Health and Environmental Impacts of Cyanobacteria and Cyanotoxins from Freshwater to Seawater
Abstract
:1. Introduction
2. Cyanotoxins
2.1. Hepatotoxins
2.1.1. Microcystins (MCs)
| Cyanotoxin | Cyanobacterial-Producers | Cell Quota | Toxic Effects | Cyanotoxin-Producers | References |
|---|---|---|---|---|---|
| Microcystins (MCs) | Aphanizomenon Dolichospermum Limnothrix Microcystis Nostoc Oscillatoria Phormidium Planktothrix Gloeotrichia Hapalosiphon Radiocystis | <150 to 850 fg/cell | diarrhea, vomiting, stomach cramps, nausea, acute liver failure, chronic kidney disease, respiratory symptoms, abdominal pain, sore throat, dry cough, blistering at the mouth, headache, flulike symptoms, irritation and rashes (colorectal cancer). 1 | Microcystis spp., M. aeruginosa, M. viridis, Dolichospermum sp., Anabaena flos-aquae, A. subcylindrica, A. variabilis, Oscillatoria (Planktothrix) agardhii, Nostoc sp., Nostoc spongiaeforme, Anabaenopsis sp., Hapalosiphon sp., Gloeotrichia echinulata, Plectonema boryanum, Phormidium corium, Phormidium splendidum, Rivularia biasolettiana, R. haematites, Tolypothrix distorta, Arthrospira fusiformis. | [12,90,91,92] |
| Nodularins (NODs) 2 | Nodularia Noctoc | 60–500 fg/cel | Allergic reactions, skin rashes, gastrointestinal illness, nausea, liver damage, bleeding. | Nodularia spumigena, Nostoc sp. | [2,12,91] |
| Cylindrospermopsins (CYNs) | Anabaena Cylindrospermopsis Aphanizomenon Chrysosporum Raphidiopsis Umezakia | <1.9 to 196 fg/cell | Nausea, vomiting, diarrhea, stomach cramps, hepatomegaly, kidney dysfunction. | Cylindrospermopsis raciborskii, Aphanizomenon ovalisporum, Dolichospermum sp., Anabaena lapponica, Raphidiopsis curvata, Umezakia natans. | [12,90,91] |
| Anatoxin-a (ATX) | Anabaena Aphanizomenon Cylindrospermum Microcystis Oscillatoria Phormidium Planktothrix | 0.1–500 fg/cell | Convulsions, fatigue, paralysis, respiratory failure. | Arthrospira fusiformis, Anabaena spp., Aphanizomenon sp., Phormidium sp., Anabaena flos-aquae, Anabaena planktonica, Cylindrospermum sp., Oscillatoria sp., Raphidiopsis meditteranea, Phormidium formosum. | [90,91] |
| Saxitoxins (STXs) | Cuspidothrix Dolichospermum Microseira RaphidiopsisPlanktothrix Oxynema | 120–1300 fg/cell | Tingling sensation around the lips, convulsions, headaches, dizziness, nausea, vomiting fatigue, paralysis, respiratory failure. | Aphanizomenon flos-aquae, Microseira sp. Dolichospermum circinale, Cylindrospermopsis raciborskii, Planktothrix sp. | [90,91] |
| Cyanotoxin | Primary Toxicity | Mode of Action | Lifetime Drinking Water (µg/L) | Short-Term Drinking Water (µg/L) | Recreational Water (µg/L) | LD50 | TDI (µg/kg/day) | NOAEL (µg/kg bw/day) | LOAEL (µg/kg bw/day) | Classification | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Microcystins | Hepatotoxicity | Inhibition of protein phosphatases | 1.0 | 12.0 | 24.0 | 5.0–10.9 (oral; µg/kg) | 0.04 | 40.0 | 50.0 | Group 2B—possibly carcinogenic to humans. | [2,53,87,93,94,95] |
| Nodularins | Hepatotoxicity | Inhibition of protein phosphatases | 1.0 | 12.0 1 | n.d. | 50.0 (intraperitoneal; µg/kg) | 0.04 | 100.0 | n.d. | Group 3— not classifiable as to its carcinogenicity to humans. | [50,53,93,96] |
| Cylindrospermopsin | Hepatotoxicity | Inhibition of protein phosphatases | 0.7 (0.01) | 3.0 | 6.0 | 4.4–6.9 (oral; µg/kg) | 0.03 2 | 30.0 | 150.0 | n.d. (potential for carcinogenicity) | [52,53,97,98] |
| Anatoxin-a | Neurotoxicity | Nicotinic acetylcholine receptor agonists | 1.0 | 30 3 | 60.0 | >5000.0 (oral; µg/kg) | 0.1 2 | 98.0 | n.d. | n.d. | [52,53,97,99] |
| Anatoxin-a(s) | Neurotoxicity | Inhibition of acetylcholinesterase | 1.0 | n.d. | n.d. | 20.0–40.0 (intraperitoneal; µg/kg) | n.d | n.d | n.d | n.d. | [52,100,101,102] |
| Saxitoxins | Neurotoxicity | Blocking of sodium channels | 3.0 | 0.3 | 30.0 | 35.0 (oral; µg/kg) | 0.05 2 | 0.5 | 1.5 | n.d. | [52,53,97,101,103] |
2.1.2. Nodularins (NODs)
2.2. Neurotoxins
2.2.1. Anatoxins (ATXs)
2.2.2. Saxitoxins (STXs)
2.3. Cytotoxin
Cylindrospermopsins (CYNs)
| Country/Organization | Cyanotoxin | Maximum Concentration in Drinking Water | Maximum Concentration in Recreational Water | References |
|---|---|---|---|---|
| WHO | Microcystin-LR | 1.0 μg/L | 24.0 μg/L | [188] |
| Cylindrospermopsin | 0.7 μg/L | 6.0 μg/L | ||
| Saxitoxins | 3.0 μg/L | 30.0 μg/L | ||
| Anatoxin-a | 3.0 μg/L | 60.0 μg/L | ||
| USEPA | Microcystin | 0.3 μg/L 1 1.6 μg/L 2 | <8.0 μg/L | [189,190,191,192] |
| Cylindrospermopsin | 0.7 μg/L 1 3.0 μg/L 2 | <15.0 μg/L | ||
| Saxitoxins | 0.3 μg/L 1 1.6 μg/L 2 | 8.0 μg/L | ||
| Anatoxin-a | 0.7 μg/L 1 3.0 μg/L 2 | 15.0 µg/L | ||
| EU | Microcystin-LR | 1.0 μg/L | [192] | |
| China | Microcystin-LR | 1.0 μg/L 4 | ND | [190] |
| Australia | Microcystin | 1.3 μg/L | ≤10.0 μg/L | [191] |
| Nodularin | 1.3 μg/L | ≤10.0 μg/L | ||
| Cylindrospermopsin | 0.9 μg/L | ND | ||
| Anatoxin-a | 3.1 μg/L | ND | ||
| Saxitoxins | 3.1 μg/L | ND | ||
| Brazil | Microcystin | 1.0 μg/L | ND | [193] |
| Cylindrospermopsin | 15.0 μg/L | ND | ||
| Saxitoxins | 3.0 μg/L (STX equiv.) | ND | ||
| Nodularin | 1.0 μg/L | ND | ||
| Canada | Microcystin-LR | 1.5 μg/L 3 | 10 μg/L | [194] |
| Denmark | Microcystin | 1.0 μg/L 3 | 20 μg/L | [195] |
| Belgium and Luxembourg | Microcystin-LR | 1.0 μg/L 3 | 20 μg/L | [196] |
| France | Microcystin-LR | 1.0 μg/L 3 | ≤25.0 μg/L 13.0 μg/L | [197] |
| Finland | Microcystin | <1.0 µg/L 3 | ND | [198] |
| Germany | Microcystin | <1.0 µg/L 3 | <10.0 µg/L | [199] |
| Greece | Microcystin-LR | 1.0 μg/L 3 | ND | [200] |
| Italy | Microcystin | ND 3 | < 25.0 µg/L | [201] |
| Poland | Microcystin-LR | 1.0 μg/L 3 | 20.0 μg/L | [202] |
| Czech Republic | Microcystin-LR | 1.0 μg/L 3 | ND | [203] |
| Portugal | Microcystin-LR | 1.0 μg/L 3 | ND | [204] |
| Netherlands | Microcystin | 1.0 μg/L 3 | <20.0 µg/L | [205] |
| New Zealand | Microcystin | 1.0 μg/L | ≤12.0 μg/L | [206] |
| Cylindrospermopsin | 1.0 μg/L | ND | ||
| Saxitoxins | 3.0 μg/L | ND | ||
| Anatoxin-a | 6.0 μg/L | ND | ||
| Anatoxin-a(s) | 1.0 μg/L | ND | ||
| Homoanatoxin-a | 2.0 μg/L | ND | ||
| Nodularin | 1.0 μg/L | ND | ||
| Singapore | Microcystin | 1.0 μg/L 4 | ND | [207] |
| Spain | Microcystin | 1.0 μg/L 4 | ND | [208] |
| Turkey | Microcystin-LR | 1.0 μg/L 3 | <25.0 µg/L | [209] |
| Cylindrospermopsin | 1.0 μg/L | ND | ||
| Uruguay | Microcystin-LR | 1.0 μg/L | 20.0 μg/L | [210] |
| Cylindrospermopsin | 0.5 μg/L | ND | ||
| Saxitoxins | 3.0 μg/L | ND | ||
| South Africa | Microcystin-LR | ND | <10.0 µg/L | [211] |
| Perú | Microcystin-LR | 1.0 μg/L 4 | ND | [212] |
| Argentina | Microcystin-LR | 1.0 μg/L 4 | ND | [213] |
| Mexico | Microcystin-LR | 1.0 μg/L 4 | ND | [214] |
| Costa Rica | Microcystin-LR | 1.0 μg/L 4 | ND | [215] |
| Paraguay | Microcystin-LR | 1.0 μg/L 4 | ND | [216] |
| Panama | Microcystin-LR | 1.0 μg/L 4 | ND | [217] |
3. Food Web Transfer
3.1. Freshwater Vectors
3.2. Marine Vectors
3.3. Non-Traditional Vectors
Cyanotoxins in Crops
4. Human Exposure to Cyanotoxins
5. Risks of cyanoHABs
6. Discussion
7. Conclusions and Future Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cirés, S.; Casero, M.C.; Quesada, A. Toxicity at the Edge of Life: A Review on Cyanobacterial Toxins from Extreme Environments. Mar. Drugs 2017, 15, 233. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto António, D.; Loos, R.; Lettieri, T. Cyanotoxins: Methods and Approaches for Their Analysis and Detection; Joint Research Centre: Brussels, Belgium, 2017. [Google Scholar]
- Jacinavicius, F.R.; Geraldes, V.; Fernandes, K.A.; Crnkovic, C.M.; Gama, W.A.; Pinto, E. Toxicological effects of cyanobacterial metabolites on zebrafish larval development. Harmful Algae 2023, 125, 102430. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Testai, E.; Funari, E.; Svirev, Z. Cyanobacteria, cyanotoxins, and human health. In Water Treatment for Purification from Cyanobacteria and Cyanotoxins; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 37–68. [Google Scholar]
- Reinl, K.L.; Brookes, J.D.; Carey, C.C.; Harris, T.D.; Ibelings, B.W.; Morales-Williams, A.M.; de Senerpont Domis, L.N.; Atkins, K.S.; Isles, P.D.F.; Mesman, J.P.; et al. Cyanobacterial blooms in oligotrophic lakes: Shifting the high-nutrient paradigm. Freshw. Biol. 2021, 66, 1846–1859. [Google Scholar] [CrossRef]
- Harris, T.D.; Reinl, K.L.; Azarderakhsh, M.; Berger, S.A.; Castro Berman, M.; Bizic, M.; Bhattacharya, R.; Burnet, S.H.; Cianci-Gaskill, J.A.; de Senerpont Domis, L.N.; et al. What makes a cyanobacterial bloom disappear? A review of the abiotic and biotic cyanobacterial bloom loss factors. Harmful Algae 2024, 133, 102599. [Google Scholar] [CrossRef] [PubMed]
- Huertas Romera, M.J.; Mallén Ponce, M.J. Dark side of cyanobacteria: Searching for strategies to control blooms. Microb. Biotechnol. 2021, 15, 1321–1323. [Google Scholar] [CrossRef]
- Bouteiller, P.; Lance, E.; Guérin, T.; Biré, R. Analysis of total-forms of cyanotoxins microcystins in biological matrices: A methodological review. Toxins 2022, 14, 550. [Google Scholar] [CrossRef]
- Facey, J.A.; Apte, S.C.; Mitrovic, S.M. A review of the effect of trace metals on freshwater cyanobacterial growth and toxin production. Toxins 2019, 11, 643. [Google Scholar] [CrossRef]
- Burford, M.A.; Carey, C.C.; Hamilton, D.P.; Huisman, J.; Paerl, H.W.; Wood, S.; Wulff, A. Perspective: Advancing the research agenda for improving understanding of cyanobacteria in a future of global change. Harmful Algae 2020, 91, 101601. [Google Scholar] [CrossRef]
- Dai, R.; Li, Z.; Yan, F.; An, L.; Du, W.; Li, X. Evaluation of changes in M. aeruginosa growth and microcystin production under phosphorus starvation via transcriptomic surveys. Sci. Total Environ. 2023, 893, 164848. [Google Scholar] [CrossRef]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Humbert, J.F.; Fastner, J. Ecology of cyanobacteria. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 9–18. [Google Scholar]
- Janssen, A.B.G.; Janse, J.H.; Beusen, A.H.W.; Chang, M.; Harrison, J.A.; Huttunen, I.; Kong, X.; Rost, J.; Teurlincx, S.; A Troost, T.; et al. How to model algal blooms in any lake on earth. Curr. Opin. Environ. Sustain. 2019, 36, 1–10. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Igwaran, A.; Kayode, A.J.; Moloantoa, K.M.; Khetsha, Z.P.; Unuofin, J.O. Cyanobacteria harmful algae blooms: Causes, impacts, and risk management. Water Air Soil Pollut. 2024, 235, 71. [Google Scholar] [CrossRef]
- Wood, S.; Kelly, L.; Bouma-Gregson, K.; Humbert, J.F.; Laughinghouse, H.D., IV; Lazorchak, J.; McAllister, T.; McQueen, A.; Pokrzywinski, K.; Puddick, J.; et al. Toxic benthic freshwater cyanobacterial proliferations: Challenges and solutions for enhancing knowledge and improving monitoring and mitigation. Freshw. Biol. 2020, 65, 1824–1842. [Google Scholar] [CrossRef]
- Schets, F.M.; Van der Oost, R.; Van de Waal, D.B. Cyanobacteria Protocol 2020; RIVM: Bilthoven, The Netherlands, 2020. [Google Scholar]
- Khiari, D. Managing Cyanotoxins; The ater Research Foundation: Denver, CO, USA, 2019. [Google Scholar]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev. Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Reinl, K.L.; Harris, T.D.; Elfferich, I.; Coker, A.; Zhan, Q.; Domis, L.N.D.S.; Morales-Williams, A.M.; Bhattacharya, R.; Grossart, H.P.; North, R.L.; et al. The role of organic nutrients in structuring freshwater phytoplankton communities in a rapidly changing world. Water Res. 2022, 219, 118573. [Google Scholar] [CrossRef]
- Lyche Solheim, A.; Gundersen, H.; Mischke, U.; Skjelbred, B.; Nejstgaard, J.C.; Guislain, A.L.; Sperfeld, E.; Giling, D.P.; Haande, S.; Ballot, A.; et al. Lake browning counteracts cyanobacteria responses to nutrients: Evidence from phytoplankton dynamics in large enclosure experiments and comprehensive observational data. Glob. Change Biol. 2024, 30, 17013. [Google Scholar] [CrossRef]
- Paerl, H.W.; Scott, J.T.; McCarthy, M.J.; Newell, S.E.; Gardner, W.S.; Havens, K.E.; Hoffman, D.K.; Wilhelm, S.W.; Wurtsbaugh, W.A. It takes two to tango: When and where dual nutrient (N & P) reductions are needed to protect lakes and downstream ecosystems. Environ. Sci. Technol. 2016, 50, 10805–10813. [Google Scholar]
- Deng, D.; Meng, H.; Ma, Y.; Guo, Y.; Wang, Z.; He, H.; Xie, W.; Liu, J.E.; Zhang, L. The cumulative impact of temperature and nitrogen availability on the potential nitrogen fixation and extracellular polymeric substances secretion by Dolichospermum. Harmful Algae 2024, 135, 102633. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.; Yuan, L.; Wang, Y.; Ma, Y.; Wang, R.; Chen, X.; Losiewicz, M.D.; Guo, H.; Zhang, H. The Diversity of Cyanobacterial Toxins on Structural Characterization, Distribution and Identification: A Systematic Review. Toxins 2019, 11, 530. [Google Scholar] [CrossRef]
- Hayes, N.M.; Haig, H.A.; Simpson, G.L.; Leavitt, P.R. Effects of lake warming on the seasonal risk of toxic cyanobacteria exposure. Limnol. Oceanogr. Lett. 2020, 5, 393–402. [Google Scholar] [CrossRef]
- Turner, A.D.; Hatfield, R.G.; Maskrey, B.H.; Algoet, M. Evaluation of the new European Union reference method for paralytic shellfish toxins in shellfish: A review of twelve years regulatory monitoring using pre-column oxidation LC-FLD. Trends Anal. Chem. 2019, 113, 124–139. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, M.; Yu, Y.; Shi, X. Temperature triggers the annual cycle of Microcystis, comparable results from the laboratory and a large shallow lake. Chemosphere 2020, 260, 127543. [Google Scholar] [CrossRef]
- Rigosi, A.; Carey, C.C.; Ibelings, B.W.; Brookes, J.D. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 2014, 59, 99–114. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Backer, L.C.; Kardinaal, E.A.; Chorus, I. Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae 2015, 49, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Redouane, E.M.; Freitas, M.; Amaral, S.; Azevedo, T.; Loss, L.; Máthé, C.; Mohamed, Z.A.; Oudra, B.; Vasconcelos, V. Impacts of microcystins on morphological and physiological parameters of agricultural plants: A review. Plants 2021, 10, 639. [Google Scholar] [CrossRef]
- Di Pofi, G. High Resolution Mass Spectrometry: New Methods of Analysis for Risk Assessment by Cyanotoxins and Cyromazine in Water for Human Consumption. Ph.D. Thesis, Sapienza University of Rome, Rome, Italy, 2020. [Google Scholar]
- Hilborn, E.D.; Beasley, V.R. One health and cyanobacteria in freshwater systems: Animal illnesses and deaths are sentinel events for human health risks. Toxins 2015, 7, 1374–1395. [Google Scholar] [CrossRef]
- Hitzfeld, B.C.; Höger, S.J.; Dietrich, D.R. Cyanobacterial toxins: Removal during drinking water treatment, and human risk assessment. Environ. Health Perspect. 2000, 108, 113–122. [Google Scholar] [PubMed]
- Caen, A.; Mathias, J.D.; Latour, D. How do seasonal temperature variations influence interplay between toxic and non-toxic cyanobacterial blooms? Evidence from modeling and experimental data. Harmful Algae 2024, 134, 102606. [Google Scholar] [CrossRef]
- Falfushynska, H.; Kasianchuk, N.; Siemens, E.; Henao, E.; Rzymski, P.A. Review of Commonv Cyanotoxins and Their Effects on Fish. Toxics 2023, 11, 118. [Google Scholar] [CrossRef]
- Leavitt, P.R.; Findlay, D.L. Comparison of fossil pigments with 20 years of phytoplankton data from eutrophic Lake 227, Experimental Lakes area, Ontario. Can. J. Fish. Aquat. Sci. 1994, 51, 2286–2299. [Google Scholar] [CrossRef]
- Waters, M.N.; Brenner, M.; Curtis, J.H.; Romero-Oliva, C.S.; Dix, M.; Cano, M. Harmful algal blooms and cyanotoxins in Lake Amatitlán, Guatemala, coincided with ancient Maya occupation in the watershed. Proc. Natl. Acad. Sci. USA 2021, 118, e2109919118. [Google Scholar] [CrossRef] [PubMed]
- Heathcote, A.J.; Taranu, Z.E.; Tromas, N.; MacIntyre-Newell, M.; Leavitt, P.R.; Pick, F.R. Sedimentary DNA and pigments show increasing abundance and toxicity of cyanoHABs during the Anthropocene. Freshw. Biol. 2024, 68, 1859–1874. [Google Scholar] [CrossRef]
- Dick, G.J.; Duhaime, M.B.; Evans, J.T.; Errera, R.M.; Godwin, C.M.; Kharbush, J.J.; Nitschky, H.S.; Powers, M.A.; Vanderploeg, H.A.; Schmidt, K.C.; et al. The genetic and ecophysiological diversity of Microcystis. Environ. Microbiol. 2021, 23, 7278–7313. [Google Scholar] [CrossRef]
- Xiao, M.; Li, M.; Reynolds, C.S. Colony formation in the cyanobacterium Microcystis. Biol. Rev. 2018, 93, 1399–1420. [Google Scholar] [CrossRef]
- Poulickova, A.; Lysakova, M.; Hasler, P.; Lelkova, E. Fishpond sediments—The source of palaeoecological information and algal “seed banks”. Nova Hedwig. 2008, 86, 141–153. [Google Scholar] [CrossRef]
- Kaplan-Levy, R.N.; Hadas, O.; Summers, M.L.; Rücker, J.; Sukenik, A. Akinetes: Dormant cells of cyanobacteria. In Dormancy and Resistance in Harsh Environments; Springer Science & Business Media: Berlin, Germany, 2010; pp. 5–27. [Google Scholar]
- Kravchuk, E.S.; Ivanova, E.A.; Gladyshev, M.I. Seasonal dynamics of akinetes of Anabaena flos-aquae in bottom sediments and water column of small Siberian reservoir. Aquat. Ecol. 2006, 40, 325–336. [Google Scholar] [CrossRef]
- Salazar Torres, G.; Adámek, Z. Factors promoting the recruitment of benthic cyanobacteria resting stages: A review. Croat. J. Fish. Ribar. 2013, 71, 182–186. [Google Scholar] [CrossRef]
- Gyllstrom, M.; Hansson, L.A. Dormancy in freshwater zooplankton: Induction, termination and the importance of benthicpelagic coupling. Aquat. Sci. 2004, 66, 274–295. [Google Scholar] [CrossRef]
- Suikkanen, S.; Kaartokallio, H.; Hällfors, S.; Huttunen, M.; Laamanen, M. Life cycle strategies of bloom-forming, filamentous cyanobacteria in the Baltic Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2010, 57, 199–209. [Google Scholar] [CrossRef]
- Cottingham, K.L.; Weathers, K.C.; Ewing, H.A.; Greer, M.L.; Carey, C.C. Predicting the effects of climate change on freshwater cyanobacterial blooms requires consideration of the complete cyanobacterial life cycle. J. Plankton Res. 2021, 43, 10–19. [Google Scholar] [CrossRef]
- Amzil, Z.; Derrien, A.; Terre Terrillon, A.; Savar, V.; Bertin, T.; Peyrat, M.; Duval, A.; Lhaute, K.; Arnich, N.; Hort, V.; et al. Five years monitoring the emergence of unregulated toxins in shellfish in France (EMERGTOX 2018–2022). Mar. Drugs 2023, 21, 435. [Google Scholar]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.B.; Bass, B.; Sawyer, D.; Depew, D.; Watson, S.B. Estimating the economic costs of algal blooms in the Canadian Lake Erie Basin. Harmful Algae 2019, 87, 101624. [Google Scholar] [CrossRef]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- WHO. Guidelines on Recreational Water Quality. Volume 1: Coastal and Fresh Waters; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Holland, A.; Kinnear, S. Interpreting the possible ecological role (s) of cyanotoxins: Compounds for competitive advantage and/or physiological aide? Mar. Drugs 2013, 11, 2239–2258. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Lee, S.H.; Yun, M.R.; Oh, S.E.; Hwang, C.W.; Yoon, M.H.; Park, H.D. Draft genome sequence of Raphidiopsis raciborskii strain GIHE 2018, isolated from a shallow freshwater pond in South Korea. Microbiol. Resour. Announc. 2020, 9, e01545-19. [Google Scholar] [CrossRef]
- Christensen, V.G.; Khan, E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Sci. Total Environ. 2020, 736, 139515. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; de Senerpont Domis, L.; Wilk-Woniak, E.; Koreivien, J.; Warming, T.P. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef]
- Hinojosa, M.G.; Gutiérrez-Praena, D.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Cameán, A.M. Neurotoxicity induced by microcystins and cylindrospermopsin: A review. Sci. Total Environ. 2019, 668, 547–565. [Google Scholar] [CrossRef]
- Van Apeldoorn, M.E.; Van Egmond, H.P.; Speijers, G.J.; Bakker, G.J. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G. Cyanobacterial toxins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: Abingdon, UK, 1999; Volume 1, pp. 43–112. [Google Scholar]
- Funari, E.; Testai, E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef]
- Jones, G.J.; Orr, P.T. Release and degradation of microcystin following algicide treatment of a Microcystis aeruginosa bloom in a recreational lake, as determined by HPLC and protein phosphatase inhibition assay. Water Res. 1994, 28, 871–876. [Google Scholar] [CrossRef]
- Chorus, I.; Bartram, J. (Eds.) Toxic Cyanobacteria in Water—A Guide to Their Public Health Consequences, Monitoring and Management; E & FN Spon for the World Health Organization: New York, NY, USA, 1999. [Google Scholar]
- Roy-Lachapelle, A.; Solliec, M.; Sauvé, S.; Gagnon, C. Evaluation of ELISA-based method for total anabaenopeptins determination and comparative analysis with on-line SPE-UHPLC-HRMS in freshwater cyanobacterial blooms. Talanta 2021, 223, 121802. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, D.; Fang, D. Nodularins in poisoning. Clin. Chim. Acta 2013, 425, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Azevedo, S.M.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef]
- Kalaitzidou, M.; Petridou, E.; Economou, V.; Theodoridis, A.; Angelidis, P. Toxic cyanobacteria. A biological hazard for animal and public health: A review. Air Water Borne Dis. 2016, 5, 130. [Google Scholar] [CrossRef]
- Thajuddin, F.; Rasheed, A.S.; Thajuddin, N.; Dhanasekaran, D. Identification of Microcystin, Nodularin Synthatase Gene Clusters in Toxic Cyanobacteria Using AntiSMASH Pipeline. In Protocols for Cyanobacteria Sampling and Detection of Cyanotoxin; Springer Nature: Singapore, 2023; pp. 387–395. [Google Scholar]
- Abdallah, M.F.; Van Hassel, W.H.; Andjelkovic, M.; Wilmotte, A.; Rajkovic, A. Cyanotoxins and food contamination in developing countries: Review of their types, toxicity, analysis, occurrence and mitigation strategies. Toxins 2021, 13, 786. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Puerto, M.; Gutiérrez-Praena, D.; Llana-Ruiz-Cabello, M.; Jos, A.; Cameán, A.M. Microcystin-RR: Occurrence, content in water and food and toxicological studies. A review. Environ. Res. 2019, 168, 467–489. [Google Scholar] [CrossRef]
- Kalaitzidou, M.P.; Nannou, C.I.; Lambropoulou, D.A.; Papageorgiou, K.V.; Theodoridis, A.M.; Economou, V.K.; Giantsis, I.A.; Angelidis, P.G.; Kritas, S.K.; Petridou, E.J. First report of detection of microcystins in farmed mediterranean mussels Mytilus galloprovincialis in Thermaikos gulf in Greece. J. Biol. Res. 2021, 28, 8. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Eschedor, J.T.; Patterson, G.M.; Moore, R.E. Toxicity and partial structure of a hepatotoxic peptide produced by the cyanobacterium Nodularia spumigena Mertens emend. Appl. Environ. Microbiol. 1988, 54, 2257–2263. [Google Scholar] [CrossRef]
- Falconer, I.R.; Humpage, A.R. Health risk assessment of cyanobacterial (blue-green algal) toxins in drinking water. Int. J. Environ. Res. Public Health 2005, 2, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.R.; Beversdorf, L.J.; Weirich, C.A.; Bartlett, S.L. Cyanobacterial Toxins of the Laurentian Great Lakes, Their Toxicological Effects, and Numerical Limits in Drinking Water. Mar. Drugs 2017, 15, 160. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, Y.L.; Conklin, A.; Westrick, J.; Weavers, L.K.; Dionysiou, D.D.; Lenhart, J.J.; Mouser, P.J.; Szlag, D.; Walker, H.W. Toxic cyanobacteria and drinking water: Impacts, detection, and treatment. Harmful Algae 2016, 54, 174–193. [Google Scholar] [CrossRef]
- Krishnamurthy, T.; Carmichael, W.W.; Sarver, E.W. Toxic peptides from freshwater cyanobacteria (blue-green algae). I. Isolation, purification and characterization of peptides from Microcystis aeruginosa and Anabaena flos-aquae. Toxicon 1986, 24, 865–873. [Google Scholar] [CrossRef]
- Watanabe, M.F.; Oishi, S.; Harada, K.I.; Matsuura, K.; Kawai, H.; Suzuki, M. Toxins contained in Microcystis species of cyanobacteria (blue-green algae). Toxicon 1988, 26, 1017–1025. [Google Scholar] [CrossRef]
- Lyon-Colbert, A.; Su, S.; Cude, C. A Systematic Literature Review for Evidence of Aphanizomenon flos-aquae Toxigenicity in Recreational Waters and Toxicity of Dietary Supplements: 2000–2017. Toxins 2018, 10, 254. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Reis, M.P.; Mateus, M.C. Liquid chromatography/negative electrospray ionization ion trap MS2 mass spectrometry application for the determination of microcystins occurrence in Southern Portugal water reservoirs. Toxicon 2013, 74, 8–18. [Google Scholar] [CrossRef]
- Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef]
- Fontanillo, M.; Köhn, M. Microcystins: Synthesis and structure-activity relationship studies toward PP1 and PP2A. Bioorg. Med. Chem. 2018, 26, 1118–1126. [Google Scholar] [CrossRef]
- Swingle, M.R.; Honkanen, R.E. Inhibitors of serine/threonine protein phosphatases: Biochemical and structural studies provide insight for further development. Curr. Med. Chem. 2019, 26, 2634–2660. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Runnegar, M.; Berndt, N.; Kong, S.M.; Lee, E.Y.C.; Zhang, L.F. In-vivo and in-vitro binding of microcystin to protein phosphatase-1 and phosphatase-2a. Biochem. Biophys. Res. Commun. 1995, 216, 162–169. [Google Scholar] [CrossRef]
- Carmichael, W.W. Cyanobacteria secondary metabolites-the cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Arman, T.; Clarke, J.D. Microcystin toxicokinetics, molecular toxicology, and pathophysiology in preclinical rodent models and humans. Toxins 2021, 13, 537. [Google Scholar] [CrossRef] [PubMed]
- Jos, A.; Pichardo, S.; Prieto, A.I.; Repetto, G.; Vázquez, C.M.; Moreno, I.; Cameán, A.M. Toxic cyanobacterial cells containing microcystins induce oxidative stress in exposed tilapia fish (Oreochromis sp.) under laboratory conditions. Aquat. Toxicol. 2005, 72, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, N.; Zhang, Z.; Gao, Y.; Dong, J.; Gao, X.; Yuan, H.; Li, X. The Combined Effects of Toxic Microcystis aeruginosa and Thermal Stress on the Edible Clam (Corbicula fluminea): Insights into Oxidative Stress Responses and Molecular Networks. Antioxidants 2023, 12, 1901. [Google Scholar] [CrossRef]
- WHO. Cyanobacterial toxins: Microcystins. In Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020; (WHO/HEP/ECH/WSH/2020.6). [Google Scholar]
- Melaram, R.; Newton, A.R.; Lee, A.; Herber, S.; El-Khouri, A.; Chafin, J. A review of microcystin and nodularin toxins derived from freshwater cyanobacterial harmful algal blooms and their impact on human health. Toxicol. Environ. Health Sci. 2024, 16, 233–241. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, D.; Yu, H.; Chen, K.; Shen, G.; Shen, Y.; Ruan, Y.; Ding, X. Drinking water types, microcystins and colorectal cancer. Zhonghua Yu Fang Yi Xue Za Zhi Chin. J. Prev. Med. 2000, 34, 224–226. [Google Scholar]
- IARC. Monographs on the Identification of Carcinogenic Hazards to Humans; IARC: Lyon, France, 2024.
- Yoshida, T.; Makita, Y.; Nagata, S.; Tsutsumi, T.; Yoshida, F.; Sekijima, M.; Tamura, S.; Ueno, Y. Acute oral toxicity of microcystin-LR, a cyanobacterial hepatotoxin, in mice. Nat. Toxins 1997, 5, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Nehring, S. Mechanisms for Recurrent Nuisance Algal Blooms in Coastal Zones: Resting Cyst Formation as Life-Strategy of Dinoflagellates; Lang: Waukesha, WI, USA, 1993; pp. 454–467. [Google Scholar]
- Farrer, D.; Counter, M.; Hillwig, R.; Cude, C. Health-Based Cyanotoxin Guideline Values Allow for Cyanotoxin-Based Monitoring and Efficient Public Health Response to Cyanobacterial Blooms. Toxins 2015, 7, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Humpage, A.R.; Falconer, I.R. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male Swiss albino mice: Determination of no observed adverse effect level for deriving a drinking water guideline value. Environ. Toxicol. 2003, 18, 94–103. [Google Scholar] [CrossRef]
- Stevens, D.K.; Krieger, R.I. Stability studies on the cyanobacterial nicotinic alkaloid anatoxin-a. Toxicon 1991, 29, 167–179. [Google Scholar] [CrossRef]
- Cook, W.O.; Dellinger, J.A.; Singh, S.S.; Dahlem, A.M.; Carmichael, W.W.; Beasley, V.R. Regional brain cholinesterase activity in rats injected intraperitoneally with anatoxin-a(s) or paraoxon. Toxicol. Lett. 1989, 49, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Testai, E.; Buratti, F.M.; Funari, E.; Manganelli, M.; Vichi, S.; Arnich, N.; Biré, R.; Fessard, V.; Sialehaamoa, A. Review and analysis of occurrence, exposure and toxicity of cyanobacteria toxins in food. EFSA Support. Publ. 2016, 13, 998E. [Google Scholar] [CrossRef]
- Melo, E.; Moura e Silva, S.; Paula, F.R. Molecular modelling and quantitative structure-activity relationship studies of anatoxin-a and epibatidine derivatives with affinity to rodent nAChR receptors. Chem. Pap. 2014, 68, 1121–1131. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Marine biotoxins in shellfish-Saxitoxin group. EFSA J. 2009, 7, 1019. [Google Scholar]
- Simiyu, B.M.; Oduor, S.O.; Rohrlack, T.; Sitoki, L.; Kurmayer, R. Microcystin Content in Phytoplankton and in Small Fish from Eutrophic Nyanza Gulf, Lake Victoria, Kenya. Toxins 2018, 10, 275. [Google Scholar] [CrossRef]
- Calado, S.L.D.M.; Santos, G.S.; Leite, T.P.B.; Wojciechowski, J.; Nadaline, M.; Bozza, D.C.; Freitas de Magalhães, V.; Cestari, M.M.; Prodocimo, V.; Silva de Assis, H.C. Depuration time and sublethal effects of microcystins in a freshwater fish from water supply reservoir. Chemosphere 2018, 210, 805–815. [Google Scholar] [CrossRef]
- Miller, M.A.; Kudela, R.M.; Mekebri, A.; Crane, D.; Oates, S.C.; Tinker, M.T.; Staedler, M.; Miller, A.; Toy-Choutka, S.; Dominik, C.; et al. Evidence for a Novel Marine Harmful Algal Bloom: Cyanotoxin (Microcystin) Transfer from Land to Sea Otters. PLoS ONE 2010, 5, 12576. [Google Scholar] [CrossRef]
- Van der Merwe, D.; Sebbag, L.; Nietfeld, J.C.; Aubel, M.T.; Foss, A.; Carney, E. Investigation of a Microcystis aeruginosa cyanobacterial freshwater harmful algal bloom associated with acute microcystin toxicosis in a dog. J. Vet. Diagn. Investig. 2012, 24, 679–687. [Google Scholar] [CrossRef]
- Singo, A.; Myburgh, J.G.; Laver, P.N.; Venter, E.A.; Ferreira, G.C.H.; Rösemann, G.M.; Botha, C.J. Vertical transmission of microcystins to Nile crocodile (Crocodylus niloticus) eggs. Toxicon 2017, 134, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Malbrouck, C.; Kestemont, P. Effects of microcystins on fish. Environ. Toxicol. Chem. Int. J. 2006, 25, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Pitois, F.; Vezie, C.; Thoraval, I.; Baurès, E. Improving microcystin monitoring relevance in recreative waters: A regional case-study (Brittany, Western France, Europe). Int. J. Hyg. Environ. Health 2016, 219, 288–293. [Google Scholar] [CrossRef]
- Schaefer, A.M.; Yrastorza, L.; Stockley, N.; Harvey, K.; Harris, N.; Grady, R.; Sullivan, J.; McFarland, M.; Reif, J.S. Exposure to microcystin among coastal residents during a cyanobacteria bloom in Florida. Harmful Algae 2020, 92, 101769. [Google Scholar] [CrossRef]
- Fischer, W.J.; Altheimer, S.; Cattori, V.; Meier, P.J.; Dietrich, D.R.; Hagenbuch, B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharmacol. 2005, 203, 257–263. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Zhang, X.; Xie, P. A review of reproductive toxicity of microcystins. J. Hazard. Mater. 2015, 301, 381–399. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.; Zheng, P.; Li, J.; Li, J.; Li, T.; Wang, X.; Wang, D.; Xian, J.; Zhang, Z.; et al. Effects of microcystin-LR on behavior, histopathology, oxidative stress, non-specific immunity and gene expression of red claw crayfish (Cherax quadricarinatus). Aquacult. Rep. 2023, 33, 101805. [Google Scholar] [CrossRef]
- Hernandez, B.Y.; Zhu, X.; Risch, H.A.; Lu, L.; Ma, X.; Irwin, M.L.; Lim, J.K.; Taddei, T.H.; Pawlish, K.S.; Stroup, A.M.; et al. Oral Cyanobacteria and Hepatocellular Carcinoma. Cancer Epidemiol. Biomark. Prev. 2022, 31, 221–229. [Google Scholar] [CrossRef]
- Christophoridis, C.; Zervou, S.K.; Manolidi, K.; Katsiapi, M.; Moustaka-Gouni, M.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. Occurrence and diversity of cyanotoxins in Greek lakes. Sci. Rep. 2018, 8, 17877. [Google Scholar] [CrossRef] [PubMed]
- Foss, A.J.; Butt, J.; Fuller, S.; Cieslik, K.; Aubel, M.T.; Wertz, T. Nodularin from benthic freshwater periphyton and implications for trophic transfer. Toxicon 2017, 140, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K.; Kononen, K.; Carmichael, W.W.; Dalem, A.M.; Rinehart, K.L.; Kiviranta, J.; Niemela, S.I. Occurrence of the hepatotoxic cyanobacterium Nodularia spumigena in the Baltic Sea and structure of the toxin. Appl. Environ. Microbiol. 1989, 55, 1990–1995. [Google Scholar] [CrossRef]
- Bownik, A. Harmful algae: Effects of cyanobacterial cyclic peptides on aquatic invertebrates-a short review. Toxicon 2016, 124, 26–35. [Google Scholar] [CrossRef]
- Konkel, R.; Toruska-Sitarz, A.; Cegowska, M.; Ežerinskis, Ž.; Šapolaitė, J.; Mažeika, J.; Mazur-Marzec, H. Blooms of toxic cyanobacterium Nodularia spumigena in Norwegian fjords during Holocene warm periods. Toxins 2020, 12, 257. [Google Scholar] [CrossRef]
- Kaur, H.P.; Prasad, B.; Kaur, S. A review on applications of biosurfactants produced from unconventional inexpensive wastes in food and agriculture industry. World J. Pharm. Res. 2015, 4, 827. [Google Scholar]
- Lopes, V.R.; Vasconcelos, V.M. Planktonic and benthic cyanobacteria of European brackish waters: A perspective on estuaries and brackish seas. Eur. J. Phycol. 2011, 46, 292–304. [Google Scholar] [CrossRef]
- Ufelmann, H.; Krüger, T.; Luckas, B.; Schrenk, D. Human and rat hepatocyte toxicity and protein phosphatase 1 and 2A inhibitory activity of naturally occurring desmethyl-microcystins and nodularins. Toxicology 2012, 293, 59–67. [Google Scholar] [CrossRef]
- Bagu, J.R.; Sykes, B.D.; Craig, M.M.; Holmes, C.B. A molecular basis for different interactions of marine toxins with protein phosphatase-1: Molecular models for bound motuporin, microcystins, okadaic acid, and calyculin A. J. Biol. Chem. 1997, 272, 5087–5097. [Google Scholar] [CrossRef]
- Teikari, J.E.; Hou, S.; Wahlsten, M.; Hess, W.R.; Sivonen, K. Comparative Genomics of the Baltic Sea Toxic Cyanobacteria Nodularia spumigena UHCC 0039 and Its Response to Varying Salinity. Front. Microbiol. 2018, 9, 356. [Google Scholar] [CrossRef]
- Liu, P.; Lu, Z.; Liu, L.; Li, R.; Liang, Z.; Shen, M.; Xu, H.; Ren, D.; Ji, M.; Yuan, S.; et al. NOD-like receptor signaling in inflammation-associated cancers: From functions to targeted therapie. Phytomedicine 2019, 64, 152925. [Google Scholar] [CrossRef]
- Carmichael, W.W. Health effects of toxin producing cyanobacteria: “The CyanoHABS”. Hum. Ecol. Risk Assess. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Stucken, K.; John, U.; Soto-Liebe, K.; Vásquez, M. Impact of nitrogen sources on gene expression and toxin production in the diazotroph Cylindrospermopsis raciborskii CS-505 and non-diazotroph Raphidiopsis brookii D9. Toxins 2014, 6, 1896–1915. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, G.; Pan, Q.; Yang, Y.; Li, R. Identification of genes for Anatoxin-a biosynthesis in Cuspidothrix issatschenkoi. Harmful Algae 2015, 46, 43–48. [Google Scholar] [CrossRef]
- Loftin, K.A.; Graham, J.L.; Hilborn, E.; Lehmann, S.; Meyer, M.T.; Dietze, J.E.; Griffith, C. Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational health risks in the EPA National Lakes Assessment 2007. Harmful Algae 2016, 56, 77–90. [Google Scholar] [CrossRef]
- Salmaso, N.; Cerasino, L.; Boscaini, A.; Capelli, C. Planktic Tychonema (Cyanobacteria) in the large lakes south of the Alps: Phylogenetic assessment and toxigenic potential. FEMS Microbiol. Ecol. 2016, 92, fiw155. [Google Scholar] [CrossRef]
- Smith, C.; Sutton, A. The Persistence of Anatoxin-A in Reservoir Water; UK Report No. FR0427; Foundation for Water Research: Denver, CO, USA, 1993. [Google Scholar]
- WHO. Cyanobacterial toxins: Anatoxin-a and analogues. In Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020; (WHO/HEP/ECH/WSH/2020.1). [Google Scholar]
- Al-Sammak, M.A.; Hoagland, K.D.; Cassada, D.; Snow, D.D. Co-occurrence of the cyanotoxins BMAA, DABA and anatoxin-a in Nebraska reservoirs, fish, and aquatic plants. Toxins 2014, 6, 488–508. [Google Scholar] [CrossRef]
- Jaramillo, M.; O’Shea, K.E. Analytical methods for assessment of cyanotoxin contamination in drinking water sources. Curr. Opin. Environ. Sci. Health 2019, 7, 45–51. [Google Scholar] [CrossRef]
- Plata-Calzado, C.; Prieto, A.I.; Cameán, A.M.; Jos, A. Analytical Methods for Anatoxin-a Determination: A Review. Toxins 2024, 16, 198. [Google Scholar] [CrossRef]
- Patoka, J.; Gupta, R.C.; Kua, K. Anatoxin-a(s): Natural organophosphorus anticholinesterase agent. Mil. Med. Sci. Lett. 2011, 80, 129–139. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.D.S.; Kozlowsky-Suzuki, B. Cyanotoxins: Bioaccumulation and effects on aquatic animals. Mar. Drugs 2011, 9, 2729–2772. [Google Scholar] [CrossRef]
- Matsunaga, S.; Moore, R.E.; Niemczura, W.P.; Carmichael, W.W. Anatoxin-a (s), a potent anticholinesterase from Anabaena flos-aquae. J. Am. Chem. Soc. 1989, 111, 8021–8023. [Google Scholar] [CrossRef]
- McAllister, T.G.; Wood, S.; Hawes, I. The rise of toxic benthic Phormidium proliferations: A review of their taxonomy, distribution, toxin content and factors regulating prevalence and increased severity. Harmful Algae 2016, 55, 282–294. [Google Scholar] [CrossRef]
- Colas, S.; Marie, B.; Lance, E.; Quiblier, C.; Tricoire-Leignel, H.; Mattei, C. Anatoxin-a: Overview on a harmful cyanobacterial neurotoxin from the environmental scale to the molecular target. Environ. Res. 2021, 193, 110590. [Google Scholar] [CrossRef]
- Botana, L.M.; Hess, P.; Munday, R.; Nathalie, A.; DeGrasse, S.L.; Feeley, M.; Suzuki, T.; van den Berg, M.; Fattori, V.; Garrido Gamarro, E.; et al. Derivation of toxicity equivalency factors for marine biotoxins associated with Bivalve Molluscs. Trends Food Sci. Technol. 2017, 59, 15–24. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; O’Neill, A.; Coates, L.; Lewis, A.; Lewis, K. Analysis of Microcystins in Cyanobacterial Blooms from Freshwater Bodies in England. Toxins 2018, 10, 39. [Google Scholar] [CrossRef]
- Oyaneder-Terrazas, J.; Contreras, H.R.; García, C. Prevalence, Variability and Bioconcentration of Saxitoxin-Group in Different Marine Species Present in the Food Chain. Toxins 2017, 9, 190. [Google Scholar] [CrossRef]
- Andrinolo, D.; Iglesias, V.; García, C.; Lagos, N. Toxicokinetics and toxicodynamics of gonyautoxins after an oral toxin dose in cats. Toxicon 2002, 40, 699–709. [Google Scholar] [CrossRef]
- Zhang, D.W.; Deng, X.; Xie, P.; Chen, J.; Guo, L. Risk assessment of microcystins in silver carp (Hypophthalmichthys molitrix) from eight eutrophic lakes in China. Food Chem. 2013, 140, 17–21. [Google Scholar] [CrossRef]
- Fuentes-Valdés, J.J.; Soto-Liebe, K.; Pérez-Pantoja, D.; Tamames, J.; Belmar, L.; Pedrós-Alió, C.; Garrido, D.; Vásquez, M. Draft genome sequences of Cylindrospermopsis raciborskii strains CS-508 and MVCC14, isolated from freshwater bloom events in Australia and Uruguay. Stand. Genom. Sci. 2018, 13, 26. [Google Scholar] [CrossRef]
- Fedorova, G.A.; Kuzmin, A.V.; Zubkov, I.N.; Tikhonova, I.V.; Shtykova, Y.R.; Butina, T.V.; Belykh, O.I.; Grachev, M.A. Determination of saxitoxin in water of Lake Baikal. Acta Biol. Sib. 2019, 5, 77–83. [Google Scholar]
- Jones, G.J.; Negri, A.P. Persistence and degradation of cyanobacterial paralytic shellfish poisons (PSPs) in freshwaters. Water Res. 1997, 31, 525–533. [Google Scholar] [CrossRef]
- Pereira, P.; Onodera, H.; Andrinolo, D.; Franca, S.; Araújo, F.; Lagos, N.; Oshima, Y. Paralytic shellfish toxins in the freshwater cyanobacterium Aphanizomenon flos-aquae, isolated from Montargil reservoir, Portugal. Toxicon 2000, 38, 1689–1702. [Google Scholar] [CrossRef]
- Figueiredo, D.R.; Azeiteiro, U.M.; Esteves, S.M.; Gonçalves, F.J.M.; Pereira, M.J. Microcystin-producing blooms-a serious global public health issue. Ecotoxicol. Environ. Saf. 2004, 59, 151–163. [Google Scholar] [CrossRef]
- Harland, F.; Wood, S.A.; Broady, P.; Williamson, W.; Gaw, S. Changes in saxitoxin production through growth phases in the metaphytic cyanobacterium Scytonema cf. crispum. Toxicon 2015, 103, 74–79. [Google Scholar] [CrossRef]
- Batoréu, M.C.; Dias, E.; Pereira, P.; Franca, S. Risk of human exposure to paralytic toxins of algal origin. Environ. Toxicol. Pharmacol. 2005, 19, 401–406. [Google Scholar] [CrossRef]
- Trainer, V.L.; Hardy, F.J. Integrative monitoring of marine and freshwater harmful algae in Washington State for public health protection. Toxins 2015, 7, 1206–1234. [Google Scholar] [CrossRef]
- Aguilera, A.; Giannuzzi, L. Book Review: Cyanobacteria as Environmental Determinants of Health; Frontiers: Lausanne, Switzerland, 2018. [Google Scholar]
- Garcia, M.R.; Mirlean, N.; Baisch, P.R.; Caramao, E.B. Assessment of polycyclic aromatic hydrocarbon influx and sediment contamination in an urbanized estuary. Environ. Monit. Assess. 2010, 168, 269–276. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Bravo, M.C.; Lagos, M.; Lagos, N. Paralytic shellfish poisoning: Post-mortem analysis of tissue and body fluid samples from human victims in the Patagonia fjords. Toxicon 2004, 43, 149–158. [Google Scholar] [CrossRef]
- García, C.; Rodriguez-Navarro, A.; Díaz, J.C.; Torres, R.; Lagos, N. Evidence of in vitro glucuronidation and enzymatic transformation of paralytic shellfish toxins by healthy human liver microsomes fraction. Toxicon 2009, 53, 206–213. [Google Scholar] [CrossRef]
- Garcia, C.; Barriga, A.; Diaz, J.C.; Lagos, M.; Lagos, N. Route of metabolization and detoxication of paralytic shellfish toxins in humans. Toxicon 2010, 55, 135–144. [Google Scholar] [CrossRef]
- Masias, D.; Gómez, K.; Contreras, C.; Gaete, L.; Garcia, C. Rapid Screening Fluorescence method applied to detection and quantitation of Paralytic Shellfish Toxins in invertebrate marine vectors. Food Addit. Contam. Part A 2019, 36, 1118–1137. [Google Scholar] [CrossRef]
- EU. Commission regulation (EC) No 2017/1980 of 31 October 2017 amending Annex III to Regulation (EC) No 2074/2005 as regards paralytic shellfish poison (PSP) detection method. Off. J. Eur. Union 2017, 258, 285–288. [Google Scholar]
- WHO. Cyanobacterial toxins: Saxitoxins. In Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020; (WHO/HEP/ECH/WSH/2020.8). [Google Scholar]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. UK 2015, 96, 61–91. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Poniedziałek, B. In search of environmental role of cylindrospermopsin: A review on global distribution and ecology of its producers. Water Res. 2014, 66, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, I.; Moore, R.E.; Runnegar, M.T.C. Cylindrospermopsin: A potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J. Am. Chem. Soc. 1991, 114, 7941–7942. [Google Scholar] [CrossRef]
- WHO. Cyanobacterial toxins: Cylindrospermopsins. In Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020; (WHO/HEP/ECH/WSH/2020.4). [Google Scholar]
- Sant Anna, C.; de Carvalho, L.R.; Fiore, M.F.; Silva-Stenico, M.E.; Lorenzi, A.S.; Rios, F.R.; Konno, K.; Garcia, C.; Lagoa, N. Highly toxic Microcystis aeruginosa strain, isolated from Sao Paulo-Brazil, produce Hepatotoxins and Paralytic Shellfish Toxins. Neurotox. Res. 2011, 19, 389–402. [Google Scholar] [CrossRef]
- Davis, D.A.; Mondo, K.; Stern, E.; Annor, A.K.; Murch, S.J.; Coyne, T.M.; Brand, L.E.; Niemeyer, M.E.; Sharp, S.; Bradley, W.G.; et al. Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins. PLoS ONE 2019, 14, e0213346. [Google Scholar] [CrossRef]
- Rücker, J.; Stüken, A.; Nixdorf, B.; Fastner, J.; Chorus, I.; Wiedner, C. Concentrations of particulate and dissolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon 2007, 50, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Chiswell, R.K.; Shaw, G.R.; Eaglesham, G.; Smith, M.J.; Norris, R.L.; Seawright, A.A.; Moore, M.R. Stability of cylindrospermopsin, the toxin from the cyanobacterium, Cylindrospermopsis raciborskii: Effect of pH, temperature, and sunlight on decomposition. Environ. Toxicol. Int. J. 1999, 14, 155–161. [Google Scholar] [CrossRef]
- Wormer, L.; Cirés, S.; Carrasco, D.; Quesada, A. Cylindrospermopsin is not degraded by co-occurring natural bacterial communities during a 40-day study. Harmful Algae 2008, 7, 206–213. [Google Scholar] [CrossRef]
- Sukenik, A.; Kaplan, A. Cyanobacterial harmful algal blooms in aquatic ecosystems: A comprehensive outlook on current and emerging mitigation and control approaches. Microorganisms 2021, 9, 1472. [Google Scholar] [CrossRef] [PubMed]
- Terao, K.; Ohmori, S.; Igarashi, K.; Ohtani, I.; Watanabe, M.F.; Harada, K.I.; Ito, E.; Watanabe, M. Electron microscopic studies on experimental poisoning in mice induced by cylindrospermopsin isolated from blue-green alga Umezakia natans. Toxicon 1994, 32, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Froscio, S.M.; Humpage, A.R.; Burcham, P.C.; Falconer, I.R. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. Int. J. 2003, 18, 243–251. [Google Scholar] [CrossRef]
- Runnegar, M.T.; Xie, C.; Snider, B.B.; Wallace, G.A.; Weinreb, S.M.; Kuhlenkamp, J. In vitro hepatotoxicity of the cyanobacterial alkaloid cylindrospermopsin and related synthetic analogues. Toxicol. Sci. 2002, 67, 81–87. [Google Scholar] [CrossRef]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological effects of selected cyanobacterial secondary metabolites a short review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218. [Google Scholar] [CrossRef]
- Chong, M.W.K.; Wong, B.S.F.; Lam, P.K.S.; Shaw, G.R.; Seawright, A.A. Toxicity and uptake mechanism of cylindrospermopsin and lophyrotomin in primary rat hepatocytes. Toxicon 2002, 40, 205–211. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, G.; Chen, Y.; Jia, N.; Li, R. Four decades of progress in cylindrospermopsin research: The ins and outs of a potent cyanotoxin. J. Hazard. Mater. 2021, 406, 124653. [Google Scholar] [CrossRef]
- Štraser, A.; Filipič, M.; Žegura, B. Genotoxic effects of the cyanobacterial hepatotoxin cylindrospermopsin in the HepG2 cell line. Arch. Toxicol. 2011, 85, 1617–1626. [Google Scholar] [CrossRef]
- Puerto, M.; Prieto, A.I.; Maisanaba, S.; Gutiérrez-Praena, D.; Mellado-García, P.; Jos, Á.; Cameán, A.M. Mutagenic and genotoxic potential of pure Cylindrospermopsin by a battery of in vitro tests. Food Chem. Toxicol. 2018, 121, 413–422. [Google Scholar] [CrossRef]
- Pichardo, S.; Cameán, A.M.; Jos, A. In vitro toxicological assessment of cylindrospermopsin: A review. Toxins 2017, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Falconer, I.R.; Humpage, A.R. Preliminary evidence for in vivo tumour initiation by oral administration of extracts of the blue-green alga Cylindrospermopsis raciborskii containing the toxin cylindrospermopsin. Environ. Toxicol. Int. J. 2001, 16, 192–195. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cyanobacterial Toxins: Cylindrospermopsins; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Huguet, A.; Lanceleur, R.; Quenault, H.; Le Hégarat, L.; Fessard, V. Identification of key pathways involved in the toxic response of the cyanobacterial toxin cylindrospermopsin in human hepatic HepaRG cells. Toxicol. Vitr. 2019, 58, 69–77. [Google Scholar] [CrossRef]
- Khomutovska, N.; Sandzewicz, M.; Łach, Ł.; Suska-Malawska, M.; Chmielewska, M.; Mazur-Marzec, H.; Cegłowska, M.; Niyatbekov, T.; Wood, S.A.; Puddick, J.; et al. Limited microcystin, anatoxin and cylindrospermopsin production by cyanobacteria from microbial mats in cold deserts. Toxins 2020, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Quiblier, C.; Wood, S.; Echenique-Subiabre, I.; Heath, M.; Villeneuve, A.; Humbert, J.F. A review of current knowledge on toxic benthic freshwater cyanobacteria—Ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar]
- Belykh, O.I.; Tikhonova, I.V.; Kuzmin, A.V.; Sorokovikova, E.G.; Fedorova, G.A.; Khanaev, I.V.; Sherbakova, T.A.; Timoshkin, O.A. First detection of benthic cyanobacteria in Lake Baikal producing paralytic shellfish toxins. Toxicon 2016, 121, 36–40. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Cyanobacteria and Cyanotoxins: Information for Drinking Water Systems; EPA: Washington, DC, USA, 2019.
- European Union. Diario Oficial de la Unión Europea. In Relativa a la Calidad de las Aguas Destinadas al Consumo Humano; DIRECTIVA (UE) 2020/2184; European Union: Brussels, Belgium, 2020. [Google Scholar]
- Australian Government. Australian Drinking Water Guidelines 6 2011; National Health and Medical Research Council: Canberra, Australia, 2017; Volume 3, p. 4.
- Ministério de Sáude. Portaria n° 1.469 Controle e Vigilância da Qualidade da Água para Consumo Humano e seu Padrão de Potabilidade; FUNASA: Paraíba, Brazil, 2001.
- Oregon Health Autority OHA. Public Health Advisory Guidelines, Harmful Algae Blooms in Freshwater Bodies; OHA: Salem, OR, USA, 2021.
- Health Canada. Guidelines for Canadian Recreational Water Quality Guideline Technical Document Cyanobacteria and Their Toxins; Health Canada: Ottawa, ON, Canada, 2022.
- Christoffersen, K. DENMARK: Occurrence, Monitoring and Management of Toxic Cyanobacteria. In Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries; Umweltbundesamt: Berlin, Germany, 2005; p. 41. [Google Scholar]
- Willame, R.; Jurczak, T.; Iffly, J.; Kull, T.; Meriluoto, J.; Hoffman, L. Distribution of Hepatotoxic Cyanobacterial Blooms in Belgium and Luxembourg. Hydrobiologia 2005, 551, 99–117. [Google Scholar] [CrossRef]
- Arnich, N. Regulation, Risk Management, Risk Assessment and Research on Cyanobacteria and Cyanotoxins; Federal Environment Agency (Umweltbundesamt): Dessau-Roßlau, Germany, 2012; Volume 63, pp. 63–70.
- Rapala, J.; Kilponen, J.; Järvinen, M.; Lahti, K. Guidelines for Monitoring of Cyanobacteria and Their Toxins; Federal Environment Agency (Umweltbundesamt): Dessau-Roßlau, Germany, 2012; Volume 63, pp. 54–62.
- Chorus, I. Approaches to assessing and managing the cyanotoxin risk. In Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries; Umweltbundesamt: Berlin, Germany, 2012; pp. 37–39. [Google Scholar]
- Kagalou, I.; Kormas, K.; Papadimitriou, T.; Katsiapi, M.; Genitsaris, S.; Moustaka-GounI, M. Occurrence, Monitoring and Risk Management of Cyanobacteria and Cyanotoxins. In Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries; Umweltbundesamt: Berlin, Germany, 2012; pp. 71–78. [Google Scholar]
- Funari, E.; Gramaccioni, L.; Scardala, S. Management of Potenially Toxic Cyanobacteria. In Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries; Umweltbundesamt: Berlin, Germany, 2012; pp. 79–81. [Google Scholar]
- Mankiewicz-Boczek, J.; Kobos, J.; Gągała, I.; Izydorczyk, K. Management and Regulation of Toxic Cyanobacteria. In Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries; Umweltbundesamt: Berlin, Germany, 2012; pp. 109–114. [Google Scholar]
- Maršálek, B.; Bláha, L.; Bláhová, L.; Babica, P. Management and Regulation of Cyanobacteria and Cyanotoxins. In Federal Environmental Agency (Umweltbundesamt), Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries; Umweltbundesamt: Berlin, Germany, 2005; pp. 37–39. [Google Scholar]
- Ministério do Ambiente, do Ordenamento do Território e do Desenvolvimento Regional. Decreto-Lei, Nº 306; Ministério do Ambiente, do Ordenamento do Território e do Desenvolvimento Regional: Lisbon, Portugal, 2007.
- Schürmann, Q.J.; Visser, P.M.; Sollie, S.; Kardinaal, W.E.A.; Faassen, E.J.; Lokmani, R.; van der Oost, R.; Van de Waal, D.B. Risk assessment of toxic cyanobacterial blooms in recreational waters: A comparative study of monitoring methods. Harmful Algae 2024, 138, 102683. [Google Scholar] [CrossRef]
- Wood, S.; Williamson, W. New Zealand: Regulation and Management of Cyanobacteria. In Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries; Umweltbundesamt: Berlin, Germany, 2012; pp. 97–108. [Google Scholar]
- Harn Te, S.; Yew-Hoong Gin, K. The dynamics of cyanobacteria and microcystin production in a tropical reservoir of Singapore. Harmful Algae 2011, 10, 319–329. [Google Scholar]
- Quesada, A.; de Hoyos, C.; Martínez, G. Cyanobacteria and Cyanotoxins—Legislation and Current Situation. In Current Approaches to Cyanotoxin Risk Assessment, Risk Management and Regulations in Different Countries; Umweltbundesamt: Berlin, Germany, 2012; pp. 118–126. [Google Scholar]
- Koker, L.; Akcaalan, R.; Oguz, A.; Gaygusuz, O.; Gurevin, C.; AkatKose, C.; Gucver, S.; Karaaslan, Y.; Erturk, A.; Albay, M.; et al. Distribution of toxic cyanobacteria and cyanotoxins in turkish waterbodies. J. Environ. Prot. Ecol. 2017, 18, 425–432. [Google Scholar]
- Instituto Uruguayo de Normas Técnicas. UNIT 833: Agua Potable—Requisitos; Instituto Uruguayo de Normas Técnicas: Montevideo, Uruguay, 2008. [Google Scholar]
- SANS241; Drinking Water Quality. South African Bureau of Standards: Pretoria, South Africa, 2022.
- Ministerio del Ambiente. Decreto Supremo N° 004-2017-MINAM; Ministerio del Ambiente: Lima, Peru, 2017.
- Ministerio de Salud. Factores ambientales y antropogénicos que afectan la formación de floraciones de cianobacterias y cianotoxinas. Cianobacterias como determinantes ambientales de la salud. Temas Salud Ambient. 2017, 5, 79–94. [Google Scholar]
- Cantoral, E.; Asencio, A.; Aboal, M. Cianotoxinas: Efectos ambientales y sanitarios. Medidas de prevención. Hidrobiológica 2017, 27, 241–251. [Google Scholar] [CrossRef]
- Avendaño, A.; Arguedas, C. Microcystin in plants that treat water for human consumption in a tropical environment: The Metropolitan Area of Costa Rica. Rev. Biol. Trop. 2006, 54, 711–716. [Google Scholar] [CrossRef]
- Secretaría del Ambiente. Resolución Nº 222/02; Secretaría del Ambiente: Asunción, Paraguay, 2002.
- Vicepresidencia de Administración del Recurso Hídrico. Informe de Calidad de agua de la Cuenca del Canal 2021; Autoridad del Canal de Panamá: Panama City, Panama, 2022. [Google Scholar]
- Flores, N.M.; Miller, T.R.; Stockwell, J.D. A global analysis of the relationship between concentrations of microcystins in water and fish. Front. Mar. Sci. 2018, 5, 30. [Google Scholar] [CrossRef]
- Poste, A.E.; Hecky, R.E.; Guildford, S.J. Evaluating microcystin exposure risk through fish consumption. Environ. Sci. Technol. 2011, 45, 5806–5811. [Google Scholar] [CrossRef]
- Shahmohamadloo, R.S.; Bhavsar, S.P.; Almirall, X.O.; Marklevitz, S.A.; Rudman, S.M.; Sibley, P.K. Lake Erie fish safe to eat yet afflicted by algal hepatotoxins. Sci. Total Environ. 2023, 861, 160474. [Google Scholar] [CrossRef] [PubMed]
- Weirich, C.A.; Miller, T.R. Freshwater harmful algal blooms: Toxins and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 2014, 44, 2–24. [Google Scholar] [CrossRef]
- Lürling, M.; van der Grinten, E. Life-history characteristics of Daphnia exposed to dissolved microcystin-LR and to the cyanobacterium Microcystis aeruginosa with and without microcystins. Environ. Toxicol. Chem. Int. J. 2003, 22, 1281–1287. [Google Scholar] [CrossRef]
- Dao, T.S.; Cronberg, G.; Nimptsch, J.; Do-Hong, L.C.; Wiegand, C. Toxic cyanobacteria from Tri An Reservoir, Vietnam. Nova Hedwig. 2010, 90, 433. [Google Scholar] [CrossRef]
- Cerbin, S.; Kraak, M.H.S.; De Voogt, P.; Visser, P.M.; Van Donk, E. Combined and single effects of pesticide carbaryl and toxic Microcystis aeruginosa on the life history of Daphnia pulicaria. Hydrobiologia 2010, 643, 129–138. [Google Scholar] [CrossRef]
- Miles, C.O.; Strand, D.A.; Rusch, J.C.; Ballot, A.; Haande, S.; Løvberg, K.L.E.; Vrålstad, T.; Samdal, I.A. Microcystin profiles in European noble crayfish Astacus astacus and water in Lake Steinsfjorden, Norway. Environ. Res. 2024, 242, 117623. [Google Scholar] [CrossRef]
- Berry, J.P.; Gibbs, P.D.; Schmale, M.C.; Saker, M.L. Toxicity of cylindrospermopsin, and other apparent metabolites from Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum, to the zebrafish (Danio rerio) embryo. Toxicon 2009, 53, 289–299. [Google Scholar] [CrossRef]
- Silva, C.; Anselmo, A.; Mac’ario, I.P.E.; de Figueiredo, D.; Gonçalves, F.J.M.; Pereira, J.L. The bad against the villain: Suitability of Corbicula fluminea as a bioremediation agent towards cyanobacterial blooms. Ecol. Eng. 2020, 152, 105881. [Google Scholar] [CrossRef]
- e Silva, F.A.; Giani, A. Population dynamic of bloom-forming Microcystis aeruginosa in the presence of the invasive bivalve Limnoperna fortunei. Harmful Algae 2018, 73, 148–156. [Google Scholar] [CrossRef]
- MacKeigan, P.W.; Taranu, Z.E.; Pick, F.R.; Beisner, B.E.; Gregory-Eaves, I. Both biotic and abiotic predictors explain significant variation in cyanobacteria biomass across lakes from temperate to subarctic zones. Limnol. Oceanogr. 2023, 68, 1360–1375. [Google Scholar] [CrossRef]
- Anderson, L.; Fabbro, L.D.; Cowden, K. Assessment of Blue-Green Algal Toxins in Barramundi, Red Clay and Mussels from Awoonga Dam; Central Queensland University: Gladstone, Australia, 2003. [Google Scholar]
- Saker, M.L.; Metcalf, J.S.; Codd, G.A.; Vasconcelos, V.M. Accumulation and depuration of the cyanobacterial toxin cylindrospermopsin in the freshwater mussel Anodonta cygnea. Toxicon 2004, 43, 185–194. [Google Scholar] [CrossRef]
- Jeon, B.S.; Han, J.; Kim, S.K.; Ahn, J.H.; Oh, H.C.; Park, H.D. An overview of problems cyanotoxins produced by cyanobacteria and the solutions thereby. J. Korean Soc. Environ. Eng. 2015, 37, 657–667. [Google Scholar] [CrossRef]
- Chen, J.; Xie, P.; Li, L.; Xu, J. First Identification of the Hepatotoxic Microcystins in the Serum of a Chronically Exposed Human Population Together with Indication of Hepatocellular Damage. Toxicol. Sci. 2009, 108, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Sasner, J.J.; Ikawa, M.; Foxall, T.L. Studies on aphanizomenon and microcystis toxins. Seaf. Toxins 1984, 33, 391–406. [Google Scholar]
- Negri, A.P.; Jones, G.J. Bioaccumulation of paralytic shellfish poisoning (PSP) toxins from the cyanobacterium Anabaena circinalis by the freshwater mussels Alathrya condola. Toxicon 1995, 33, 667–678. [Google Scholar] [CrossRef]
- Karlson, A.M.L.; Mozūraitis, R. Deposit-feeders accumulate the cyanobacterial toxin nodularin. Harmful Algae 2011, 12, 77–81. [Google Scholar] [CrossRef]
- Strogyloudi, E.; Giannakourou, A.; Legrand, C.; Ruehl, A.; Granéli, E. Estimating the accumulation and transfer of Nodularia spumigena toxins by the blue mussel Mytilus edulis: An appraisal from culture and mesocosm experiments. Toxicon 2006, 48, 359–372. [Google Scholar] [CrossRef]
- España Amórtegui, J.C.; Pekar, H.; Chico Retrato, M.D.; Persson, M.; Karlson, B.; Bergquist, J.; Zuberovic-Muratovic, A. LC-MS/MS Analysis of Cyanotoxins in Bivalve Mollusks-Method Development, Validation and First Evidence of Occurrence of Nodularin in Mussels (Mytilus edulis) and Oysters (Magallana gigas) from the West Coast of Sweden. Toxins 2023, 15, 329. [Google Scholar] [CrossRef]
- Sipiä, V.; Kankaanpää, H.T.; Flinkman, J.; Lahti, K.; Meriluoto, J.A.O. Time-dependent accumulation of cyanobacterial hepatotoxins in flounders (Platichthys flesus) and Mussels (Mytilus edulis) from the Northern Baltic Sea. Environ. Toxicol. 2001, 16, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Kankaanpää, H.T.; Leiniö, S.; Olin, M.; Sjövall, O.; Meriluoto, J.; Lehtonen, K.K. Accumulation and depuration of cyanobacterial toxin nodularin and biomarker responses in the mussel Mytilus edulis. Chemosphere 2007, 68, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- White, S.H.; Duivenvoorden, L.J.; Fabbro, L.D.; Eaglesham, G.K. Influence of intracellular toxin concentrations on cylindrospermopsin bioaccumulation in a freshwater gastropod (Melanoidestuberculata). Toxicon 2006, 47, 497–509. [Google Scholar] [CrossRef]
- Vareli, K.; Zarali, E.; Zacharioudakis, G.S.; Vagenas, G.; Varelis, V.; Pilidis, G.; Briasoulis, E.; Sainis, I. Microcystin producing cyanobacterial communities in Amvrakikos Gulf (Mediterranean Sea, NW Greece) and toxin accumulation in mussels (Mytilus galloprovincialis). Harmful Algae 2012, 15, 109–118. [Google Scholar] [CrossRef]
- Preece, E.P.; Moore, B.C.; Joan Hardy, F. Transfer of microcystin from freshwater lakes to Puget Sound, WA and toxin accumulation in marine mussels (Mytilus trossulus). Ecotoxicol. Environ. Saf. 2015, 122, 98–105. [Google Scholar] [CrossRef]
- Wood, S.; Phillips, N.R.; Winton, M.D.; Gibbs, M.M. Consumption of benthic cyanobacterial mats and nodularin-R accumulation in freshwater crayfish (Paranephrops planifrons) in Lake Tikitapu (Rotorua, New Zealand). Harmful Algae 2012, 20, 175–179. [Google Scholar] [CrossRef]
- Saker, M.L.; Eaglesham, G.K. The accumulation of cylindrospermopsin from the cyanobacterium Cylindrospermopsis raciborskii in tissues of the Redclaw crayfish Cherax quadricarinatus. Toxicon 1999, 37, 1065–1077. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, P.; Chen, J.; Dai, M.; Qiu, T.; Liu, Y.; Liang, G. Determination of microcystin-LR and its metabolites in snail (Bellamya aeruginosa), shrimp (Macrobrachium nipponensis) and silver carp (Hypophthalmichthys molitrix) from Lake Taihu, China. Chemosphere 2009, 76, 974–981. [Google Scholar] [CrossRef]
- Chen, J.; Xie, P.; Guo, L.; Zheng, L.; Ni, L. Tissue distributions and seasonal dynamics of the hepatotoxic microcystins-LR and -RR in a freshwater snail (Bellamya aeruginosa) from a large shallow, eutrophic lake of the subtropical China. Environ. Pollut. 2005, 134, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Poullain, V.; Lance, E.; Acou, A.; Brient, L.; Carpentier, A. Influence of toxic cyanobacteria on community structure and microcystin accumulation of freshwater molluscs. Environ. Pollut. 2009, 157, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Lance, E.; Neffling, M.R.; Gérard, C.; Meriluoto, J.; Bormans, M. Accumulation of free and covalently bound microcystins in tissues of Lymnaea stagnalis (Gastropoda) following toxic cyanobacteria or dissolved microcystin-LR exposure. Environ. Pollut. 2010, 158, 674–680. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Song, Z.; Xie, Z.; Li, L.; Song, L. Bioaccumulation of microcystins in two freshwater gastropods from a cyanobacteria-bloom plateau lake, Lake Dianchi. Environ. Pollut. 2012, 164, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Lance, E. Decline of freshwater gastropods exposed to recurrent interacting stressors implying cyanobacterial proliferations and droughts. Aquat. Ecol. 2019, 53, 79–96. [Google Scholar] [CrossRef]
- Berry, J.P.; Lind, O. First evidence of “paralytic shellfish toxins” and cylindrospermopsin in a Mexican freshwater system, Lago Catemaco, and apparent bioaccumulation of the toxins in “tegogolo” snails (Pomacea patula catemacensis). Toxicon 2010, 55, 930–938. [Google Scholar] [CrossRef]
- Xie, L.; Yokoyama, A.; Nakamura, K.; Park, H. Accumulation of microcystins in various organs of the freshwater snail Sinotaia histrica and three fishes in a temperate lake, the eutrophic Lake Suwa, Japan. Toxicon 2007, 49, 646–652. [Google Scholar] [CrossRef]
- Engström-öst, J.; Lehtinienmi, M.; Green, S.; Kozlowsky-Suzuki, B.; Viitassalo, M. Does cyanobacterial toxin accumulate in mysid shrimps and fish via copepods? J. Exp. Mar. Biol. Ecol. 2002, 276, 95–107. [Google Scholar] [CrossRef]
- Garcia, A.C.; Bargu, S.; Dash, P.; Rabalais, N.N.; Sutor, M.M.; Morrison, W.; Walker, N.D. Evaluating the potential risk of microcystins to blue crab (Callinectes sapidus) fisheries and human health in a eutrophic estuary. Harmful Algae 2010, 9, 134–143. [Google Scholar] [CrossRef]
- Berry, J.P.; Jaja-Chimedza, A.; Dávalos-Lind, L.; Lind, O. Apparent bioaccumulation of cylindrospermopsin and paralytic shellfish toxins by finfish in Lake Catemaco (Veracruz, Mexico). Food Addit. Contam. 2011, 29, 314–321. [Google Scholar] [CrossRef]
- Kankaanpää, H.T.; Holliday, J.; Schröder, H.; Goddard, T.J.; Von Fister, R.; Carmichael, W.W. Cyanobacteria and prawn farming in northern New South Wales, Australia-a case study on cyanobacteria diversity and hepatotoxin bioaccumulation. Toxicol. Appl. Pharmacol. 2005, 203, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Xie, P.; Wang, Q.; Ma, Z. Simultaneous determination of microcystin contaminations in various vertebrates (fish, turtle, duck and water bird) from a large eutrophic Chinese lake, Lake Taihu, with toxic Microcystis blooms. Sci. Total Environ. 2009, 407, 3317–3322. [Google Scholar] [CrossRef] [PubMed]
- Pawlik-Skowrońska, B.; Toporowska, M.; Rechulicz, J. Simultaneous accumulation of anatoxin-a and microcystins in three fish species indigenous to lakes affected by cyanobacterial blooms. Oceanol. Hydrobiol. Stud. 2012, 41, 53–65. [Google Scholar] [CrossRef]
- Sipiä, V.; Kankaanpää, H.; Peltonen, H.; Vinni, M.; Meriluoto, J. Transfer of nodularin to three-spined stickleback (Gasterosteus aculeatus L.), herring (Clupea harengus L.), and salmon (Salmo salar L.) in the northern Baltic Sea. Ecotoxicol. Environ. Saf. 2007, 66, 421–425. [Google Scholar] [CrossRef]
- Clemente, Z.; Busato, R.H.; Oliveira Ribeiro, C.A.; Cestari, M.M.; Ramsdorf, W.A.; Magalhães, V.F.; Wosiack, A.C.; Silva de Assis, H.C. Analyses of paralytic shellfish toxins and biomarkers in a southern Brazilian reservoir. Toxicon 2010, 55, 396–406. [Google Scholar] [CrossRef]
- Roegner, A.F.; Corman, J.R.; Sitoki, L.M.; Kwena, Z.A.; Ogari, Z. Impacts of algal blooms and microcystins in fish on small-scalefishers in Winam Gulf, Lake Victoria: Implications for health and livelihood. Ecol. Soc. 2023, 28, 49. [Google Scholar] [CrossRef] [PubMed]
- Scarlett, K.R.; Kim, S.; Lovin, L.M.; Chatterjee, S.; Scott, J.T.; Brooks, B.W. Global scanning of cylindrospermopsin: Critical review and analysis of aquatic occurrence, bioaccumulation, toxicity and health hazards. Sci. Total Environ. 2020, 738, 139807. [Google Scholar] [CrossRef]
- Cadel-Six, S.; Moyenga, D.; Magny, S.; Trotereau, S.; Edery, M.; Krys, S. Detection of free and covalently bound microcystins in different tissues (liver, intestines, gills, and muscles) of rainbow trout (Oncorhynchus mykiss) by liquid chromatography-tandem mass spectrometry: Method characterization. Environ. Pollut. 2014, 185, 333–339. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Bakr, A. Concentrations of cylindrospermopsin toxin in water and tilapia fish of tropical fishponds in Egypt, and assessing their potential risk to human health. Environ. Sci. Pollut. Res. 2018, 25, 36287–36297. [Google Scholar] [CrossRef]
- Wilson, A.E.; Gossiaux, D.C.; Höök, T.O.; Berry, J.P.; Landrum, P.F.; Dyble, J.; Guildford, S.J. Evaluation of the human health threat associated with the hepatotoxin microcystin in the muscle and liver tissues of yellow perch (Perca flavescens). Can. J. Fish. Aquat. Sci. 2008, 65, 1487–1497. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Bruning, K.; de Jonge, J.; Wolfstein, K.; Dionisio Pires, L.M.; Postma, J.; Burger, T. Distribution of Microcystins in a Lake Foodweb: No Evidence for Biomagnification. Microb. Ecol. 2005, 49, 487–500. [Google Scholar] [CrossRef]
- Sipiä, V.; Sjövall, O.; Valtonen, T.; Barnaby, D.L.; Codd, G.A.; Metcalf, J.S.; Kilpi, M.; Mustonen, O.; Meriluoto, J.A.O. Analysis of nodularin-R in eider (Somateria mollissima), roach (Rutilus rutilus L.), and flounder (Platichthys flesus L.) liver and muscle samples from the western Gulf of Finland, northern Baltic Sea. Environ. Toxicol. Chem. 2006, 25, 2834–2839. [Google Scholar] [CrossRef] [PubMed]
- Kankaanpää, H.T.; Vuorinen, P.J.; Sipiä, V.; Keinänen, M. Acute effects and bioaccumulation of nodularin in sea trout (Salmo trutta m. trutta L.) exposed orally to Nodularia spumigena under laboratory conditions. Aquat. Toxicol. 2002, 61, 155–168. [Google Scholar] [CrossRef]
- Soares, R.M.; Magalhães, V.F.; Azevedo, S. Accumulation and depuration of microcystins (cyanobacteria hepatotoxins) in Tilapia rendalli (Cichlidae) under laboratory conditions. Aquat. Toxicol. 2004, 70, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Acou, A.; Robinet, T.; Lance, E.; Gérard, C.; Mounaix, B.; Brient, L.; Le Rouzic, B.; Feunteun, E. Evidence of silver eels contamination by microcystin-LR at the onset of their seaward migration: What consequences for breeding potential? J. Fish. Biol. 2008, 72, 753–762. [Google Scholar] [CrossRef]
- Stewart, I.; Eaglesham, J.K.; McGregor, G.B.; Chong, R.; Seawright, A.A.; Wickramasinghe, W.A.; Sadler, R.; Hunt, L.; Graham, G. First Report of a Toxic Nodularia spumigena (Nostocales/Cyanobacteria) Bloom in Sub-Tropical Australia. II. Bioaccumulation of Nodularin in Isolated Populations of Mullet (Mugilidae). Int. J. Environ. Res. Public Health 2012, 9, 2412–2443. [Google Scholar] [CrossRef]
- Mazur-Marzec, H.; Tymińska, A.; Szafranek, J.; Pliński, M. Accumulation of nodularin in sediments, mussels, and fish from the Gulf of Gdańsk, southern Baltic Sea. Environ. Toxicol. 2007, 22, 101–111. [Google Scholar] [CrossRef]
- Sipiä, V.; Kankaanpää, H.T.; Pflugmacher, S.; Flinkman, J.; Furey, A.; James, K.J. Bioaccumulation and Detoxication of Nodularin in Tissues of Flounder (Platichthys flesus), Mussels (Mytilus edulis, Dreissena polymorpha), and Clams (Macoma balthica) from the Northern Baltic Sea. Ecotoxicol. Environ. Saf. 2002, 53, 305–311. [Google Scholar] [CrossRef]
- Persson, K.J.; Legrand, C.; Olsson, T. Detection of nodularin in European flounder (Platichthys flesus) in the west coast of Sweden: Evidence of nodularin mediated oxidative stress. Harmful Algae 2009, 8, 832–838. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.A.; Zhao, Q.; Pu, C.; Qiu, Z.; Zhang, R.; Shu, W. A cross-sectional investigation of chronic exposure to microcystin in relationship to childhood liver damage in the Three Gorges Reservoir Region, China. Environ. Health Perspect. 2011, 119, 1483–1488. [Google Scholar] [CrossRef]
- Azevedo, S.; Carmichael, W.W.; Jochimsen, E.M.; Rinehart, K.L.; Lau, S.; Shaw, G.R.; Eaglesham, G.K. Human intoxication by microcystins during renal dialysis treatment in Caruaru-Brazil. Toxicology 2002, 181, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, E.D.; Carmichael, W.W.; Yuan, M.; Azevedo, S.M.F.D.O.E. A simple colorimetric method to detect biological evidence of human exposure to microcystins. Toxicon 2005, 46, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.M.; Yuan, M.; Servaites, J.C.; Delgado, A.; Magalhães, V.F.; Hilborn, E.D.; Carmichael, W.W.; Azevedo, S. Sublethal exposure from microcystins to renal insufficiency patients in Rio de Janeiro, Brazil. Environ. Toxicol. 2006, 21, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Dreher, T.W.; Collart, L.P.; Mueller, R.S.; Halsey, K.H.; Bildfell, R.J.; Schreder, P.; Sobhakumari, A.; Ferry, R. Anabaena/Dolichospermum as the source of lethal microcystin levels responsible for a large cattle toxicosis event. Toxicon X 2019, 1, 100003. [Google Scholar] [CrossRef]
- Badar, M.; Batool, F.; Shah Khan, S.; Khokhar, I.; Qamar, M.K.; Yasir, C. Effects of microcystins toxins contaminated drinking water on hepatic problems in animals (cows and buffalos) and toxins removal chemical method. Buffalo Bull. 2017, 36, 43–56. [Google Scholar]
- Handeland, K.; Østensvik, Ø. Microcystin poisoning in roe deer (Capreolus capreolus). Toxicon 2010, 56, 1076–1078. [Google Scholar] [CrossRef]
- Foss, A.J.; Aubel, M.T.; Gallagher, B.; Mettee, N.; Miller, A.; Fogelson, S.B. Diagnosing Microcystin Intoxication of Canines: Clinicopathological Indications, Pathological Characteristics, and Analytical Detection in Postmortem and Antemortem Samples. Toxins 2019, 11, 456. [Google Scholar] [CrossRef]
- Turner, A.D.; Turner, F.R.I.; White, M.; Hartnell, D.; Crompton, C.G.; Bates, N.; Egginton, J.; Branscombe, L.; Lewis, A.M.; Maskrey, B.H. Confirmation Using Triple Quadrupole and High-Resolution Mass Spectrometry of a Fatal Canine Neurotoxicosis following Exposure to Anatoxins at an Inland Reservoir. Toxins 2022, 14, 804. [Google Scholar] [CrossRef]
- Fredrickson, A.; Richter, A.; Perri, K.A.; Manning, S.R. First Confirmed Case of Canine Mortality Due to Dihydroanatoxin-a in Central Texas, USA. Toxins 2023, 15, 485. [Google Scholar] [CrossRef]
- Faassen, E.J.; Harkema, L.; Begeman, L.; Lurning, M. First report of (homo)anatoxin-a and dog neurotoxicosis after ingestion of benthic cyanobacteria in The Netherlands. Toxicon 2012, 60, 378–384. [Google Scholar]
- Gugger, M.; Lenoir, S.; Berger, C.; Ledreux, A.; Druart, J.C.; Humbert, J.F.; Guette, C.; Bernard, C. First report in a river in France of the benthic cyanobacterium Phormidium favosum producing anatoxin-a associated with dog neurotoxicosis. Toxicon 2005, 45, 919–928. [Google Scholar] [CrossRef]
- McCarron, P.; Rafuse, C.; Scott, S.; Lawrence, J.E.; Bruce, M.R.; Douthwright, E.; Murphy, C.; Reith, M.; Beach, D.G. Anatoxins from benthic cyanobacteria responsible for dog mortalities in New Brunswick, Canada. Toxicon 2023, 227, 107086. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.O.; Foss, A.; Miller, M.A.; Gibson, Q.A. Detection of cyanotoxins (microcystins/nodularins) in livers from estuarine and coastal bottlenose dolphins (Tursiops truncatus) from Northeast Florida. Harmful Algae 2018, 76, 22–34. [Google Scholar] [CrossRef]
- Greer, B.; Meneely, J.P.; Elliott, C.T. Uptake and accumulation of Microcystin-LR based on exposure through drinking water: An animal model assessing the human health risk. Sci. Rep. 2018, 8, 4913. [Google Scholar] [CrossRef]
- White, S.H.; Duivenvoorden, L.J.; Fabbro, L.D.; Eaglesham, G.K. Mortality and toxin bioaccumulation in Bufo marinus following exposure to Cylindrospermopsis raciborskii cell extracts and live cultures. Environ. Pollut. 2007, 147, 158–167. [Google Scholar] [CrossRef]
- Su, R.C.; Meyers, C.M.; Warner, E.A.; Garcia, J.A.; Refsnider, J.M.; Lad, A.; Breidenbach, J.D.; Modyanov, N.; Malhotra, D.; Haller, S.T.; et al. Harmful algal bloom toxicity in Lithobates catesbeiana tadpoles. Toxins 2020, 12, 378. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; El Herry, S.; Bouaïcha, N. First reported case of turtle deaths during a toxic Microcystis spp. bloom in Lake Oubeira, Algeria. Ecotoxicol. Environ. Saf. 2008, 71, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yuan, L.; Zhang, L.; Qiu, T.; Liao, Q.; Xiang, J.; Luo, L.; Xiong, X. Pathological and biochemical characterizations of microcystin-LR-induced liver and kidney damage in chickens after acute exposure. Toxicon 2022, 220, 106952. [Google Scholar] [CrossRef]
- Sipiä, V.; Karlsson, K.M.; Meriluoto, J.A.O.; Kankaanpää, H.T. Eiders (Somateria mollissima) obtain nodularin, a cyanobacterial hepatotoxin, in baltic sea food web. Environ. Toxicol. Chem. 2009, 23, 1256–1260. [Google Scholar] [CrossRef]
- Krienitz, L.; Ballot, A.; Kotut, K.; Wiegand, C.; Pütz, S.; Metcalf, J.S.; Codd, G.A.; Pflugmacher, S. Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiol. Ecol. 2003, 43, 141–148. [Google Scholar] [CrossRef]
- Alonso-Andicoberry, C.; García-Villada, L.; López-Rodas, V.; Costas, E. Catastrophic mortality of flamingos in a Spanish national park caused by cyanobacteria. Vet. Rec. 2002, 151, 706–707. [Google Scholar] [PubMed]
- Pflugmacher, S. Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environ. Toxicol. 2002, 17, 407–413. [Google Scholar] [CrossRef]
- Biré, R.; Bertin, T.; Dom, I.; Hort, V.; Schmitt, C.; Diogène, J.; Lemée, R.; De Haro, L.; Nicolas, M. First Evidence of the Presence of Anatoxin-a in Sea Figs Associated with Human Food Poisonings in France. Mar. Drugs 2020, 18, 285. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.E.; Brena, B.M.; Luquet, C.M.; San Julian, M.; Pirez, M.; de Molina, M. Microcystin accumulation and antioxidant responses in the freshwater clam Diplodon chilensis patagonicus upon subchronic exposure to toxic Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2011, 74, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E. Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxication. Biochim. Biophys. Acta BBA-Gen. Subj. 1998, 1425, 527–533. [Google Scholar] [CrossRef]
- Peuthert, A.; Wiegand, C. Glutathione involvement in the biotransformation of microcystins in Dreissena polymorpha. In Poster Abstract, Proceedings of the 6th International Conference on Toxic Cyanobacteria (ICTC), Bergen, Norway, 21–27 June 2004; School of Chemistry and Chemical Engineering: Nanjing, China, 2024; pp. 21–27. [Google Scholar]
- Grosse, Y.; Baan, R.; Straif, K.; Secretan, B.; El Ghissassi, F.; Cogliano, V. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol. 2006, 7, 628–629. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Chorus, I. Accumulation of cyanobacterial toxins in freshwater “seafood” and its consequences for public health: A review. Environ. Pollut. 2007, 150, 177–192. [Google Scholar] [CrossRef]
- Butler, N.; Carlisle, J.; Linville, R. Toxicological Summary and Suggested Action Levels to Reduce Potential Adverse Health Effects of Six Cyanotoxins; Office of Environmental Health Hazard Assessment, California Environmental Protection Agency: Sacramento, CA, USA, 2012.
- Williams, D.E.; Dawe, S.C.; Kent, M.L.; Andersen, R.J.; Craig, M.; Holmes, C.F.B. Bioaccumulation and clearance of microcystins from salt water mussels, Mytilus edulis, and in vivo evidence for covalently bound microcystins in mussel tissues. Toxicon 1997, 35, 1617–1625. [Google Scholar] [CrossRef]
- Miles, C.O.; Sandvik, M.; Nonga, H.E.; Ballot, A.; Wilkins, A.L.; Rise, F.; Jaabaek, A.H.; Loader, J.I. Conjugation of microcystins with thiols is reversible: Base-catalyzed deconjugation for chemical analysis. Chem. Res. Toxicol. 2016, 29, 860–870. [Google Scholar] [CrossRef]
- Shahmohamadloo, R.S.; Frenken, T.; Rudman, S.M.; Ibelings, B.W.; Trainer, V.L. Diseases and disorders in fish due to harmful algal blooms. In Climate Change on Diseases and Disorders of Finfish in Cage Culture; CABI: Wallingford, UK, 2023; pp. 387–429. [Google Scholar]
- Ernst, B.; Hoeger, S.J.; O’Brien, E.; Dietrich, D.R. Oral toxicity of the microcystin-containing cyanobacterium Planktothrix rubescens in European whitefish (Coregonus lavaretus). Aquat Toxicol. 2006, 79, 31–40. [Google Scholar] [CrossRef]
- de Lima Pinheiro, M.M.; Santos, B.L.T.; Dantas Filho, J.V.; Pedroti, V.P.; Cavali, J.; Dos Santos, R.B.; Oliveira Carreira Nishiyama, A.C.; Guedes, E.A.C.; de Vargas Schons, S. First monitoring of cyanobacteria and cyanotoxins in freshwater from fish farms in Rondônia state, Brazil. Heliyon 2023, 9, e18518. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Barrios, C.A.; Nandini, S.; Sarma, S. Bioaccumulation of microcystins in seston, zooplankton and fish: A case study in Lake Zumpango, Mexico. Environ. Pollut. 2019, 249, 267–276. [Google Scholar] [CrossRef]
- Berry, J.P.; Lee, E.; Walton, K.; Wilson, A.E.; Bernal-Brooks, F. Bioaccumulation of microcystins by fish associated with a persistent cyanobacterial bloom in Lago de Patzcuaro (Michoacan, Mexico). Environ. Toxicol. Chem. 2011, 30, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Dolamore, B.; Puddick, J.; Wood, S. Accumulation of nodularin in New Zealand shortfin eel (Anguilla australis): Potential consequences for human consumption. N. Z. J. Mar. Freshw. Res. 2016, 51, 321–332. [Google Scholar] [CrossRef]
- Bukaveckas, P.A.; Lesutienė, J.; Gasiūnaitė, Z.R.; Ložys, L.; Olenina, I.; Pilkaitytė, R.; Pūtys, Ž.; Tassone, S.; Wood, J. Microcystin in aquatic food webs of the Baltic and Chesapeake Bay regions. Estuar. Coast. Shelf Sci. 2017, 191, 50–59. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Medrano-Padial, C.; Llana-Ruiz-Cabello, M.; Cătunescu, G.M.; Moyano, R.; Risalde, M.A.; Cameán, A.M.; Jos, A. Cylindrospermopsin-microcystin-LR combinations may induce genotoxic and histopathological damage in rats. Toxins 2020, 12, 348. [Google Scholar] [CrossRef]
- Kruk, C.; Martínez, A.; de la Escalera, G.M.; Trinchin, R.; Manta, G.; Segura, A.M.; Piccini, C.; Brena, B.; Yannicelli, B.; Fabiano, G.; et al. Rapid freshwater discharge on the coastal ocean as a mean of long distance spreading of an unprecedented toxic cyanobacteria bloom. Sci. Total Environ. 2021, 754, 142362. [Google Scholar] [CrossRef]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in Estuarine and Marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef]
- Mulvenna, V.; Dale, K.; Priestly, B.; Mueller, U.; Humpage, A.; Shaw, G.; Allinson, G.; Falconer, I. Health risk assessment for cyanobacterial toxins in seafood. Int. J. Environ. Res. Public Health 2012, 9, 807–820. [Google Scholar] [CrossRef]
- Gibble, C.M.; Peacock, M.B.; Kudela, R.M. Evidence of freshwater algal toxins in marine shellfish: Implications for human and aquatic health. Harmful Algae 2016, 59, 59–66. [Google Scholar] [CrossRef]
- Fristachi, A.; Sinclair, J.L.; Hall, S.; Berkman, J.A.H.; Boyer, G.; Burkholder, J.; Burns, J.; Carmichael, W.; DuFour, A.; Frazier, W.; et al. Occurrence of cyanobacterial harmful algal blooms workgroup report. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer Science & Business Media: Berlin, Germany, 2008; pp. 45–103. [Google Scholar]
- Vuorinen, P.J.; Sipiä, V.O.; Karlsson, K.; Keinänen, M.; Furey, A.; Allis, O.; James, K.; Perttilä, U.; Rimaila-Pärnänen, E.; Meriluoto, J.A.O. Accumulation and effects of nodularin from a single and repeated oral doses of cyanobacterium Nodularia spumigena on flounder (Platichthys flesus L.). Arch. Environ. Contam. Toxicol. 2009, 57, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Al-Tebrineh, J.; Mihali, T.K.; Pomati, F.; Neilan, B.A. Detection of saxitoxin-producing cyanobacteria and Anabaena circinalis in environmental water blooms by quantitative PCR. Appl. Environ. Microbiol. 2010, 76, 7836–7842. [Google Scholar] [CrossRef]
- Nauman, C.; Stanislawczyk, K.; Reitz, L.A.; Chaffin, J.D. The spatiotemporal distribution of potential saxitoxin-producing cyanobacteria in western Lake Erie. J. Great Lakes Res. 2024, 50, 102342. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Li, L.; Bhatia, M.; Ryu, H.; Santo Domingo, J.W.; Brown, J.; Goetz, J.; Seo, Y. Impact of harmful algal bloom severity on bacterial communities in a full-scale biological filtration system for drinking water treatment. Sci. Total Environ. 2024, 927, 171301. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, G.; Han, S.; Kim, M.J.; Shin, J.H.; Lee, S. Microbial communities in aerosol generated from cyanobacterial bloom-affected freshwater bodies: An exploratory study in Nakdong River, South Korea. Front. Microbiol. 2023, 14, 1203317. [Google Scholar] [CrossRef]
- Montuori, E.; De Luca, D.; Penna, A.; Stalberga, D.; Lauritano, C. Alexandrium spp.: From Toxicity to Potential Biotechnological Benefits. Mar. Drugs 2023, 22, 31. [Google Scholar] [CrossRef]
- García, C.; Pérez, F.; Contreras, C.; Figueroa, D.; Barriga, A.; López-Rivera, A.; Araneda, O.F.; Contreras, H.R. Saxitoxins and okadaic acid group: Accumulation and distribution in invertebrate marine vectors from Southern Chile. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 984–1002. [Google Scholar] [CrossRef]
- Seguel, M.; Molinet, C.; Díaz, M.; Álvarez, G.; García, C.; Marín, A.; Millanao, M.O.; Díaz, P.A. Paralytic shellfish toxins in the gastropod Concholepas concholepas: Variability, toxin profiles and mechanisms for toxicity reduction. Mar. Drugs 2023, 21, 44. [Google Scholar] [CrossRef]
- Zamorano, R.; Marín, M.; Cabrera, F.; Figueroa, D.; Contreras, C.; Barriga, A.; Lagos, N.; García, C. Determination of the Variability of both Hydrophilic and Lipophilic Toxins in Endemic Bivalves and Carnivores from Austral Pacific’s Fjords. Food Addit. Contam. Part A 2013, 30, 1660–1677. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, X.; Hu, J.; Chang, X.; Qian, Y. Adverse effects of Microcystis aeruginosa exudates on the filtration, digestion, and reproduction organs of benthic bivalve Corbicula fluminea. Sci. Rep. 2024, 14, 10934. [Google Scholar] [CrossRef]
- Álvarez, G.; Díaz, P.A.; Godoy, M.; Araya, M.; Ganuza, I.; Pino, R.; Álvarez, F.; Rengel, J.; Hernández, C.; Uribe, E.; et al. Paralytic Shellfish Toxins in Surf Clams Mesodesma donacium during a Large Bloom of Alexandrium catenella Dinoflagellates Associated to an Intense Shellfish Mass Mortality. Toxins 2019, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Vilariño, N.; Botana, L.M. The problem of cyanotoxins in freshwaters: From climate change to detoxification. In Freshwater Researches: Fundamentals, Trends and Perspectives; İstanbul University Press: Istanbul, Turkey, 2024. [Google Scholar]
- Anderson, M.; Valera, M.; Schnetzer, A. Co-occurrence of freshwater and marine phycotoxins: A record of microcystins and domoic acid in Bogue Sound, North Carolina (2015 to 2020). Harmful Algae 2023, 125, 102412. [Google Scholar] [CrossRef] [PubMed]
- Bormans, M.; Amzil, Z.; Mineaud, E.; Brient, L.; Savar, V.; Robert, E.; Lance, E. Demonstrated transfer of cyanobacteria and cyanotoxins along a freshwater-marine continuum in France. Harmful Algae 2019, 87, 101639. [Google Scholar] [CrossRef] [PubMed]
- Overlingė, D.; Kataržytė, M.; Vaičiūtė, D.; Gyraite, G.; Gečaitė, I.; Jonikaitė, E.; Mazur-Marzec, H. Are there concerns regarding cHAB in coastal bathing waters affected by freshwater-brackish continuum? Mar. Pollut. Bull. 2020, 159, 111500. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Havens, K.E. Cyanobacterial toxins: A qualitative meta-analysis of concentrations, dosage and effects in freshwater, estuarine and marine biota. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer Science & Business Media: Berlin, Germany, 2008; pp. 675–732. [Google Scholar]
- Jia, J.; Luo, W.; Lu, Y.; Giesy, J.P. Bioaccumulation of microcystins (MCs) in four fish species from Lake Taihu, China: Assessment of risks to humans. Sci. Total Environ. 2014, 487, 224–232. [Google Scholar] [CrossRef]
- Carmichael, W.W. The cyanotoxins. Adv. Bot. Res. 1997, 27, 211–256. [Google Scholar]
- Griffiths, D.J.; Saker, M.L. The Palm Island mystery disease 20 years on: A review of research on the cyanotoxin cylindrospermopsin. Environ. Toxicol. Int. J. 2003, 18, 78–93. [Google Scholar] [CrossRef]
- Foss, A.J.; Butt, J.; Aubel, M.T. Benthic periphyton from Pennsylvania, USA is a source for both hepatotoxins (microcystins/nodularin) and neurotoxins (anatoxin-a/homoanatoxin-a). Toxicon 2008, 150, 13–16. [Google Scholar] [CrossRef]
- Cordeiro-Araújo, M.K.; Chia, M.A.; Bittencourt-Oliveira, M.d.C. Potential human health risk assessment of cylindrospermopsin accumulation and depuration in lettuce and arugula. Harmful Algae 2017, 68, 217–223. [Google Scholar] [CrossRef]
- Haida, M.; El Khalloufi, F.; Mugani, R.; Essadki, Y.; Campos, A.; Vasconcelos, V.; Oudra, B. Microcystin Contamination in Irrigation Water and Health Risk. Toxins 2024, 16, 196. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Banack, S.A.; Lindsay, J.; Morrison, L.F.; Cox, P.A.; Codd, G.A. Co-occurrence of beta-N-methylamino-L-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environ. Microbiol. 2008, 10, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.; Bell, S.; Kaya, K.; Ward, C.; Beattie, K.; Metcalf, J. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Redouane, E.M.; Tazart, Z.; Lahrouni, M.; Mugani, R.; Elgadi, S.; Zine, H.; El Amrani Zerrifi, S.; Haida, M.; Martins, J.C.; Campos, A.; et al. Health risk assessment of lake water contaminated with microcystins for fruit crop irrigation and farm animal drinking. Environ. Sci. Pollut. Res. 2023, 30, 80234–80244. [Google Scholar] [CrossRef]
- Hereman, T.C.; Bittencourt-Oliveira, M. Bioaccumulation of Microcystins in Lettuce. J. Phycol. 2012, 48, 1535–1537. [Google Scholar] [CrossRef] [PubMed]
- Bullerjahn, G.S.; McKay, R.M.; Davis, T.W.; Baker, D.B.; Boyer, G.L.; D’Anglada, L.V.; Doucette, G.J.; Ho, J.C.; Irwin, E.G.; Kling, C.L.; et al. Global solutions to regional problems: Collecting global expertise to address the problem of harmful cyanobacterial blooms. A Lake Erie case study. Harmful Algae 2016, 54, 223–238. [Google Scholar] [CrossRef]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Mijović, B.; Codd, G.A.; Meriluoto, J. Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Arch. Toxicol. 2017, 91, 621–650. [Google Scholar] [CrossRef]
- Manganelli, M.; Testai, E.; Tazart, Z.; Scardala, S.; Codd, G.A. Co-occurrence of taste and odor compounds and cyanotoxins in cyanobacterial blooms: Emerging risks to human health? Microorganisms 2023, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Plaas, H.E.; Paerl, H.W. Toxic cyanobacteria: A growing threat to water and air quality. Environ. Sci. Technol. 2020, 55, 44–64. [Google Scholar] [CrossRef]
- Ressom, R. Health Effects of Toxic Cyanobacteria (Blue-Green Algae); National Health and Medical Research Council: Canberra, Australia, 1994. [Google Scholar]
- Kondo, F.; Matsumoto, H.; Yamada, S.; Ishikawa, N.; Ito, E.; Nagata, S.; Ueno, Y.; Suzuki, M.; Harada, K. Detection and identification of metabolites of microcystins formed in vivo in mouse and rat livers. Chem. Res. Toxicol. 1996, 9, 1355–1359. [Google Scholar] [CrossRef]
- Hilborn, E.D.; Carmichael, W.W.; Soares, R.M.; Yuan, M.; Servaites, J.C.; Barton, H.A.; Azevedo, S.M.F.O. Serologic evaluation of human microcystin exposure. Environ. Toxicol. 2007, 22, 459–463. [Google Scholar] [CrossRef]
- Blyth, S. Palm Island mystery disease. Med. J. Aust. 1980, 2, 40–42. [Google Scholar]
- Hawkins, P.R.; Runnegar, M.T.; Jackson, A.R.; Falconer, I. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Appl. Environ. Microbiol. 1985, 50, 1292–1295. [Google Scholar] [CrossRef]
- Mchau, G.; Machunda, R.; Kimanya, M.; Makule, E.; Gong, Y.; Mpolya, E.; Meneely, J.; Elliott, C.; Greer, B. First Report of the Co-occurrence of Cylindrospermopsin, Nodularin and Microcystins in the Freshwaters of Lake Victoria, Tanzania. Expo Health 2021, 13, 185–194. [Google Scholar] [CrossRef]
- Behm, D. Coroner cites algae in teen’s death. Milwaukee J. Sentin. 2003, 5, 1–2. [Google Scholar]
- Giannuzzi, L.; Sedan, D.; Echenique, R.; Andrinolo, D. An acute case of intoxication with cyanobacteria and cyanotoxins in recreational water in Salto Grande Dam, Argentina. Mar. Drugs 2011, 9, 2164–2175. [Google Scholar] [CrossRef]
- Vidal, F.; Sedan, D.; D’Agostino, D.; Cavalieri, M.L.; Mullen, E.; Parot Varela, M.M.; Flores, C.; Caixach, J.; Andrinolo, D. Recreational exposure during algal bloom in Carrasco Beach, Uruguay: A liver failure case report. Toxins 2017, 9, 267. [Google Scholar] [CrossRef]
- Bloch, R.A.; Beuhler, M.C.; Hilborn, E.D.; Faulkner, G.; Rhea, S. Epidemiologic and clinical features of cyanobacteria harmful algal bloom exposures reported to the National Poison Data System, United States, 2010–2022: A descriptive analysis. Environ. Health 2024, 23, 80. [Google Scholar] [CrossRef]
- Kubickova, B.; Babica, P.; Hilscherová, K.; Šindlerová, L. Effects of cyanobacterial toxins on the human gastrointestinal tract and the mucosal innate immune system. Environ. Sci. Eur. 2019, 31, 31. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Jiang, S.C. Can cyanotoxins penetrate human skin during water recreation to cause negative health effects? Harmful Algae 2020, 98, 101872. [Google Scholar] [CrossRef]
- Kholafazad Kordasht, H.; Hassanpour, S.; Baradaran, B.; Nosrati, R.; Hashemzaei, M.; Mokhtarzadeh, A.; de la Guardia, M. Biosensing of microcystins in water samples; recent advances. Biosens. Bioelectron. 2020, 165, 112403. [Google Scholar]
- French, B.W.; Kaul, R.; George, J.; Haller, S.T.; Kennedy, D.J.; Mukundan, D. A Case Series of Potential Pediatric Cyanotoxin Exposures Associated with Harmful Algal Blooms in Northwest Ohio. Infect. Dis. Rep. 2023, 15, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Drobac Backović, D.; Tokodi, N.; Marinović, Z.; Lujić, J.; Dulić, T.; Simić, S.B.; Đorđević, N.B.; Kitanović, N.; Šćekić, I.; Urbányi, B.; et al. Cyanobacteria, cyanotoxins, and their histopathological effects on fish tissues in Fehérvárcsurgó reservoir, Hungary. Environ. Monit. Assess. 2021, 193, 554. [Google Scholar] [CrossRef]
- Gaget, V.; Humpage, A.R.; Huang, Q.; Monis, P.; Brookes, J.D. Benthic cyanobacteria: A source of cylindrospermopsin and microcystin in Australian drinking water reservoirs. Water Res. 2017, 124, 454–464. [Google Scholar] [CrossRef]
- Pham, T.L.; Shimizu, K.; Dao, T.S.; Motoo, U. First report on free and covalently bound microcystins in fish and bivalves from Vietnam: Assessment of risks to humans. Environ. Toxicol. Chem. 2017, 36, 2953–2957. [Google Scholar] [CrossRef]
- Díez-Quijada, L.; Guzmán-Guillén, R.; Prieto Ortega, A.I.; Llana-Ruíz-Cabello, M.; Campos, A.; Vasconcelos, V.; Jos, A.; Cameán, A.M. New method for simultaneous determination of microcystins and cylindrospermopsin in vegetable matrices by SPE-UPLC-MS/MS. Toxins 2018, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Downing, A.; Niu, Y. Distribution and composition of cyanobacteria and microalgae associated with biological soil crusts in the Gurbantunggut Desert, China. Arid Land Res. Manag. 2011, 25, 275–293. [Google Scholar] [CrossRef]
- Ibelings, B.W.; Kurmayer, R.; Azevedo, S.M.; Wood, S.A.; Chorus, I.; Welker, M. Understanding the occurrence of cyanobacteria and cyanotoxins. In Toxic Cyanobacteria in Water; CRC Press: Boca Raton, FL, USA, 2021; pp. 213–294. [Google Scholar]
- Plaas, H.E.; Paerl, R.W.; Baumann, K.; Karl, C.; Popendorf, K.J.; Barnard, M.A.; Chang, N.Y.; Curtis, N.P.; Huang, H.; Mathieson, O.L.; et al. Harmful cyanobacterial aerosolization dynamics in the airshed of a eutrophic estuary. Sci. Total Environ. 2022, 852, 158383. [Google Scholar] [CrossRef] [PubMed]
- Fleming, E.D.; Bebout, B.M.; Castenholz, R.W. Photochemical changes during rehydration of an intertidal cyanobacterial mat exposed to variations in salinity and light intensity. Microbiology 2021, 167, 001075. [Google Scholar] [CrossRef]
- Reichwaldt, E.S.; Stone, D.; Barrington, D.J.; Sinang, S.C.; Ghadouani, A. Development of toxicological risk assessment models for acute and chronic exposure to pollutants. Toxins 2016, 8, 251. [Google Scholar] [CrossRef]
- Walls, J.T.; Wyatt, K.H.; Doll, J.C.; Rubenstein, E.M.; Rober, A.R. Hot and toxic: Temperature regulates microcystin release from cyanobacteria. Sci. Total Environ. 2018, 610, 786–795. [Google Scholar] [CrossRef]
- Corbel, S.; Mougin, C.; Bouaïcha, N. Cyanobacterial toxins: Modes of actions, fate in aquatic and soil ecosystems, phytotoxicity and bioaccumulation in agricultural crops. Chemosphere 2014, 96, 1–15. [Google Scholar] [CrossRef]
- Glibert, P.M.; Maranger, R.; Sobota, D.J.; Bouwman, L. The Haber Bosch-harmful algal bloom (HB-HAB) link. Environ. Res. Lett. 2014, 9, 105001. [Google Scholar] [CrossRef]
- Lyu, K.; Gu, L.; Li, B.; Lu, Y.; Wu, C.; Guan, H.; Yang, Z. Stress-responsive expression of a glutathione S-transferase(delta) gene in waterflea Daphnia magna challenged by microcystin-producing and microcystin-free Microcystis aeruginosa. Harmful Algae 2016, 56, 1–8. [Google Scholar] [CrossRef]
- Olano, H.; Martigani, F.; Somma, A.; Aubriot, L. Wastewater discharge with phytoplankton may favor cyanobacterial development in the main drinking water supply river in Uruguay. Environ. Monit. Assess. 2019, 191, 146. [Google Scholar] [CrossRef]
- Portner, H.O.; Masson-Delmotte, V.; Roberts, D.; Zhai, P. IPCC Special Report on the Ocean and Crysphere in a Changing Climate; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Chen, N.; Liu, L.; Chen, M.S.; Li, Y.F.; Xing, X.G.; Lv, Y.Y. Effects of benthic bioturbation on phytoplankton in eutrophic water: A laboratory experiment. Fundam. Appl. Limnol. 2016, 188, 25–39. [Google Scholar] [CrossRef]
- Guo, Y.; Meng, H.; Zhao, S.; Wang, Z.; Zhu, L.; Deng, D.; Liu, J.; He, H.; Xie, W.; Wang, G.; et al. How does Microcystis aeruginosa respond to elevated temperature? Sci. Total Environ. 2023, 889, 164277. [Google Scholar] [CrossRef]
- Jiang, M.; Zheng, Z. Effects of multiple environmental factors on the growth and extracellular organic matter production of Microcystis aeruginosa: A central composite design response surface model. Environ. Sci. Pollut. Res. 2018, 25, 23276–23285. [Google Scholar] [CrossRef]
- Martin, R.M.; Moniruzzaman, M.; Stark, G.F.; Gann, E.R.; Derminio, D.S.; Wei, B.; Hellweger, F.L.; Pinto, A.; Boyer, G.L.; Wilhelm, S.W. Episodic decrease in temperature increases mcy gene transcription and cellular microcystin in continuous cultures of Microcystis aeruginosa PCC 7806. Front. Microbiol. 2020, 11, 601864. [Google Scholar] [CrossRef]
- Rodell, M.; Famiglietti, J.S.; Wiese, D.N.; Reager, J.T.; Beaudoing, H.K.; Landerer, F.W.; Lo, M.H. Emerging trends in global freshwater availability. Nature 2018, 557, 651–659. [Google Scholar] [CrossRef]
- Torremorell, A.; Hegoburu, C.; Brandimarte, A.L.; Rodrigues, E.H.C.; Pompêo, M.; da Silva, S.C.; Moschini-Carlos, V.; Caputo, L.; Fierro, P.; Mojica, J.I.; et al. Current and future threats for ecological quality management of South American freshwater ecosystems. Inland Waters 2021, 11, 125–140. [Google Scholar] [CrossRef]
- Mignot, A.; Ferrari, R.; Claustre, H. Floats with bio-optical sensors reveal what processes trigger the North Atlantic bloom. Nat. Commun. 2018, 9, 190. [Google Scholar] [CrossRef]
- Cai, P.; Cai, Q.; He, F.; Huang, Y.; Tian, C.; Wu, X.; Wang, C.; Xiao, B. Flexibility of Microcystisoverwintering strategy in response to winter temperatures. Microorganisms 2021, 9, 2278. [Google Scholar] [CrossRef]
- Gobler, C.J. Climate change and harmful algal blooms: Insights and perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef]
- Lad, A.; Breidenbach, J.D.; Su, R.C.; Murray, J.; Kuang, R.; Mascarenhas, A.; Najjar, J.; Patel, S.; Hegde, P.; Youssef, M.; et al. As we drink and breathe: Adverse health effects of microcystins and other harmful algal bloom toxins in the liver, gut, lungs and beyond. Life 2022, 12, 418. [Google Scholar] [CrossRef]
- Chia, M.A.; Kramer, B.J.; Jankowiak, J.G.; Bittencourt-Oliveira, M.D.C.; Gobler, C.J. The Individual and Combined Effects of the Cyanotoxins, Anatoxin-a and Microcystin-LR, on the Growth, Toxin Production, and Nitrogen Fixation of Prokaryotic and Eukaryotic Algae. Toxins 2019, 11, 43. [Google Scholar] [CrossRef]
- Zepernick, B.N.; Denison, E.R.; Chaffin, J.D.; Bullerjahn, G.S.; Pennacchio, C.P.; Frenken, T.; Peck, D.H.; Anderson, J.T.; Niles, D.; Zastepa, A.; et al. Metatranscriptomic sequencing of winter and spring planktonic communities from Lake Erie, a Laurentian Great Lake. Microbiol. Resour. Announc. 2022, 11, e00351-22. [Google Scholar] [CrossRef]
- Howard, M.D.; Smith, J.; Caron, D.A.; Kudela, R.M.; Loftin, K.; Hayashi, K.; Fadness, R.; Fricke, S.; Kann, J.; Roethler, M. Integrative monitoring strategy for marine and freshwater harmful algal blooms and toxins across the freshwater to marine continuum. Integr. Environ. Assess. Manag. 2022, 10, 1002. [Google Scholar] [CrossRef]
- Gaget, V.; Lau, M.; Sendall, B.; Froscio, S.; Humpage, A.R. Cyanotoxins: Which detection technique for an optimus risk assessment? Water Res. 2017, 118, 227–238. [Google Scholar] [CrossRef]
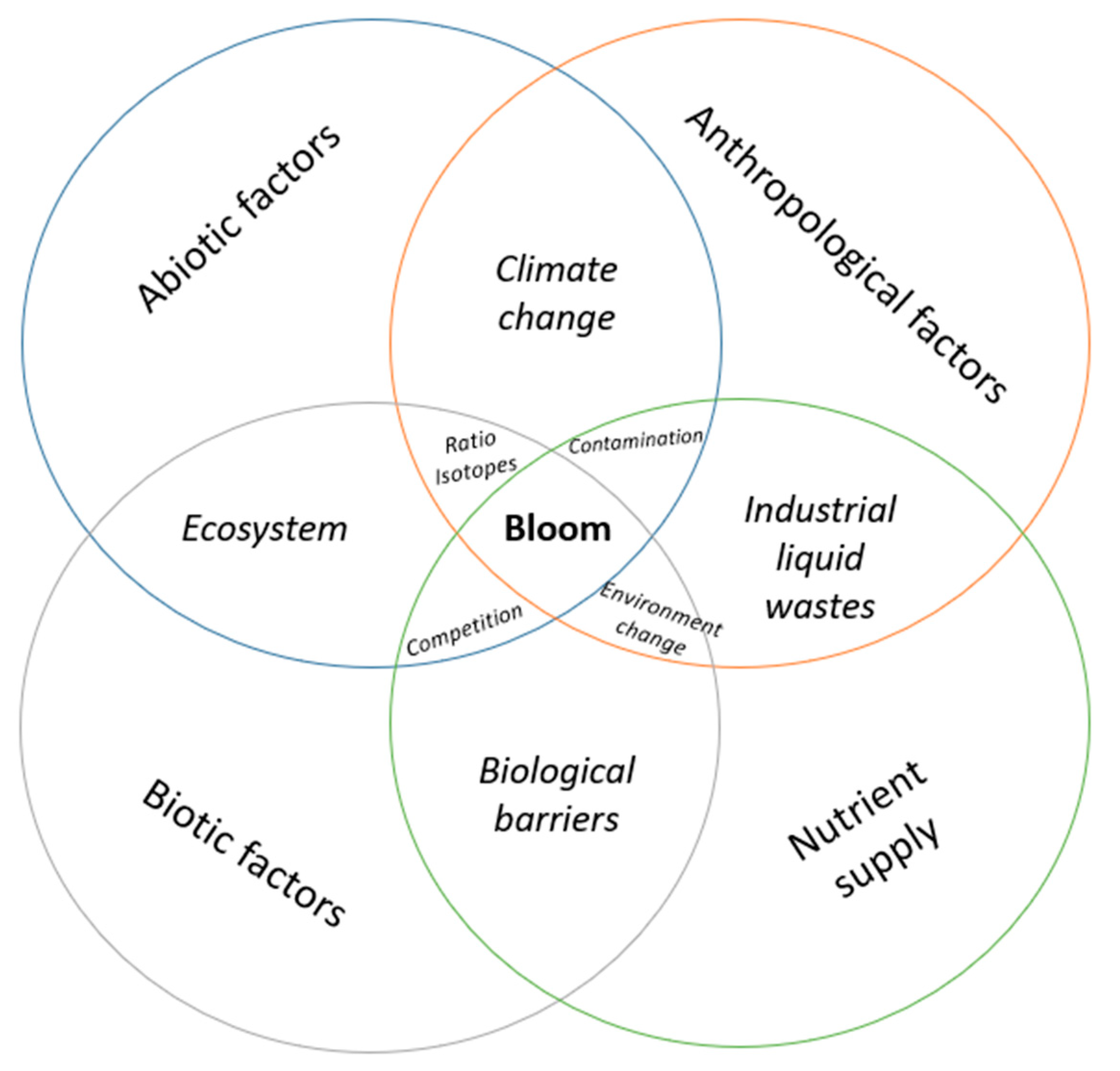
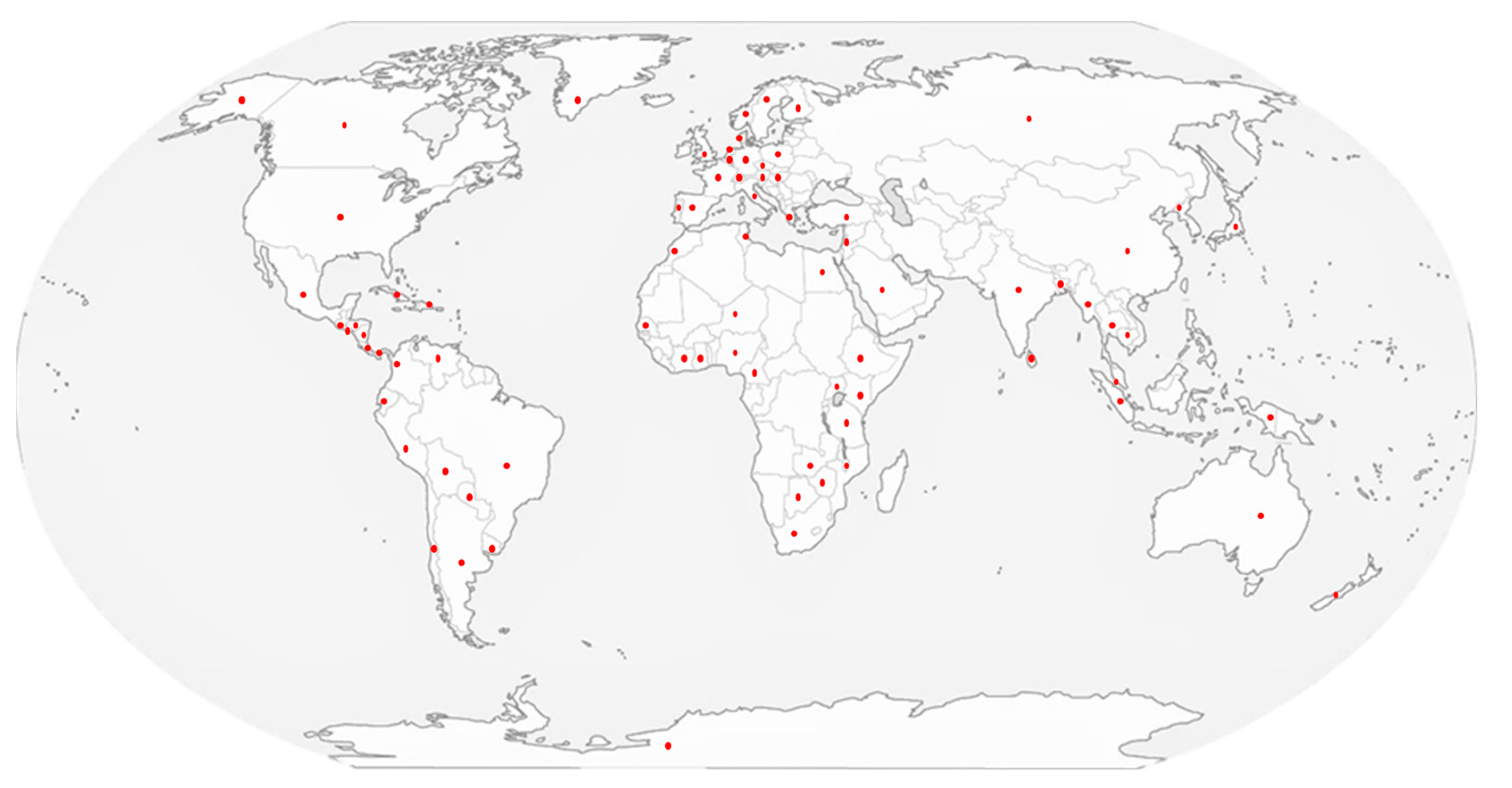


| Typical Species | |||||
|---|---|---|---|---|---|
| Species | Cyanotoxins | Toxin Accumulation | Method of Detection | References | |
| Bivalvia | |||||
| Freshwater | |||||
| Bivalve | Alathyria pertexta | CYN | 130.0–560.0 μg CYN/kg | HPLC | [229] |
| Anodonta cygnea | CYN | 2.9–61.5 μg/g DW | HPLC | [230] | |
| Anodonta woodiana | MC | 12.6 μg/kg DW | HPLC | [231] | |
| Cristaria plicata | MC-LR/YR/RR | 0.07 μg/g DW | LC-MS/MS | [232] | |
| Unio douglasiae | MC | 420.0 μg/kg DW | HPLC | [231] | |
| Corbicula fluminea | STX | 0.4–0.6 μg/g DW | HPLC | [233] | |
| Seawater | |||||
| Bivalve | Alanthyria condola | STX | 0.8–6.2 μg/g DW | HPLC | [234] |
| Macoma balthica | NOD | 0.16–30.0 μg/g DW | LC-ESI-MS | [235] | |
| Macoma balthica | NOD | 320.0 μg/kg DW | HPLC-DAD | [236] | |
| Magallana gigas | NOD | 24.1–397.3 μg/kg | LC-MS/MS | [237] | |
| Mytilus edulis | NOD | 0.28–13.8 μg/g DW | LC-ESI-MS | [236] | |
| Mytilus edulis | NOD | 2200.0 μg/kg DW | ELISA-LC-MS | [238] | |
| Mytilus edulis | NOD | 400.0–1100.0 μg/kg DW | LC-MS/MS | [239] | |
| Mytilus galloprovinciales | MC-LR/YR/RR | 0.7–53.9 ng/g | ELISA/UHPLC-HRMS | [71] | |
| Mytilus galloprovinciales | ATX | 6.6 ng/g DW | HPLC | [240] | |
| Mytilus galloprovinciales | MC-LR | 45.0–141.5 ng/g | ELISA | [241] | |
| Mytilus trossulus | MC-LR/LA/LW | 1.9–32.3 μg/kg DW | LC-MS/MS | [242] | |
| Gastropoda | |||||
| Freshwater | |||||
| Crayfish | Paranephrops planifrons | NOD | 9.7–225.3 μg/kg WW | LC-MS | [243] |
| Lobster | Cherax quadricarinatus | CYN | 0.54–4.3 μg/g | HPLC | [244] |
| Snail | Bellamya aeruginosa | MC-LR | 6.61 μg/g DW | LC-MS/MS | [245] |
| Bellamya aeruginosa | MC-LR/RR | 1.06–7.42 μg/g DW | LC-MS | [246] | |
| Helisoma trivolvis | MC | 37.0 μg/g DW | HPLC | [231] | |
| Hippeutis complanatus | MC | 1223.26 ng/g FM | HPLC | [247] | |
| Lymnaea stagnalis | MC-LR | 0.26 μg/g DW | ELISA | [248] | |
| Margaria melanoides | MC | 0.40 μg/g DW | ELISA | [249] | |
| Melanoides tuberculata | CYN | ND–250.0 μg/g DW | HPLC | [240] | |
| Physa acuta | MC | 1325.45 ng/g FM | HPLC | [250] | |
| Physa gyrina | MC | 129.0 μg/g DW | HPLC | [231] | |
| Planorbis planorbis | MC | 548.33 ng/g FM | HPLC | [250] | |
| Pomacea patula catemacensis | CYN | 3.35 ng/g | LC-MS/MS | [251] | |
| Pomacea patula catemacensis | STXs | 1.04–21.34 ng/g | ELISA | [251] | |
| Sinotaia histrica | MC-LR | 9.03 μg/g DW | HPLC | [252] | |
| Shrimp | Mysis relicta (Decapoda) | NOD | 0.5–0.74 μg/g DW | ELISA/PP1 | [253] |
| Seawater | |||||
| Crayfish | Callinectes sapidus | MC | 105.0 μg/L | ELISA | [254] |
| Cherax quadricarinatus | CYN | 0.9–4.3 μg/g DW | HPLC | [244] | |
| Shrimp | Macrobrachium nipponensis | MC-LR | 0.24 μg/g DW | LC-MS/MS | [245] |
| Snail | Vaughtia fenestrata | CYN | 0.8 ng/g | ELISA | [255] |
| Prawn | Penaeus monodon | NOD | 6.0–80.0 μg/kg DW | ELISA-LC-MS | [256] |
| Actinopterygii | |||||
| Freshwater | |||||
| Fish | Anguilla australis | NOD | 24.0 μg/kg | LC-MS/MS | [250] |
| Anguilla reinhardtii | NOD | 58.6 μg/kg DW (liver) | LC-MS/MS | [251] | |
| Bramocharax caballeroi | CYN | 0.81 ng/g | ELISA | [255] | |
| Ctenopharyngodon idellus | MC-LR/YR/RR | 0.04 μg/g DW | LC-MS/MS | [246] | |
| Carassius auratus | MC-LR | 150.0 ng/g DW | LC-ESI-MS | [257] | |
| Cyprinus carpio | ATX-a | 30.0 ng/g DW | GC/MS | [258] | |
| Cyprinus carpio | MC-LR/YR/RR | 0.10 μg/g DW | LC-MS/MS | [257] | |
| Gasterosteus aculeatus L. | NOD | 2.8–700.0 μg/kg | LC-MS/MS | [259] | |
| Geophagus brasiliensis | STX | 1.22–1.97 μg STX equiv/100 g | HPLC-FLD | [260] | |
| Hypophthalichthys molitrix | MC | 1.16–17.8 μg/kg DW | HPLC | [231] | |
| Hypophthalmichthys molitrix | MC-LR/YR/RR | 0.08 μg/g DW | LC-MS/MS | [257] | |
| Lates niloticus | MC-LR/YR/RR/LA | 0.7 μg/kg DW | LC-MS/MS | [261] | |
| Melanotaenia eachamensis | CYN | 1.2 μg/g DW | HPLC | [262] | |
| Oncorhynchus mykiss | MC-LR | 90.66 ng/g DW | LC-MS/MS | [263] | |
| Oreochromis niloticus | MC-LR/YR/RR/LA | 0.6–15 μg/kg DW | LC-MS/MS | [261] | |
| Oreochromis niloticus | CYN | 0.417 µg/g DW | ELISA/HPLC | [264] | |
| Perca flavescens | MC | 130.0 µg/g DW | ELISA | [265] | |
| Perca fluviatilis L. | MCs | 50.0 µg/g | HPLC-UV | [266] | |
| Rastrineobola argentea | MC-LR/YR/RR/LA | 23.4 μg/kg DW | LC-MS/MS | [261] | |
| Rutilus rutilus | NOD | 900.0 μg/kg DW | ELISA/LC-MS/MS | [267] | |
| Silurus glanis | MC-RR | 0.14 μg/g DW (muscle) | HPLC | [259] | |
| Salmo trutta | NOD | 125.0 μg/kg DW | ELISA | [268] | |
| Tilapia rendalli | MC | 2.9–67.8 µg/g PS | HPLC/ELISA | [269] | |
| Seawater | |||||
| Fish | Ariosoma mellissii | MC-LR | 28.1 μg/kg DW | ELISA | [270] |
| Clupea harengus | NOD | 6.5 μg/kg DW | ELISA | [259] | |
| Clupea harengus membras L. | NOD | 0.0–90.0 μg/kg | LC-MS/MS | [252] | |
| Gadus morhua | NOD | 0.05 µg/g DW | LC-MS/MS | [238] | |
| Melanotaenia eachamensis | CYN | 1.2 µg/g DW | HPLC | [244] | |
| Mugil cephalus | NOD | 32.3–56.8 μg/kg DW | LC-MS/MS | [271] | |
| Osmerus eperlanus | MCs | 874.0 µg/g DW | HPLC-DAD | [266] | |
| Platichthys flesus | NOD | 1.0 μg/kg DW | ELISA | [272] | |
| Platichthys flesus | NOD | 100.0–600.0 μg/kg WW | LC-MS/MALDI-TOF-MS | [273] | |
| Platichthys flesus | NOD | 22.0–557.0 μg/kg | HPLC | [274] | |
| Salmon salar | NOD | 5.0–10.0 μg/kg | ELISA/LC-MS/MS | [252] | |
| Vieja sp. | CYN | 0.42 ng/g | ELISA | [255] | |
| Atypical Species | |||||
| Species | Cyanotoxins | Toxin accumulation | Method of Detection | References | |
| Humans | |||||
| Human | Homo sapiens-sapiens. | MC | 2.03 μg daily MC intake | HPLC | [275] |
| Homo sapiens-sapiens. | MC-LR/YR/RR | 2.2–3.9 μg daily MC intake | LC-MS/MS | [232] | |
| Homo sapiens-sapiens. | MC | 2.2 ng/mL | ELISA | [276] | |
| Homo sapiens-sapiens. | MC | 7.1–31.4 ng/mL | LC-MS/MS | [277] | |
| Homo sapiens-sapiens. | MC | 0.16–0.96 ng/mL | ELISA | [278] | |
| Mammals | |||||
| Cow | Aberdeen angus | MC-LR | 7100.0 μg/L (rumen) | LC-MS/MS | [279] |
| Bos Taurus | MC | 5.7 ± 0.5 mg/L | ELISA | [280] | |
| Buffalo | Bison bison | MC | 9.7 ± 1.4 mg/L | ELISA | [237] |
| Deer | Capreolus capreolus | MC-YR/LR/RR | 1.36 μg equiv MC-LR/g | LC-MS | [281] |
| Dog | Canis lupus familiaris | MC | 100.0 mg/g DW | ELISA–LC-MS/MS | [282] |
| Flat-coat Retriever | ATX | 1.04 µg/g | LC-HRMS | [283] | |
| Golden retriever | dihydroanatoxin-a (dhATX) | 974.88 ng/g DW | LC-HRMS | [284] | |
| Labrador | Homo-ATX-a | 9.5 μg/g DW | LC-MS/MS | [285] | |
| Yorkshire terrier | ATX-a | 0.6 mg/g (liver) | HPLC-UV-LC-MS/MS | [286] | |
| Canis lupus familiaris | ATX | 357.0–785.0 mg/kg | LC-HRMS | [287] | |
| Dolphin | Tursiops truncatus | MC/NOD | 14.3 ± 5.6 ng/g DW | ELISA-LC-MS/MS | [288] |
| Pig | Sus scrofa domesticus | MC-LR | 26.4 μg/g DW | LC-MS/MS | [289] |
| Sea Otter | Enhydra lutris | MC-RR | 1.36–104.46 μg/g | LC-MS/MS | [106] |
| Amphibians | |||||
| Bufo marinus | Rhinella marina | CYN | 895.0 µg free-CYN/kg FW | LC-MS/MS | [290] |
| Bullfrog | Lithobates catesbeianus | MC | 1 μg/L | ELISA | [291] |
| Rana eperotica | Pelophylax epeiroticus | MC | 0.26–0.47 μg/g DW | PP2/ELISA | [290] |
| Reptiles | |||||
| Turtle | Emys orbicularis | MC-LR | 0.001–37.2 μg/g DW | PP2A/Limieux-GC-MS | [292] |
| Mauremys leprosa | MC-LR, -RR, -YR | 0.02–1.193 μg/g DW | PP2A/Limieux-GC-MS | [292] | |
| Pelodiscus sinensis | MC | 0.011–0.021 μg/g DW | LC-ESI-MS | [257] | |
| Birds | |||||
| Black-crowned night heron | Nycticorax nycticorax | MCs | 10.0 ng/g DW gonad | LC-ESI-MS | [257] |
| Chicken | Gallus gallus domesticus | MC-LR | 20.0 μg/kg | Bioassay | [293] |
| Domestic duck | Anas platyrhynchos | MC | 0.031 μg/g DW (liver) | HPLC | [231] |
| Anas platyrhynchos | MCs | 15.0 ng/g DW muscle | LC-ESI-MS | [257] | |
| Duck | Somateria mollissima | NOD | 3.0–180.0 μg/kg DW | LC-MS/MALDI-TOF-MS | [294] |
| Flamingo | Phoeniconaias minor | ATX-a | 7.62 μg/g DW | LC-MS/MS | [295] |
| Phoenicopterusruber | MC | 625.0 μg equiv MC-LR/mL | ELISA | [296] | |
| Plant Kingdom | |||||
| Apricot | Prunus armeniaca L. | MC | 7.20 ± 0.85 μg/kg DW | ELISA | [232] |
| Grape | Vitis vinifera L. | MC | 0.10 ± 0.02 μg/kg DW | ELISA | [232] |
| Lettuce | Lactuca sativa L. | MC | 8.31–177.8 μg/kg FW | LC-MS/MS | [233] |
| Plum | Prunus domestica L. | MC | 7.17 ± 0.39 μg/kg DW | ELISA | [232] |
| Aquatic plant | Ceratophyllum demersum | MC | 0.1–0.2 µg/L | HPLC | [297] |
| Myriophyllum spicatum | MC | 0.5 µg/L | HPLC | [297] | |
| Chordata | |||||
| Tunicate | Microcosmus sabatieri | ATX-a | 193.7–1240.2 μg/kg | LC-MS/MS | [298] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos, T.; Suárez-Isla, B.; Garcia, C. Health and Environmental Impacts of Cyanobacteria and Cyanotoxins from Freshwater to Seawater. Toxins 2025, 17, 126. https://doi.org/10.3390/toxins17030126
Villalobos T, Suárez-Isla B, Garcia C. Health and Environmental Impacts of Cyanobacteria and Cyanotoxins from Freshwater to Seawater. Toxins. 2025; 17(3):126. https://doi.org/10.3390/toxins17030126
Chicago/Turabian StyleVillalobos, Tamara, Benjamín Suárez-Isla, and Carlos Garcia. 2025. "Health and Environmental Impacts of Cyanobacteria and Cyanotoxins from Freshwater to Seawater" Toxins 17, no. 3: 126. https://doi.org/10.3390/toxins17030126
APA StyleVillalobos, T., Suárez-Isla, B., & Garcia, C. (2025). Health and Environmental Impacts of Cyanobacteria and Cyanotoxins from Freshwater to Seawater. Toxins, 17(3), 126. https://doi.org/10.3390/toxins17030126





