Morphologic, Phylogenetic and Chemical Characterization of a Brackish Colonial Picocyanobacterium (Coelosphaeriaceae) with Bioactive Properties
Abstract
:1. Introduction
2. Results
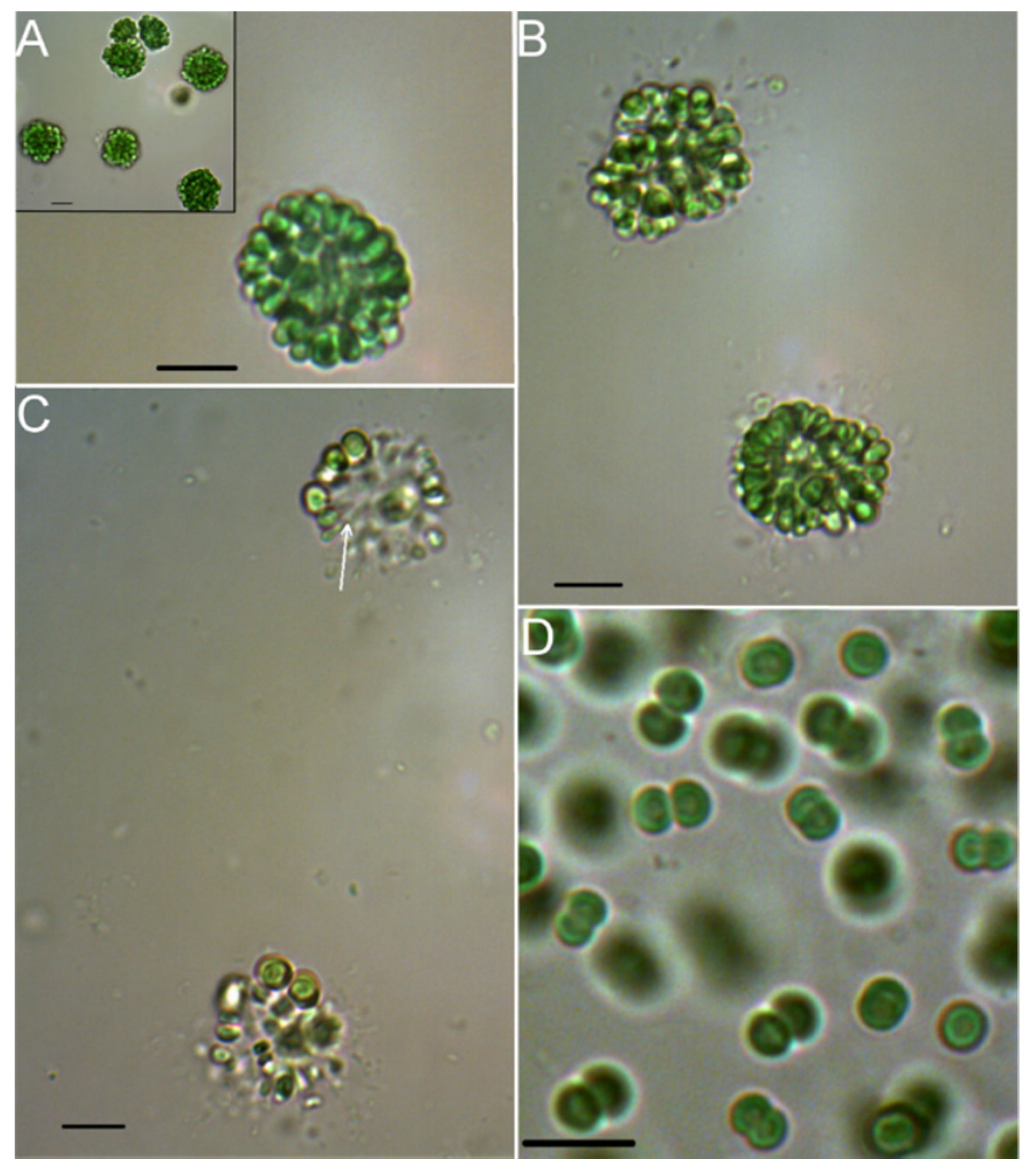
2.1. Morphologic and Phylogenetic Characterization
2.2. Analyses of Nonribosomal Oligopeptides
2.3. Analyses of Enzyme Inhibitory Activity
3. Discussion
3.1. Morphology and Phylogeny of Strain 06S067
3.2. Nonribosomal Oligopeptides Produced by Strain 06S067
3.3. Enzyme Inhibitory Activity of Fractionated Extracts from Strain 06S067
4. Conclusions
5. Materials and Methods
5.1. Cultivation
5.2. DNA-Extraction and PCR
5.3. Phylogenetic Analyses
5.4. Biomass Extraction
5.5. Analyses of Nonribosomal Oligopeptides
5.6. Enzyme Inhibition Assays
5.6.1. Carboxypeptidase A
5.6.2. Chymotrypsin
5.6.3. Elastase
5.6.4. Protein Phosphatase 1
5.6.5. Protein Phosphatase 2A
5.6.6. Thrombin
5.6.7. Trypsin
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AP | anabaenopeptin |
| BLAST | basic local alignment search tool |
| CYP | cyanopeptolin |
| NRP | nonribosomal oligopeptide |
| OSC | oscillamide |
| PCR | polymerase chain reaction |
References
- Christiansen, G.; Philmus, B.; Hemscheidt, T.; Kurmayer, R. Genetic variation of adenylation domains of the anabaenopeptin synthesis operon and evolution of substrate promiscuity. J. Bacteriol. 2011, 193, 3822–3831. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Fujii, K.; Shimada, T.; Suzuki, M.; Sano, H.; Adachi, K.; Carmichael, W.W. Two cyclic peptides, anabaenopeptins, a third group of bioactive compounds from the cyanobacterium Anabaena flos-aquae NRC 525-17. Tetrahedron Lett. 1995, 36, 1511–1514. [Google Scholar] [CrossRef]
- Welker, M.; von Döhren, H. Cyanobacterial peptides—Nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Oberer, L.; Ino, T.; Köing, W.; Busch, M.; Weckesser, J. Cyanopeptolins, new depsipeptides from the cyanobacterium Microcystis sp. PCC 7806. J. Antibiot. 1993, 46, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Spoof, L.; Błaszczyk, A.; Meriluoto, J.; Cegłowska, M.; Mazur-Marzec, H. Structures and activity of new anabaenopeptins produced by Baltic Sea cyanobacteria. Mar. Drugs 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Sivonen, K.; Börner, T. Bioactive compounds produced by cyanobacteria. In The Cyanobacteria. Molecular Biology, Genomics and Evolution; Herrero, A., Flores, E., Eds.; Caister Academic Press: Norfolk, UK, 2008; pp. 180–197. [Google Scholar]
- Rouhiainen, L. Characterization of Anabaena Cyanobacteria: Repeated Sequences and Genes Involved in Biosynthesis of Microcystins and Anabaenopeptilides. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 22 October 2004. [Google Scholar]
- Lopez-Otin, C.; Bond, J.S. Proteases: Multifunctional enzymes in life and disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef] [PubMed]
- McConnell, J.L.; Wadzinski, B.E. Targeting protein serine/threonine phosphatases for drug development. Mol. Pharmacol. 2009, 75, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki-Matsushima, R.; Ohta, T.; Nishiwaki, S.; Suganuma, M.; Kohyama, K.; Ishikawa, T.; Carmichael, W.W.; Fujiki, H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J. Cancer Res. Clin. Oncol. 1992, 118, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Sueoka, E.; Iida, N.; Komori, A.; Suganuma, M.; Nishiwaki, R.; Tatematsu, M.; Kim, S.J.; Carmichael, W.W.; Fujiki, H. Nodularin, a potent inhibitor of protein phosphatases 1 and 2A, is a new environmental carcinogen in male F344 rat liver. Cancer Res. 1994, 54, 6402–6406. [Google Scholar] [PubMed]
- Agha, R.; Quesada, A. Oligopeptides as biomarkers of cyanobacterial subpopulations. Toward an understanding of their biological role. Toxins 2014, 6, 1929–1950. [Google Scholar] [CrossRef] [PubMed]
- Gkelis, S.; Lanaras, T.; Sivonen, K. The presence of microcystins and other cyanobacterial bioactive peptides in aquatic fauna collected from Greek freshwaters. Aquat. Toxicol. 2006, 78, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Marzec, H.; Sutryk, K.; Hebel, A.; Hohlfeld, N.; Pietrasik, A.; Błaszczyk, A. Nodularia spumigena peptides—Accumulation and effect on aquatic invertebrates. Toxins 2015, 7, 4404–4420. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, O.; Henning, M.; Lippert, I.; Welker, M. Identification of peptide metabolites of Microcystis (Cyanobacteria) that inhibit trypsin-like activity in planktonic herbivorous Daphnia (Cladocera). Environ. Microbiol. 2006, 8, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Von Elert, E.; Zitt, A.; Schwarzenberger, A. Inducible tolerance to dietary protease inhibitors in Daphnia magna. J. Exp. Biol. 2012, 215, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Komárek, J.; Kastovsky, J.; Mares, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Joosten, A. Flora of the Blue-Green Algae of the Netherlands. I The Non-Filamentous Species of Inland Waters; KNNV Publishing: Utrecht, The Netherlands, 2006. [Google Scholar]
- Komárek, J. Review of the cyanobacterial genera implying planktic species after recent taxonomic revisions according to polyphasic methods: State as of 2014. Hydrobiologia 2015, 764, 1–12. [Google Scholar] [CrossRef]
- Rajaniemi-Wacklin, P.; Rantala, A.; Mugnai, M.; Turicchia, S.; Ventura, S.; Komárková, J.; Lepistö, L.; Sivonen, K. Correspondence between phylogeny and morphology of Snowella spp. and Woronichinia naegeliana, cyanobacteria commonly occurring in lakes. J. Phycol. 2006, 42, 226–232. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Teil/Part 1: Chroococcales. In Süßwasserflora von Mitteleuropa 19/1. Freshwater Flora of Central Europe; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Fischer: Stuttgart, Germany, 1999; pp. 192–215. [Google Scholar]
- Wilk-Woźniak, E.; Mazurkiewicz-Boroń, G. The autumn dominance of cyanoprokaryotes in a deep meso-eutrophic submontane reservoir. Biologia 2003, 58, 17–24. [Google Scholar]
- Willame, R.; Jurczak, T.; Iffly, J.; Kull, T.; Meriluoto, J.; Hoffmann, L. Distribution of hepatotoxic cyanobacterial blooms in Belgium and Luxembourg. Hydrobiologia 2005, 551, 99–117. [Google Scholar] [CrossRef]
- Oberholster, P.; Botha, A.; Cloete, T. Toxic cyanobacterial blooms in a shallow, artificially mixed urban lake in Colorado, USA. Lakes Reserv. Res. Manag. 2006, 11, 111–123. [Google Scholar] [CrossRef]
- Santos, M.C.; Muelle, H.; Pacheco, D.M. Cyanobacteria and microcystins in lake Furnas (S. Miguel Island-Azores). Limnetica 2012, 31, 107–118. [Google Scholar]
- Perkins, R.; Underwood, G. Partial recovery of a eutrophic reservoir through managed phosphorus limitation and unmanaged macrophyte growth. Hydrobiologia 2002, 481, 75–87. [Google Scholar] [CrossRef]
- Sivonen, K.; Niemelä, S.; Niemi, R.; Lepistö, L.; Luoma, T.; Räsänen, L. Toxic cyanobacteria (blue-green algae) in Finnish fresh and coastal waters. Hydrobiologia 1990, 190, 267–275. [Google Scholar] [CrossRef]
- Skulberg, O.M. Toxins produced by cyanophytes in Norwegian inland waters—Health and environment. In Chemical Data of Plant, Animal and Human Tissues as a Basis of Geomedical Investigations; Låg, J., Ed.; The Norwegian Academy of Science and Letters: Oslo, Norway, 1996; pp. 197–216. [Google Scholar]
- Bober, B.; Lechowski, Z.; Bialczyk, J. Determination of some cyanopeptides synthesized by Woronichinia naegeliana (Chroococcales, Cyanophyceae). Phycol. Res. 2011, 59, 286–294. [Google Scholar] [CrossRef]
- Descy, J.P.; Pirlot, S.; Verniers, G.; Viroux, L.; Lara, Y.; Wilmotte, A.; Vyverman, W.; Vanormelingen, P.; van Wichelen, J.; van Gremberghe, I.; et al. Cyanobacterial Blooms: Toxicity, Diversity, Modelling and Management “B-BLOOMS2”; Technical Report for Belgian Science Policy, Research Programme Science for a Sustainable Development: Brussels, Belgium, 2011; pp. 1–84. [Google Scholar]
- Komárek, J.; Komárková-Legnerová, J. Variability of some planktic gomphosphaerioid cyanoprocaryotes in northern lakes. Nord. J. Bot. 1992, 12, 513–524. [Google Scholar] [CrossRef]
- Sagert, S.; Rieling, T.; Eggert, A.; Schubert, H. Development of a phytoplankton indicator system for the ecological assessment of brackish coastal waters (German Baltic Sea coast). Hydrobiologia 2008, 611, 91–103. [Google Scholar] [CrossRef]
- Willame, R.; Boutte, C.; Grubisic, S.; Wilmotte, A.; Komárek, J.; Hoffmann, L. Morphological and molecular characterization of planktonic cyanobacteria from Belgium and Luxembourg. J. Phycol. 2006, 42, 1312–1332. [Google Scholar] [CrossRef]
- Lee, E.; Ryan, U.M.; Monis, P.; McGregor, G.B.; Bath, A.; Gordon, C.; Paparini, A. Polyphasic identification of cyanobacterial isolates from Australia. Water Res. 2014, 59, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Drag, M.; Salvesen, G.S. Emerging principles in protease-based drug discovery. Nat. Rev. Drug Discov. 2010, 9, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Usui, T.; Ueda, K.; Osada, H.; Kaya, K. Isolation of new protein phosphatase inhibitors from two cyanobacteria species, Planktothrix spp. J. Nat. Prod. 2001, 64, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Suzuki, S.; Itou, Y.; Kodani, S.; Ishida, K. New anabaenopeptins, potent carboxypeptidase-A inhibitors from the cyanobacterium Aphanizomenon flos-aquae. J. Nat. Prod. 2000, 63, 1280–1282. [Google Scholar] [CrossRef] [PubMed]
- Reshef, V.; Carmeli, S. Schizopeptin 791, a new anabaenopeptin-like cyclic peptide from the cyanobacterium Schizothrix sp. J. Nat. Prod. 2002, 65, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Itou, Y.; Suzuki, S.; Ishida, K.; Murakami, M. Anabaenopeptins G and H, potent carboxypeptidase A inhibitors from the cyanobacterium Oscillatoria agardhii (NIES-595). Bioorg. Med. Chem. Lett. 1999, 9, 1243–1246. [Google Scholar] [CrossRef]
- Kodani, S.; Suzuki, S.; Ishida, K.; Murakami, M. Five new cyanobacterial peptides from water bloom materials of lake Teganuma (Japan). FEMS Microbiol. Lett. 1999, 178, 343–348. [Google Scholar] [CrossRef]
- Repka, S.; Koivula, M.; Harjunpä, V.; Rouhiainen, L.; Sivonen, K. Effects of phosphate and light on growth of and bioactive peptide production by the cyanobacterium Anabaena strain 90 and its anabaenopeptilide mutant. Appl. Environ. Microbiol. 2004, 70, 4551–4560. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Matsuda, H.; Murakami, M. Micropeptins 88-A to 88-F, chymotrypsin inhibitors from the cyanobacterium Microcystis aeruginosa (NIES-88). Tetrahedron 1998, 54, 5545–5556. [Google Scholar] [CrossRef]
- Sano, T.; Kaya, K. Oscillamide Y, a chymotrypsin inhibitor from toxic Oscillatoria agardhii. Tetrahedron Lett. 1995, 36, 5933–5936. [Google Scholar] [CrossRef]
- Zafrir-Ilan, E.; Carmeli, S. Eight novel serine proteases inhibitors from a water bloom of the cyanobacterium Microcystis sp. Tetrahedron 2010, 66, 9194–9202. [Google Scholar] [CrossRef]
- Kwan, J.C.; Taori, K.; Paul, V.J.; Luesch, H. Lyngbyastatins 8–10, elastase inhibitors with cyclic depsipeptide scaffolds isolated from the marine cyanobacterium Lyngbya semiplena. Mar. Drugs 2009, 7, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Rohrlack, T.; Christoffersen, K.; Kaebernick, M.; Neilan, B.A. Cyanobacterial protease inhibitor microviridin J causes a lethal molting disruption in Daphnia pulcaria. Appl. Environ. Microbiol. 2004, 70, 5047–5050. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Spoof, L.; Meriluoto, J. SOP: Cultivation of cyanobacteria in modified Z8 medium. In TOXIC. Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Codd, G.A., Eds.; Åbo Akademi University Press: Åbo, Finland, 2005; Volume 65, pp. 87–92. [Google Scholar]
- Kotai, J. Instructions for Preparation of Modified Nutrient Solution Z8 for Algae; Norwegian Institute for Water Research: Blindern, Norway, 1972. [Google Scholar]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [PubMed]
- Lepère, C.; Wilmotte, A.; Meyer, B. Molecular diversity of Microcystis strains (Cyanophyceae, Chroococcales) based on 16S rDNA sequences. Syst. Geogr. Plants 2000, 70, 275–283. [Google Scholar] [CrossRef]
- Koskenniemi, K.; Lyra, C.; Rajaniemi-Wacklin, P.; Jokela, J.; Sivonen, K. Quantitative real-time PCR detection of toxic Nodularia cyanobacteria in the Baltic Sea. Appl. Environ. Microbiol. 2007, 73, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. BLAST >> Blastn Suite. Available online: http://blast.ncbi.nlm.nih.gov (accessed on 1 September 2015).
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Felsenstein, J. PHYLIP (Phylogeny Inference Package) Version 3.6; Department of Genome Sciences, University of Washington: Seattle, WA, USA, 2005. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Jukes, T.H.; Cantor, C.R. Evolution of protein molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; Volume 3, pp. 21–132. [Google Scholar]
- Rambaut, A. FigTree. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 16 January 2015).
- Grabowska, M.; Kobos, J.; Toruńska-Sitarz, A.; Mazur-Marzec, H. Non-ribosomal peptides produced by Planktothrix agardhii from Siemianówka dam reservoir SDR (northeast Poland). Arch. Microbiol. 2014, 196, 697–707. [Google Scholar] [CrossRef] [PubMed]
- MyCurveFit. Online Curve Fitting. Available online: http://mycurvefit.com/ (accessed on 15 October 2015).
- Hass, G.M.; Ager, S.P.; Le Tourneau, D.; Derr-Makus, J.E. Specificity of the carboxypeptidase inhibitor from potatoes. Plant Physiol. 1981, 67, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Mock, W.L.; Liu, Y.; Stanford, D.J. Arazoformyl peptide surrogates as spectrophotometric kinetic assay substrates for carboxypeptidase A. Anal. Biochem. 1996, 239, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Rapala, J.; Erkomaa, K.; Kukkonen, J.; Sivonen, K.; Lahti, K. Detection of microcystins with protein phosphatase inhibition assay, high-performance liquid chromatography-UV detection and enzyme-linked immunosorbent assay: Comparison of methods. Anal. Chim. Acta 2002, 466, 213–231. [Google Scholar] [CrossRef]
- Pluotno, A.; Carmeli, S. Banyasin A and Banyasides A and B, three novel modified peptides from a water bloom of the cyanobacterium Nostoc sp. Tetrahedron 2005, 61, 575–583. [Google Scholar] [CrossRef]


| Fraction (% MeOH) | m/z | Proposed Anabaenopeptin |
|---|---|---|
| 20 | 844 | AP A |
| 30 | 810 | AP 809 |
| 844 | AP A | |
| 858 | OSC Y | |
| 40 | 803 | AP 802 |
| 810 | AP 809 | |
| 828 | AP 827 | |
| 837 | AP B | |
| 844 | AP A | |
| 858 | OSC Y | |
| 50 | 752 | AP fragment a |
| 803 | AP 802 | |
| 837 | AP B | |
| 844 | AP A | |
| 858 | OSC Y | |
| 60 | 752 | AP fragment a |
| 803 | AP 802 | |
| 837 | AP B | |
| 844 | AP A | |
| 851 | AP F | |
| 70 | 637 | AP fragment a |
| 80 | 637 | AP fragment a |
| 90 | 637 | AP fragment a |
| 100 | 637 | AP fragment a |
| Enzyme | Fraction (% MeOH) | Inhibition (%) |
|---|---|---|
| Carboxypeptidase A | 30 | 13 |
| 40 | 8 | |
| 50 | 1 a | |
| Chymotrypsin | 50 | 5 |
| 60 | 14 a | |
| Elastase | 90 | 15 a |
| 100 | – b | |
| Protein phosphatase 1 | 40 | 0.001 |
| 50 | 0.0003 | |
| Protein phosphatase 2A | 30 | 0.003 |
| 40 | 0.001 | |
| 50 | 0.0005 | |
| Thrombin | 60 | 49 a |
| 70 | 44 | |
| Trypsin | 60 | 94 |
| 70 | 26 a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Häggqvist, K.; Toruńska-Sitarz, A.; Błaszczyk, A.; Mazur-Marzec, H.; Meriluoto, J. Morphologic, Phylogenetic and Chemical Characterization of a Brackish Colonial Picocyanobacterium (Coelosphaeriaceae) with Bioactive Properties. Toxins 2016, 8, 108. https://doi.org/10.3390/toxins8040108
Häggqvist K, Toruńska-Sitarz A, Błaszczyk A, Mazur-Marzec H, Meriluoto J. Morphologic, Phylogenetic and Chemical Characterization of a Brackish Colonial Picocyanobacterium (Coelosphaeriaceae) with Bioactive Properties. Toxins. 2016; 8(4):108. https://doi.org/10.3390/toxins8040108
Chicago/Turabian StyleHäggqvist, Kerstin, Anna Toruńska-Sitarz, Agata Błaszczyk, Hanna Mazur-Marzec, and Jussi Meriluoto. 2016. "Morphologic, Phylogenetic and Chemical Characterization of a Brackish Colonial Picocyanobacterium (Coelosphaeriaceae) with Bioactive Properties" Toxins 8, no. 4: 108. https://doi.org/10.3390/toxins8040108
APA StyleHäggqvist, K., Toruńska-Sitarz, A., Błaszczyk, A., Mazur-Marzec, H., & Meriluoto, J. (2016). Morphologic, Phylogenetic and Chemical Characterization of a Brackish Colonial Picocyanobacterium (Coelosphaeriaceae) with Bioactive Properties. Toxins, 8(4), 108. https://doi.org/10.3390/toxins8040108