Evaluation of Lipid Accumulation Using Electrical Impedance Measurement under Three-Dimensional Culture Condition
Abstract
:1. Introduction
2. Materials and Methods
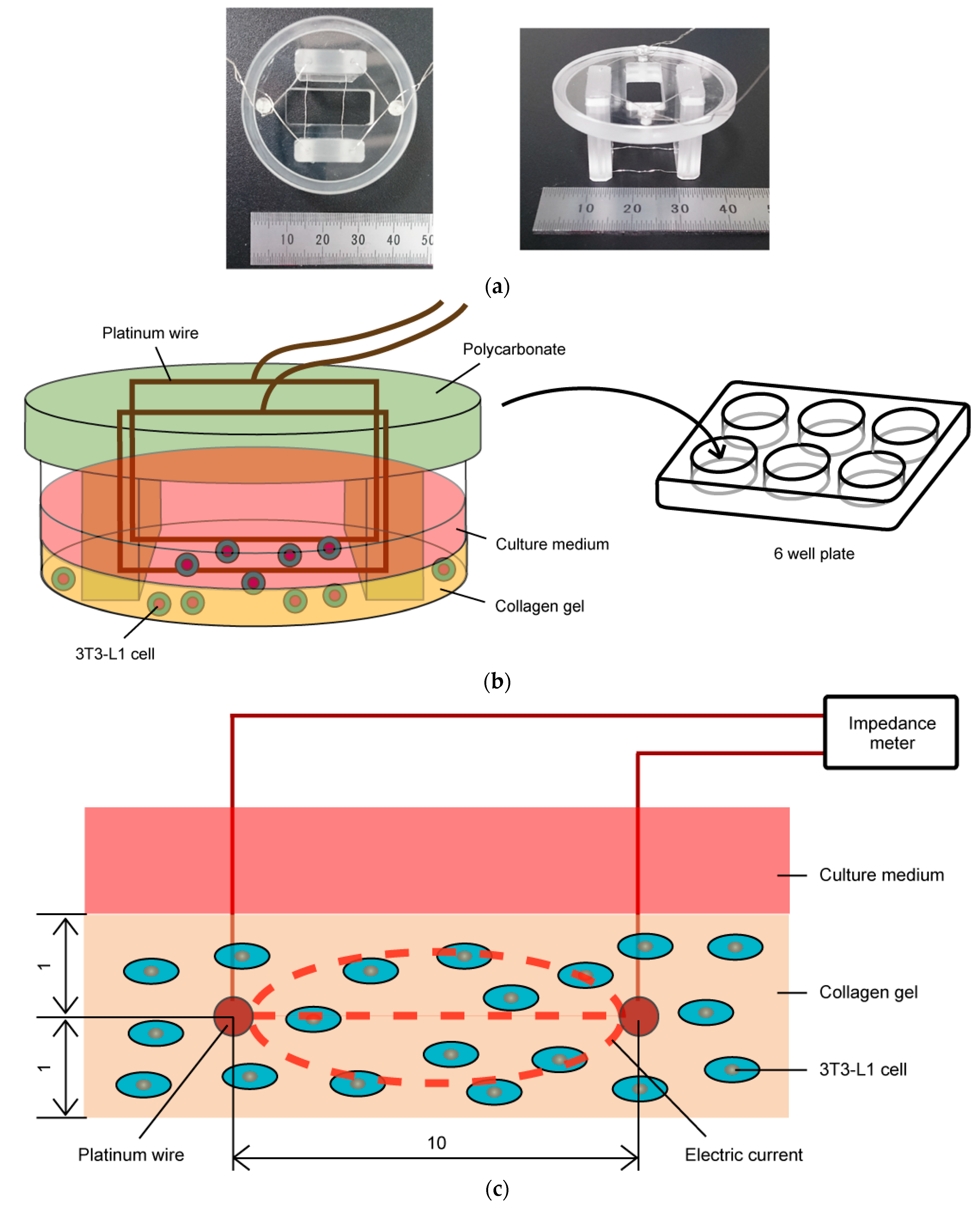
2.1. Impedance Measurement of Three-Dimensional Cell Culture
2.2. Evaluation of Characteristics of Living Cell
2.3. Cell Culture with Impedance Measurement
2.4. Biochemical Characterization
2.5. Statistical Analysis
3. Results and Discussion
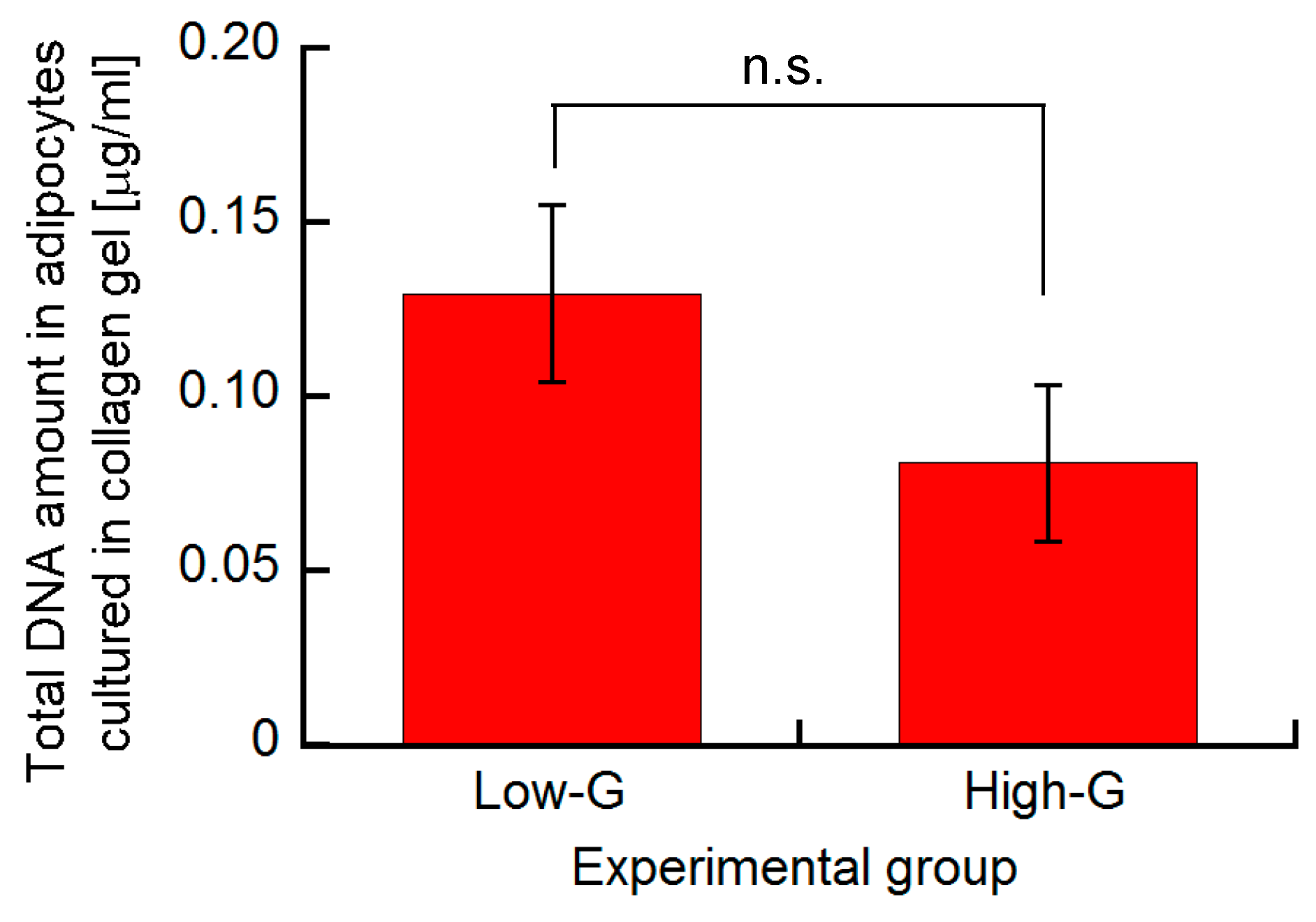
3.1. Effect of Glucose Concentration on Proliferation and Lipid Accumulation of 3T3-L1 Cells
3.2. Electrical Impedance of 3T3-L1 Cells Cultured Three-Dimensionally
3.3. Relationship between Lipid Accumulation and Electrical Impedance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platt, F.M. Emptying the stores: lysosomal diseases and therapeutic strategies. Nat. Rev. Drug Discov. 2017, 17, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Harm, T.; Levine, D.; Christopher, S.; Hostetter, S.; Snella, E.M.; Johnson, G.; Ellinwood, N.M. Novel lysosomal disease in a juvenile golden retriever. Mol. Genet. Metab. 2018, 123, S133. [Google Scholar] [CrossRef]
- Escorcia, W.; Ruter, D.L.; Nhan, J.; Curran, S.P. Quantification of Lipid Abundance and Evaluation of Lipid Distribution in Caenorhabditis elegans by Nile Red and Oil Red O Staining. J. Vis. Exp. 2018, 133, e57352. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Schaart, G.; Hesselink, M.K. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem. Cell Biol. 2001, 116, 63–68. [Google Scholar] [PubMed]
- Chyau, C.-C.; Chu, C.-C.; Chen, S.-Y.; Duh, P.-D. The inhibitory effects of djulis (chenopodium formosanum) and its bioactive compounds on adipogenesis in 3T3-L1 adipocytes. Molecules 2018, 23, 1780. [Google Scholar] [CrossRef] [PubMed]
- Giaever, I.; Keese, C.R. Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc. Natl. Acad. Sci. USA 1984, 81, 3761–3764. [Google Scholar] [CrossRef] [PubMed]
- Angstmann, M.; Brinkmann, I.; Bieback, K.; Breitkreutz, D.; Maercker, C. Monitoring human mesenchymal stromal cell differentiation by electrochemical impedance sensing. Cytotherapy 2011, 13, 1074–1089. [Google Scholar] [CrossRef]
- Jun, H.-S.; Dao, L.T.M.; Pyun, J.-C.; Cho, S. Effect of cell senescence on the impedance measurement of adipose tissue-derived stem cells. Enzym. Microb. Technol. 2013, 53, 302–306. [Google Scholar] [CrossRef]
- Xiao, C.; Lachance, B.; Sunahara, G.; Luong, J.H.T. An In-Depth Analysis of Electric Cell−Substrate Impedance Sensing to Study the Attachment and Spreading of Mammalian Cells. Anal. Chem. 2002, 74, 1333–1339. [Google Scholar] [CrossRef]
- Asphahani, F.; Zhang, M. Cellular Impedance Biosensors for Drug Screening and Toxin Detection. Analyst 2007, 132, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Basu, S.; Laue, E.; Seshia, A.A. Single cell studies of mouse embryonic stem cell (mESC) differentiation by electrical impedance measurements in a microfluidic device. Biosens. Bioelectron. 2016, 81, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.E.; Kim, D.; Koh, H.S.; Cho, S.; Sung, J.-S.; Kim, J.Y. Real-Time Monitoring of Neural Differentiation of Human Mesenchymal Stem Cells by Electric Cell-Substrate Impedance Sensing. J. Biomed. Biotechnol. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Jung, I.; Park, M.; Ha, H.; Yoo, K.H. Real-time monitoring of adipocyte differentiation using a capacitance sensor array. Lab Chip 2013, 13, 3410–3416. [Google Scholar] [CrossRef] [PubMed]
- Bagnaninchi, P.O.; Drummond, N. Real-time label-free monitoring of adipose-derived stem cell differentiation with electric cell-substrate impedance sensing. Proc. Natl. Acad. Sci. USA 2011, 108, 6462–6467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhr, G.; Müller, T.; Schnelle, T.; Hagedorn, R.; Voigt, A.; Fiedler, S.; Arnold, W.M.; Zimmermann, U.; Wagner, B.; Heuberger, A. Radio-frequency microtools for particle and liver cell manipulation. Naturwissenschaften 1994, 81, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Fuhr, G.; Shirley, S.G. Cell handling and characterization using micron and submicron electrode arrays: state of the art and perspectives of semiconductor microtools. J. Micromech. Microeng. 1995, 5, 77–85. [Google Scholar] [CrossRef]
- Puttaswamy, S.V.; Sivashankar, S.; Chen, R.-J.; Chin, C.-K.; Chang, H.-Y.; Liu, C.H.; Chen, R.; Chin, C.; Chang, H. Enhanced cell viability and cell adhesion using low conductivity medium for negative dielectrophoretic cell patterning. Biotechnol. J. 2010, 5, 1005–1015. [Google Scholar] [CrossRef]
- Macdonald, R.A.; Voge, C.M.; Kariolis, M.; Stegemann, J.P. Carbon nanotubes increase the electrical conductivity of fibroblast-seeded collagen hydrogels. Acta Biomater. 2008, 4, 1583–1592. [Google Scholar] [CrossRef]
- Da Cruz, A.G.; Góes, J.; Figueiró, S.; Feitosa, J.P.; Ricardo, N.M.P.; Sombra, A.S. On the piezoelectricity of collagen/natural rubber blend films. Eur. Polym. J. 2003, 39, 1267–1272. [Google Scholar] [CrossRef]
- Schade-Kampmann, G.; Huwiler, A.; Hebeisen, M.; Hessler, T.; Di Berardino, M. On-chip non-invasive and label-free cell discrimination by impedance spectroscopy. Cell Prolif. 2008, 41, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Aulthouse, A.L.; Freeh, E.; Newstead, S.; Stockert, A.L. Part 1: A Novel Model for Three-Dimensional Culture of 3T3-L1 Preadipocytes Stimulates Spontaneous Cell Differentiation Independent of Chemical Induction Typically Required in Monolayer. Nutr. Metab. Insights 2019, 12, 117863881984139. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Sah, R.L.; Doong, J.-Y.H.; Grodzinsky, A.J. Fluorometric assay of DNA in cartilage explants using Hoechst. Anal. Biochem. 1988, 174, 168–176. [Google Scholar] [CrossRef]
- Forsey, R.W.; Chaudhuri, J.B. Validity of DNA analysis to determine cell numbers in tissue engineering scaffolds. Biotechnol. Lett. 2009, 31, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Balaguera, E.; López-Dolado, E.; Polo, J.L. Obtaining electrical equivalent circuits of biological tissues using the current interruption method, circuit theory and fractional calculus. RSC Adv. 2016, 6, 22312–22319. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemmyo, D.; Miyata, S. Evaluation of Lipid Accumulation Using Electrical Impedance Measurement under Three-Dimensional Culture Condition. Micromachines 2019, 10, 455. https://doi.org/10.3390/mi10070455
Zemmyo D, Miyata S. Evaluation of Lipid Accumulation Using Electrical Impedance Measurement under Three-Dimensional Culture Condition. Micromachines. 2019; 10(7):455. https://doi.org/10.3390/mi10070455
Chicago/Turabian StyleZemmyo, Daiki, and Shogo Miyata. 2019. "Evaluation of Lipid Accumulation Using Electrical Impedance Measurement under Three-Dimensional Culture Condition" Micromachines 10, no. 7: 455. https://doi.org/10.3390/mi10070455




