Kang, S., Kellogg, J. J., & Wee, J. (2024). Three Ecological Models to Evaluate the Effectiveness of Trichoderma spp. for Suppressing Aflatoxigenic Aspergillus flavus and Aspergillus parasiticus. Toxins, 16(7), 314. https://doi.org/10.3390/toxins16070314






