A Review: Preparation, Performance, and Applications of Silicon Oxynitride Film
Abstract
:1. Introduction
2. Performance of SiNxOy Film
2.1. Luminescent Performance
2.2. Adjustable Refractive Index
3. Preparation of SiNxOy Film
3.1. CVD Method
3.1.1. PECVD
3.1.2. LPCVD
3.1.3. High Temperature Thermochemical Vapor Deposition (HTCVD)
3.1.4. Photochemical Vapor Deposition (Photo-CVD)
3.2. PVD
3.3. Oxynitridation
4. Applications of SiNxOy Film
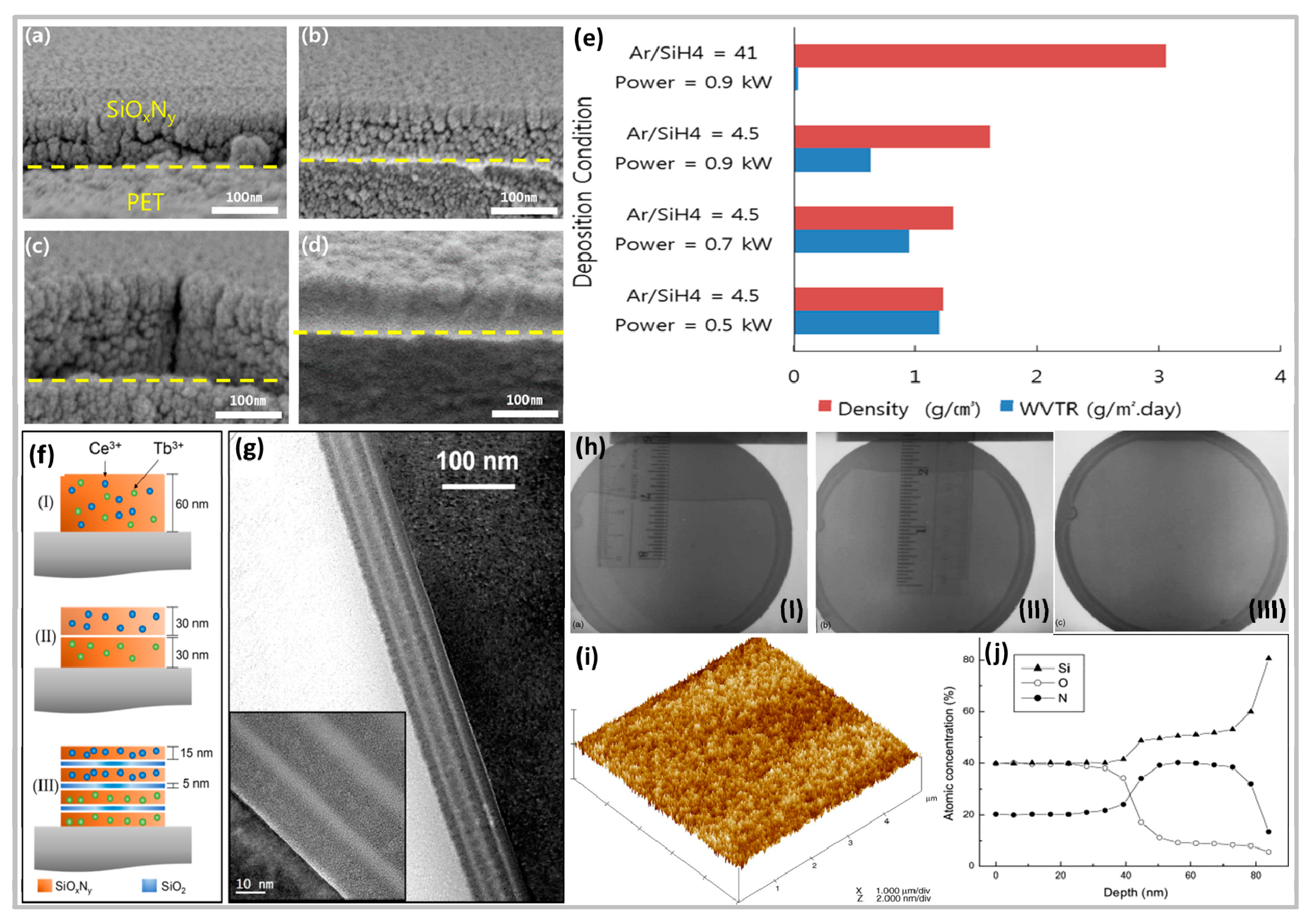
4.1. Application of Barrier Material
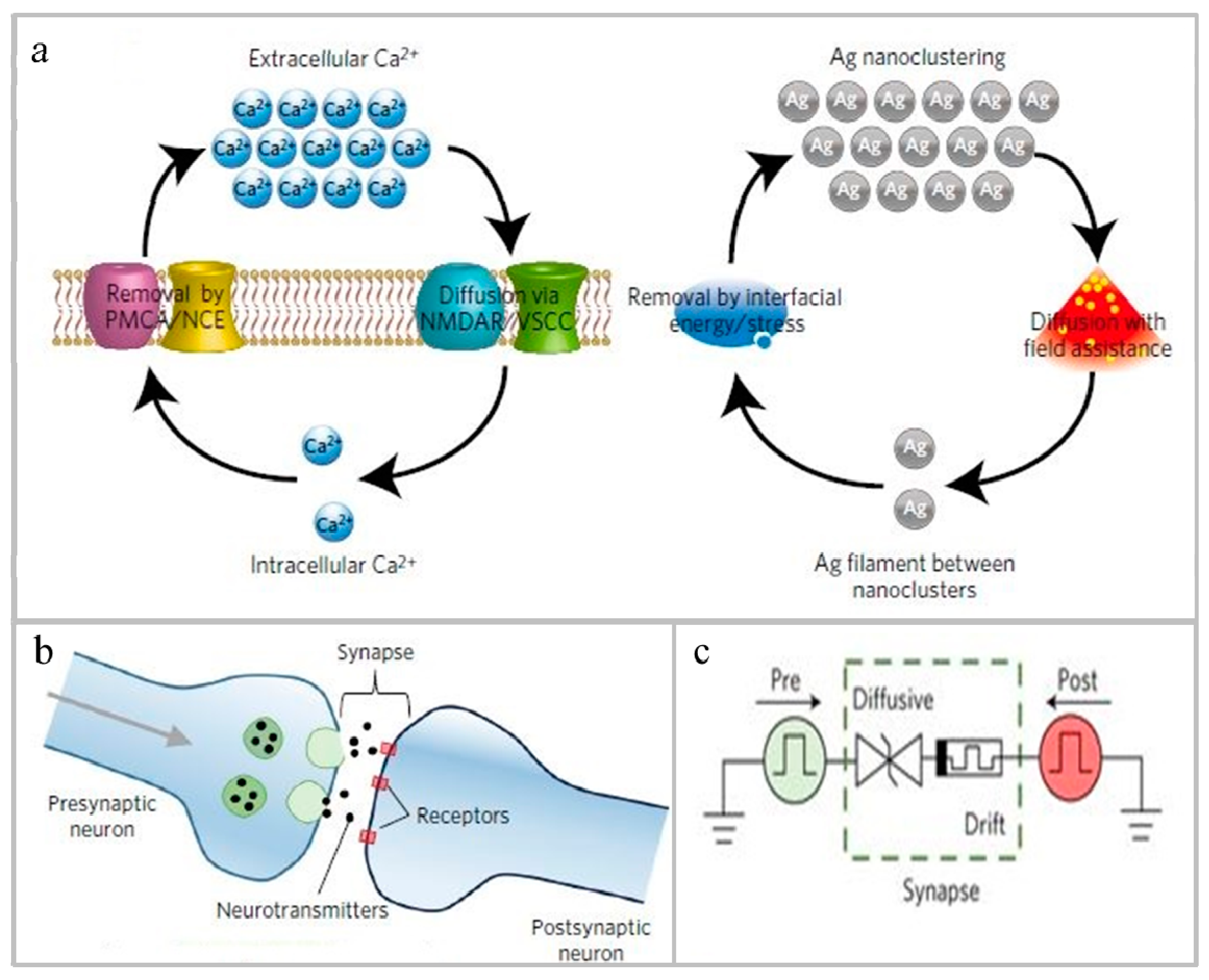
4.2. Application of Non-Volatile Semiconducting Memory
4.3. Application of Optical Devices
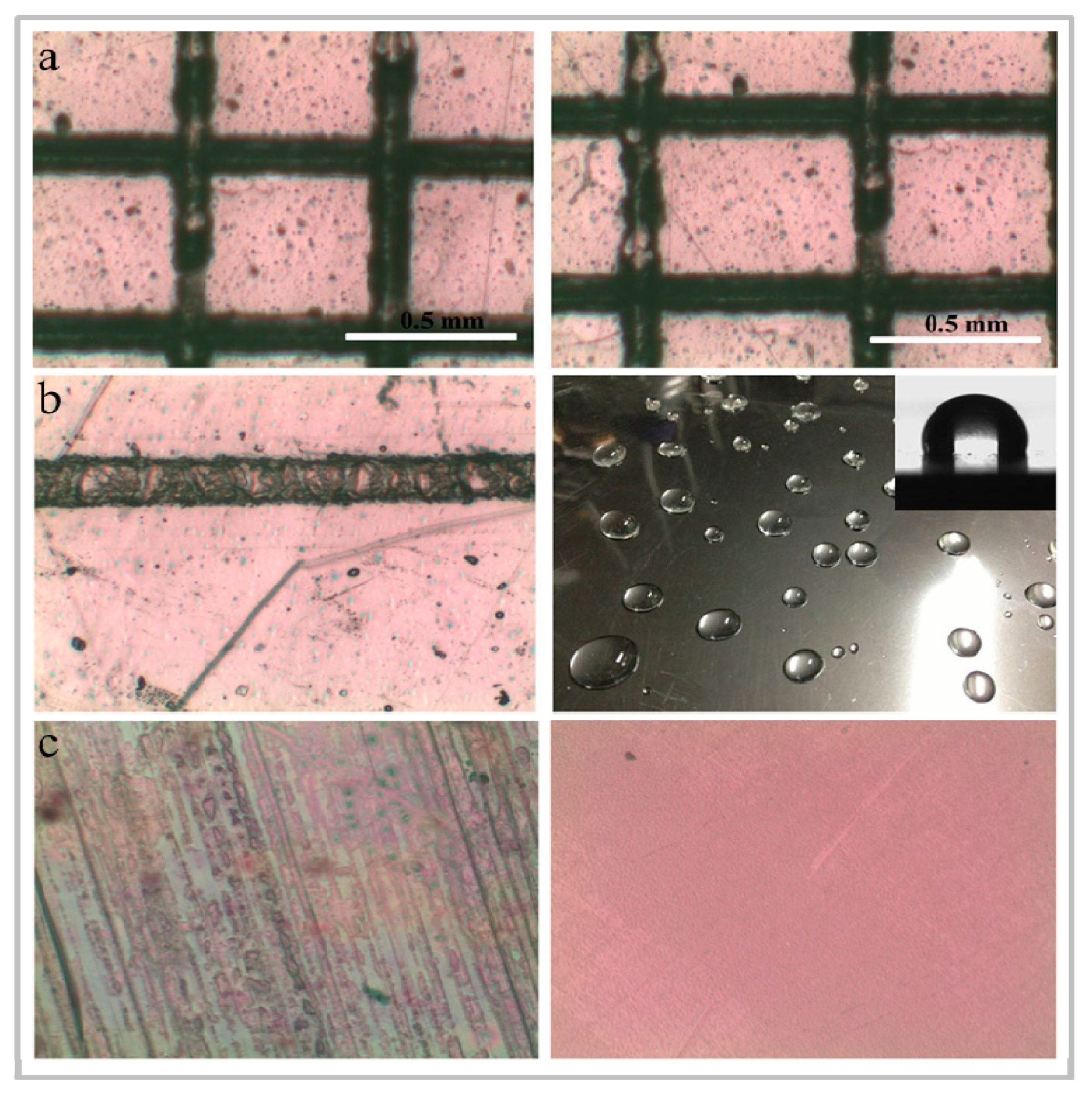
4.4. Application of Anti-Scratch Coating
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iwase, Y.; Horie, Y.; Daiko, Y.; Honda, S.; Iwamoto, Y. Synthesis of a Novel Polyethoxysilsesquiazane and Thermal Conversion into Ternary Silicon Oxynitride Ceramics with Enhanced Thermal Stability. Materials 2017, 10, 1391. [Google Scholar] [CrossRef] [PubMed]
- Meziani, S.; Moussi, A.; Mahiou, L.; Outemzabet, R. Compositional analysis of silicon oxide/silicon nitride thin films. Mater. Sci. 2016, 34, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Larker, R. Reaction Sintering and Properties of Silicon Oxynitride Densified by Hot Isostatic Pressing. J. Am. Ceram. Soc. 1992, 75, 62–66. [Google Scholar] [CrossRef]
- Ohashi, M.; Tabata, H.; Kanzaki, S. High-temperature flexural strength of hot-pressed silicon oxynitride ceramics. J. Mater. Sci. Lett. 1988, 7, 339–340. [Google Scholar] [CrossRef]
- Li, S.Q.; Pei, Y.C.; Yu, C.Q.; Li, J.L. Mechanical and Dielectric Properties of Porous Si2N2O-Si3N4 in Situ Composites. Ceram. Int. 2009, 35, 1851–1854. [Google Scholar]
- Ohashi, M.; Kanzaki, S.; Tabata, H. Processing, Mechanical Properties, and Oxidation Behavior of Silicon Oxynitride Ceramics. J. Am. Ceram. Soc. 1991, 74, 109–114. [Google Scholar] [CrossRef]
- Ohashi, M.; Kanzaki, S.; Tabata, H. Effect of additives on some properties of silicon oxynitride ceramics. J. Mater. Sci. 1991, 26, 2608–2614. [Google Scholar] [CrossRef]
- Rocabois, P.; Chatillon, C.; Bernard, C. Thermodynamics of the Si-O-N System: II, Stability of Si2N2O(s) by High-Temperature Mass Spectrometric Vaporization. J. Am. Ceram. Soc. 1996, 79, 1361–1365. [Google Scholar] [CrossRef]
- Ikeda, K.; Saperstein, R.E.; Alic, N.; Fainman, Y. Thermal and Kerr nonlinear properties of plasma-deposited silicon nitride/silicon dioxide waveguides. Opt. Express 2008, 16, 12987–12994. [Google Scholar] [CrossRef]
- Feng, Y.; Gong, H.; Zhang, Y.; Wang, X.; Che, S.; Zhao, Y.; Guo, X. Effect of BN content on the mechanical and dielectric properties of porous BNp/Si3N4 ceramics. Ceram. Int. 2016, 42, 661–665. [Google Scholar] [CrossRef]
- Lee, S.J.; Baek, S. Effect of SiO2 content on the microstructure, mechanical and dielectric properties of Si3N4 ceramics. Ceram. Int. 2016, 42, 9921–9925. [Google Scholar] [CrossRef]
- Philipp, H.; Andersen, K.; Svendsen, W.E.; Ou, H. Amorphous silicon rich silicon nitride optical waveguides for high density integrated optics. Electron. Lett. 2004, 40, 419–421. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Chen, Y.; Geng, Y.; Hong, X.; Li, X.; Cheng, Z.; Zhenzhou, C. Saturable absorption in graphene-on-waveguide devices. Appl. Phys. Express 2019, 12, 032003. [Google Scholar] [CrossRef]
- Wang, J.Q.; Cheng, Z.; Chen, Z.; Xu, J.-B.; Tsang, H.K.; Shu, C. Graphene photodetector integrated on silicon nitride waveguide. J. Appl. Phys. 2015, 117, 144504. [Google Scholar] [CrossRef]
- Levy, J.S.; Saha, K.; Okawachi, Y.; Foster, M.A.; Gaeta, A.L.; Lipson, M. High-Performance Silicon-Nitride-Based Multiple-Wavelength Source. IEEE Photonics Technol. Lett. 2012, 24, 1375–1377. [Google Scholar] [CrossRef] [Green Version]
- Gruhler, N.; Benz, C.; Jang, H.; Ahn, J.-H.; Danneau, R.; Pernice, W.H.P. High-quality Si3N4 circuits as a platform for graphene-based nanophotonic devices. Opt. Express 2013, 21, 31678–31689. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.; Chandra, S. Silicon dioxide films by RF sputtering for microelectronic and MEMS applications. J. Micromech. Microeng. 2007, 17, 1066–1077. [Google Scholar] [CrossRef]
- Ho, S.-S.; Rajgopal, S.; Mehregany, M. Thick PECVD silicon dioxide films for MEMS devices. Sens. Actuators A Phys. 2016, 240, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, Y.; Xue, C.; Zhang, C.; Chen, Y. Filtering effect of SiO2 optical waveguide ring resonator applied to optoelectronic oscillator. Opt. Express 2018, 26, 12638–12647. [Google Scholar] [CrossRef]
- Brown, D.M.; Gray, P.V.; Heumann, F.K.; Philipp, H.R.; Taft, E.A. Properties of SixOyNz Films on Si. J. Electrochem. Soc. 1968, 115, 311–317. [Google Scholar] [CrossRef]
- Gunning, W.J.; Hall, R.L.; Woodberry, F.J.; Southwell, W.H.; Gluck, N.S. Codeposition of continuous composition rugate filters. Appl. Opt. 1989, 28, 2945–2948. [Google Scholar] [CrossRef] [PubMed]
- Lipiński, M.; Kluska, S.; Czternastek, H.; Zięba, P. Graded SiOxNy layers as antireflection coatings for solar cells application. In Proceedings of the 8th International Conference on Intermolecular and Magnetic Interaction in Matter, Nałeczów, Poland, 8–10 September 2005; pp. 1009–1016. [Google Scholar]
- Hayafuji, Y.; Kajiwara, K. Nitridation of Silicon and Oxidized-Silicon. J. Electrochem. Soc. 1982, 129, 2102–2108. [Google Scholar] [CrossRef]
- Sombrio, G.; Franzen, P.L.; Maltez, R.; Matos, L.G.; Pereira, M.B.; Boudinov, H. Photoluminescence from SiNxOy films deposited by reactive sputtering. J. Phys. D Appl. Phys. 2013, 46, 235106. [Google Scholar] [CrossRef]
- Temple-Boyer, P.; Hajji, B.; Alay, J.; Morante, J.; Martinez, A. Properties of SiOxNy films deposited by LPCVD from SiH4/N2O/NH3 gaseous mixture. Sens. Actuators A Phys. 1999, 74, 52–55. [Google Scholar] [CrossRef]
- Augustine, B.H.; Hu, Y.Z.; Irene, E.A.; McNeil, L.E. An annealing study of luminescent amorphous silicon-rich silicon oxynitride thin films. Appl. Phys. Lett. 1995, 67, 3694–3696. [Google Scholar] [CrossRef]
- Dong, J.; Du, P.; Zhang, X. Characterization of the Young’s modulus and residual stresses for a sputtered silicon oxynitride film using micro-structures. Thin Solid Films 2013, 545, 414–418. [Google Scholar] [CrossRef]
- Lim, J.W.; Jin, C.K.; Lim, K.Y.; Lee, Y.J.; Kim, S.-R.; Choi, B.-I.; Kim, T.W.; Kim, D.H.; Hwang, D.K.; Choi, W.K. Transparent high-performance SiOxNy/SiOx barrier films for organic photovoltaic cells with high durability. Nano Energy 2017, 33, 12–20. [Google Scholar] [CrossRef]
- Trinh, T.T.; Jang, K.; Nguyen, V.D.; Dao, V.A.; Yi, J. Role of SiOxNy surface passivation layer on stability improvement and kink effect reduction of ELA poly silicon thin film transistors. Microelectron. Eng. 2016, 164, 14–19. [Google Scholar] [CrossRef]
- Park, J.-H.; Shin, M.-H.; Yi, J.-S. The Characteristics of Transparent Non-Volatile Memory Devices Employing Si-Rich SiOX as a Charge Trapping Layer and Indium-Tin-Zinc-Oxide. Nanomaterials 2019, 9, 784. [Google Scholar] [CrossRef]
- Soman, A.; Antony, A. Broad range refractive index engineering of SixNy and SiOxNy thin films and exploring their potential applications in crystalline silicon solar cells. Mater. Chem. Phys. 2017, 197, 181–191. [Google Scholar] [CrossRef]
- Himmler, A.; Fahland, M.; Linß, V. Roll-to-roll deposition of silicon oxynitride layers on polymer films using a rotatable dual magnetron system. Surf. Coat. Technol. 2018, 336, 123–127. [Google Scholar] [CrossRef]
- Rats, D.; Martinu, L.; Von Stebut, J. Mechanical properties of plasma-deposited SiOxNy coatings on polymer substrates using low load carrying capacity techniques. Surf. Coat. Technol. 2000, 123, 36–43. [Google Scholar] [CrossRef]
- Wang, J.; Bouchard, J.P.; Hart, G.A.; Oudard, J.F.; Paulson, C.A.; Sachenik, P.A.; Price, J.J. Silicon oxynitride based scratch resistant anti-reflective coatings. In Proceedings of the Advanced Optics for Defense Applications: UV through LWIR III (2018), Orlando, FL, USA, 15–16 April 2018; Volume 10627. [Google Scholar]
- Vivaldo, I.; Moreno, M.; Torres, A.; Ambrosio, R.; Rosales, P.; Carlos, N.; Calleja, W.; Monfil, K.; Benítez, A. A comparative study of amorphous silicon carbide and silicon rich oxide for light emission applications. J. Lumin. 2017, 190, 215–220. [Google Scholar] [CrossRef]
- Shcherban, N.D. Review on synthesis, structure, physical and chemical properties and functional characteristics of porous silicon carbide. J. Ind. Eng. Chem. 2017, 50, 15–28. [Google Scholar] [CrossRef]
- Chang, I.M.; Pan, S.C.; Chen, Y.F. Light-induced degradation on porous silicon. Phys. Rev. B 1993, 48, 8747–8750. [Google Scholar] [CrossRef] [PubMed]
- Dudel, F.P.; Gole, J.L. Stabilization of the photoluminescence from porous silicon: The competition between photoluminescence and dissolution. J. Appl. Phys. 1997, 82, 402–406. [Google Scholar] [CrossRef]
- Qin, G.; Jia, Y. Mechanism of the visible luminescence in porous silicon. Solid State Commun. 1993, 86, 559–563. [Google Scholar] [CrossRef]
- Qin, G.G.; Li, Y.J. Photoluminescence mechanism model for oxidized porous silicon and nanoscale-silicon-particle-embedded silicon oxide. Phys. Rev. B 2003, 68, 085309. [Google Scholar] [CrossRef]
- Torchynska, T.; Cano, A.D.; Rodríguez, M.M.; Khomenkova, L.; Khomenkova, L. Hot carriers and excitation of Si/SiOx interface defect photoluminescence in Si nanocrystallites. Phys. B Condens. Matter 2003, 340, 1113–1118. [Google Scholar] [CrossRef]
- Wolkin, M.V.; Jorne, J.; Fauchet, P.M.; Allan, G.; Delerue, C. Electronic States and Luminescence in Porous Silicon Quantum Dots: The Role of Oxygen. Phys. Rev. Lett. 1999, 82, 197–200. [Google Scholar] [CrossRef]
- Prokes, S.M.; Glembocki, O.J. Role of interfacial oxide-related defects in the red-light emission in porous silicon. Phys. Rev. B 1994, 49, 2238–2241. [Google Scholar] [CrossRef] [PubMed]
- Godinho, V.; De Haro, M.J.; García-López, J.; Goossens, V.; Terryn, H.; Delplancke-Ogletree, M.; Fernández, A.; De Haro, M.C.J. SiOxNy thin films with variable refraction index: Microstructural, chemical and mechanical properties. Appl. Surf. Sci. 2010, 256, 4548–4553. [Google Scholar] [CrossRef]
- Godinho, V.; Rojas, T.C.; Fernández, A. Magnetron sputtered a-SiOxNy thin films: A closed porous nanostructure with controlled optical and mechanical properties. Microporous Mesoporous Mater. 2012, 149, 142–146. [Google Scholar] [CrossRef]
- Jou, S.; Liaw, I.-C.; Cheng, Y.-C.; Li, C.-H. Light emission of silicon oxynitride films prepared by reactive sputtering of silicon. J. Lumin. 2013, 134, 853–857. [Google Scholar] [CrossRef]
- Jan, V.; Michael, G.; Sebastian, G.; Daniel, H.; Margit, Z. Photoluminescence performance limits of Si nanocrystals in silicon oxynitride matrices. J. Appl. Phys. 2017, 122, 144303. [Google Scholar]
- Goncharova, L.V.; Nguyen, P.H.; Karner, V.L.; D’Ortenzio, R.; Chaudhary, S.; Mokry, C.R.; Simpson, P.J. Si quantum dots in silicon nitride: Quantum confinement and defects. J. Appl. Phys. 2015, 118, 224302. [Google Scholar] [CrossRef]
- Augustine, B.H.; Irene, E.A. Visible light emission from thin films containing Si, O, N, and H. J. Appl. Phys. 1995, 78, 4020–4030. [Google Scholar] [CrossRef]
- Claassen, W.A.P.; Pol, H.A.J.T.; Goemans, A.H.; Kuiper, A.E.T. Characterization of Silicon-Oxynitride Films Deposited by Plasma-Enhanced CVD. J. Electrochem. Soc. 1986, 133, 1458–1464. [Google Scholar] [CrossRef]
- Alayo, M.; Pereyra, I.; Scopel, W.; Fantini, M.; Scopel, W.; Fantini, M. On the nitrogen and oxygen incorporation in plasma-enhanced chemical vapor deposition (PECVD) SiOxNy films. Thin Solid Films 2002, 402, 154–161. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Wu, Y.; Wang, S.; Ge, X. High photoluminescence quantum yields generated from N-Si-O bonding states in amorphous silicon oxynitride films. Opt. Express 2018, 26, 31617–31625. [Google Scholar] [CrossRef]
- Lee, D.K.; Shin, H.J.; Sohn, S.H. Characteristics of Silicon Oxynitride Barrier Films Grown on Poly(ethylene naphthalate) by Ion-Beam-Assisted Deposition. Jpn. J. Appl. Phys. 2010, 49, 05EA14. [Google Scholar] [CrossRef]
- Labbé, C.; An, Y.T.; Zatryb, G.; Portier, X.; Podhorodecki, A.; Marie, P.; Frilay, C.; Cardin, J.; Gourbilleau, F. Structural and emission properties of Tb3+-doped nitrogen-rich silicon oxynitride films. Nanotechnology 2017, 28, 115710. [Google Scholar] [CrossRef] [PubMed]
- Ehré, F.; Labbé, C.; Dufour, C.; Jadwisienczak, W.M.; Weimmerskirch-Aubatin, J.; Portier, X.; Doualan, J.L.; Cardin, J.; Richard, A.L.; Ingram, D.C.; et al. The Nitrogen concentration effect on Ce doped SiOxNy emission: Towards optimized Ce3+ for DEL applications. Nanoscale 2018, 10, 3823–3837. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Lin, Z.; Guo, Y.; Song, C.; Wang, X.; Lin, H.; Xu, L.; Song, J.; Li, H. Bright red, orange-yellow and white switching photoluminescence from silicon oxynitride films with fast decay dynamics. Opt. Mater. Express 2014, 4, 205–212. [Google Scholar] [CrossRef]
- Steveler, E.; Rinnert, H.; Vergnat, M. Photoluminescence of erbium in SiOxNy alloys annealed at high temperature. J. Alloy. Compd. 2014, 593, 56–60. [Google Scholar] [CrossRef]
- Kim, D.S.; Yoon, S.G.; Jang, G.E.; Suh, S.J.; Kim, H.; Yoon, D.H. Refractive index properties of SiN thin films and fabrication of SiN optical waveguide. J. Electroceram. 2006, 17, 315–318. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, P.F.; Shen, W.D.; Zou, T. Study of Silicon Oxynitride Film Deposited by RF Magnetron Sputtering. Acta Opt. Sin. 2005, 25, 567. [Google Scholar]
- Hu, J.L.; Hang, L.X.; Zhou, S. Preparation of Low Refractive Index Optical Thin Film by PECVD Technology. Surf. Technol. 2013, 42, 95–97. [Google Scholar]
- Xue, J.; Hang, L.X.; Lin, H.X. Study on controlled refractive index of optical thin films by PECVD. Opt. Tech. 2014, 40, 353–356. [Google Scholar]
- Hänninen, T.; Schmidt, S.; Jensen, J.; Hultman, L.; Högberg, H. Silicon oxynitride films deposited by reactive high power impulse magnetron sputtering using nitrous oxide as a single-source precursor. J. Vac. Sci. Technol. A 2015, 33, 05E121. [Google Scholar] [CrossRef] [Green Version]
- Farhaoui, A.; Bousquet, A.; Smaali, R.; Moreau, A.; Centeno, E.; Cellier, J.; Bernard, C.; Rapegno, R.; Réveret, F.; Tomasella, E. Reactive gas pulsing sputtering process, a promising technique to elaborate silicon oxynitride multilayer nanometric antireflective coatings. J. Phys. D Appl. Phys. 2017, 50, 015306. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Kato, K.; Omoto, H.; Tomioka, T.; Takamatsu, A. Stable deposition of silicon oxynitride thin films with intermediate refractive indices by reactive sputtering. Thin Solid Films 2012, 520, 3862–3864. [Google Scholar] [CrossRef]
- Yadav, A.; Polji, R.H.; Singh, V.; Dubey, S.; Rao, T.G. Synthesis of buried silicon oxynitride layers by ion implantation for silicon-on-insulator (SOI) structures. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2006, 245, 475–479. [Google Scholar] [CrossRef]
- Jasinski, J.M.; Meyerson, B.S.; Scott, B.A. Mechanistic Studies of Chemical Vapor Deposition. Annu. Rev. Phys. Chem. 1987, 38, 109–140. [Google Scholar] [CrossRef]
- Ouvry, S. Random Aharonov–Bohm vortices and some exactly solvable families of integrals. J. Stat. Mech. Theory Exp. 2005, 2005, 09004. [Google Scholar] [CrossRef]
- Pandey, R.; Patil, L.; Bange, J.; Patil, D.; Mahajan, A.; Patil, D.; Gautam, D.; Mahajan, A. Growth and characterization of SiON thin films by using thermal-CVD machine. Opt. Mater. 2004, 25, 1–7. [Google Scholar] [CrossRef]
- Balaji, N.; Nguyen, H.T.T.; Park, C.; Ju, M.; Raja, J.; Chatterjee, S.; Jeyakumar, R.; Yi, J. Electrical and optical characterization of SiOxNy and SiO2 dielectric layers and rear surface passivation by using SiO2/SiOxNy stack layers with screen printed local Al-BSF for c-Si solar cells. Curr. Appl. Phys. 2018, 18, 107–113. [Google Scholar] [CrossRef]
- Piqueras, J.; Hernandez, M.J.; Garrido, J.; Martinez, J. Compositional and electrical properties of ECR-CVD silicon oxynitrides. Semicond. Sci. Technol. 1997, 12, 927–932. [Google Scholar]
- Ramírez, J.M.; Wójcik, J.; Berencén, Y.; Ruiz-Caridad, A.; Estrade, S.; Peirò, F.; Mascher, P.; Garrido, B. Amorphous sub-nanometre Tb-doped SiOxNy /SiO2 superlattices for optoelectronics. Nanotechnology 2015, 26, 85203. [Google Scholar] [CrossRef]
- Bang, S.-H.; Suk, J.-H.; Kim, K.-S.; Park, J.-H.; Hwang, N.-M. Effects of radio frequency power and gas ratio on barrier properties of SiOxNy films deposited by inductively coupled plasma chemical vapor deposition. Thin Solid Films 2019, 669, 108–113. [Google Scholar] [CrossRef]
- Chen, W.; Lee, A.; Deng, W.; Liu, K. The implementation of neural network for semiconductor PECVD process. Expert Syst. Appl. 2007, 32, 1148–1153. [Google Scholar] [CrossRef]
- Varanasi, V.G.; Ilyas, A.; Velten, M.F.; Shah, A.; Lanford, W.A.; Aswath, P.B. Role of Hydrogen and Nitrogen on the Surface Chemical Structure of Bioactive Amorphous Silicon Oxynitride Films. J. Phys. Chem. B 2017, 121, 8991–9005. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, H.; Kim, D.; Nam, J.; Yang, J.; Lee, D.; Min, B.K.; Kim, K.N.; Park, S.J.; Kim, S.; et al. Continuously deposited anti-reflection double layer of silicon nitride and silicon oxynitride for selective emitter solar cells by PECVD. Curr. Appl. Phys. 2017, 17, 517–521. [Google Scholar] [CrossRef]
- Kalisz, M.; Szymanska, M.; Dębowska, A.K.; Michalak, B.; Brzozowska, E.; Górska, S.; Smietana, M. Influence of biofunctionalization process on properties of silicon oxynitride substrate layer. Surf. Interface Anal. 2014, 46, 1086–1089. [Google Scholar] [CrossRef]
- Kijaszek, W.; Oleszkiewicz, W.; Zakrzewski, A.; Patela, S.; Tłaczała, M. Investigation of optical properties of silicon oxynitride films deposited by RF PECVD method. Mater. Sci. Pol. 2016, 34, 868–871. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, K.; Nishi, H.; Tsuchizawa, T.; Hiraki, T.; Ishikawa, Y.; Wada, K.; Yamamoto, T.; Yamada, K. Optical coupling between SiOxNy waveguide and Ge mesa structures for bulk-Si photonics platform. In Proceedings of the 12th International Conference on Group IV Photonics (GFP), Vancouver, BC, Canada, 26–28 August 2015; pp. 122–123. [Google Scholar]
- Wood, R. ECR-PECVD silicon oxynitride thin films for flat panel displays. In Proceedings of the Opto-Canada: SPIE Regional Meeting on Optoelectronics, Photonics, and Imaging, Ottawa, ON, Canada, 9–10 May 2002; Volume 103133N. [Google Scholar]
- Meng, X.S.; Yuan, J.; Ma, Q.S.; Ge, M.Z.; Yang, H. Study on the Preparation Methods of Silicon Oxynitride Thin Films. Mater. Sci. Eng. 1999, 17, 10–13. [Google Scholar]
- Kaghouche, B.; Mansour, F.; Molliet, C.; Rousset, B.; Temple-Boyer, P. Investigation on optical and physico-chemical properties of LPCVD SiOxNy thin films. Eur. Phys. J. Appl. Phys. 2014, 66, 20301. [Google Scholar] [CrossRef]
- Gan, Z.; Wang, C.; Chen, Z. Material Structure and Mechanical Properties of Silicon Nitride and Silicon Oxynitride Thin Films Deposited by Plasma Enhanced Chemical Vapor Deposition. Surfaces 2018, 1, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Pernas, P.; Ruiz, E.; Garrido, J.; Piqueras, J.; Pászti, F.; Climent-Font, A.; Lifante, G.; Cantelar, E. Silicon Oxynitride ECR-PECVD Films for Integrated Optics. Mater. Sci. Forum 2005, 480, 149–154. [Google Scholar] [CrossRef]
- Rangarajan, B.; Kovalgin, A.Y.; Schmitz, J. Deposition and properties of silicon oxynitride films with low propagation losses by inductively coupled PECVD at 150 °C. Surf. Coat. Technol. 2013, 230, 46–50. [Google Scholar] [CrossRef]
- Halova, E.; Alexandrova, S.; Szekeres, A.; Modreanu, M. LPCVD-silicon oxynitride films: Interface properties. Microelectron. Reliab. 2005, 45, 982–985. [Google Scholar] [CrossRef]
- Faller, F.; Hurrle, A. High-temperature CVD for crystalline-silicon thin-film solar cells. IEEE Trans. Electron Devices 1999, 46, 2048–2054. [Google Scholar] [CrossRef]
- Lebland, F.; Licoppe, C.; Gao, Y.; Nissim, Y.; Rigo, S. Rapid thermal chemical vapour deposition of SiOxNy films. Appl. Surf. Sci. 1992, 54, 125–129. [Google Scholar] [CrossRef]
- Watanabe, J.; Hanabusa, M. Photochemical vapor deposition of silicon oxynitride films by deuterium lamp. J. Mater. Res. 1989, 4, 882–885. [Google Scholar] [CrossRef]
- Bergonzo, P.; Kogelschatz, U.; Boyd, I.W. Photo-CVD of dielectric materials by pseudo-continuous excimer sources. In Proceedings of the Laser-Assisted Fabrication of Thin Films and Microstructures, Quebec City, QC, Canada, 16–20 August 1993; pp. 174–181. [Google Scholar]
- Zhang, Z.B.; Luo, Y.M.; Xu, C.H. Research Progress on Silicon Oxynitride Films. Mater. Rev. 2009, 11, 110–114. [Google Scholar]
- Hallam, B.; Tjahjono, B.; Wenham, S. Effect of PECVD silicon oxynitride film composition on the surface passivation of silicon wafers. Sol. Energy Mater. Sol. Cells 2012, 96, 173–179. [Google Scholar] [CrossRef]
- Li, X.B.; Zhang, F.H.; Mou, Q. Effect of Plasma Enhanced Chemical Vapor Deposition Parameters on Characteristics of Silicon nitride Film. Mater. Prot. 2006, 39, 12–16. [Google Scholar]
- Mubarak, A.; Hamzah, E.; Toff, M.R.M. Review of physical vapour deposition (PVD) techniques for hard coating. J. Mek. 2005, 20, 42–51. [Google Scholar]
- Mattox, D.M. Ion plating—Past, present and future. Surf. Coat. Technol. 2000, 133, 517–521. [Google Scholar] [CrossRef]
- Nakao, H.; Hori, K.; Iwazaki, Y.; Ueno, T. Investigation of High Current Tetracene-TFT Using Surface Nitrided SiO2 Gate Insulator Film. ECS Trans. 2016, 75, 81–85. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering Physical Vapour Deposition (PVD) Coatings: A Critical Review on Process Improvement and Market Trend Demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Mattox, D.M. The Foundations of Vacuum Coating Technology; Noyes Publications: Norwich, UK, 2003; ISBN 978-0-8155-1495-4. [Google Scholar]
- Yu, D.H.; Wang, C.Y.; Cheng, X.L.; Song, Y.X. Recent development of magnetron sputtering processes. Vacuum 2009, 46, 19–25. [Google Scholar]
- Tang, C.J.; Jiang, C.C.; Tien, C.L.; Sun, W.C.; Lin, S.C. Optical, structural, and mechanical properties of silicon oxynitride films prepared by pulsed magnetron sputtering. Appl. Opt. 2017, 56, C168–C174. [Google Scholar] [CrossRef] [PubMed]
- Šimurka, L.; Čtvrtlík, R.; Roch, T.; Turutoglu, T.; Erkan, S.; Tomáštík, J.; Bange, K. Effect of deposition conditions on physical properties of sputtered silicon oxynitride thin films on float glass. Int. J. Appl. Glas. Sci. 2018, 9, 403–412. [Google Scholar] [CrossRef]
- Li, J.; Shen, G.; Chen, W.; Li, Z.; Hong, R. Preparation of SiNx multilayer films by mid-frequency magnetron sputtering for crystalline silicon solar cells. Mater. Sci. Semicond. Process. 2017, 59, 40–44. [Google Scholar] [CrossRef]
- Arnell, R.; Kelly, P. Recent advances in magnetron sputtering. Surf. Coat. Technol. 1999, 112, 170–176. [Google Scholar] [CrossRef]
- Sangines, R.; Abundiz-Cisneros, N.; Diliegros-Godines, C.; Machorro-Mejia, R.; Hernández-Utrera, O. Plasma emission spectroscopy and its relation to the refractive index of silicon nitride thin films deposited by reactive magnetron sputtering. J. Phys. D Appl. Phys. 2018, 51, 095203. [Google Scholar] [CrossRef]
- Yount, J.; Lenahan, P. Bridging nitrogen dangling bond centers and electron trapping in amorphous NH3-nitrided and reoxidized nitrided oxide films. J. Non-Cryst. Solids 1993, 164, 1069–1072. [Google Scholar] [CrossRef]
- Hori, T.; Naito, Y.; Iwasaki, H.; Esaki, H. Interface states and fixed charges in nanometer-range thin nitrided oxides prepared by rapid thermal annealing. IEEE Electron Device Lett. 1986, 7, 669–671. [Google Scholar] [CrossRef]
- Itakura, A.; Shimoda, M.; Kitajima, M. Surface stress relaxation in SiO2 films by plasma nitridation and nitrogen distribution in the film. Appl. Surf. Sci. 2003, 216, 41–45. [Google Scholar] [CrossRef]
- He, S.C.; Jia, Q.M.; Su, H.Y.; Ma, A.H.; Shan, S.Y.; Hu, T.D. Review on the Research Progress of Silicon Oxynitride Materials. Mater. Rev. 2016, 8, 80–84. [Google Scholar]
- Yamamoto, M.; Matsumae, T.; Kurashima, Y.; Takagi, H.; Suga, T.; Itoh, T.; Higurashi, E. Growth Behavior of Au Films on SiO2 Film and Direct Transfer for Smoothing Au Surfaces. Int. J. Autom. Technol. 2019, 13, 254–260. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, H.; Wang, Y.; Lv, R.; Yang, X.; Wang, J.; Li, L.; Ren, W. Improved optical damage threshold graphene Oxide/SiO2 absorber fabricated by sol-gel technique for mode-locked erbium-doped fiber lasers. Carbon 2019, 144, 737–744. [Google Scholar] [CrossRef]
- Lai, C.H.; Lin, B.C.; Chang, K.M.; Hsieh, K.Y.; Lai, Y.L. A novel process for forming an ultra-thin oxynitride film with high nitrogen topping. J. Phys. Chem. Solids 2008, 69, 456–460. [Google Scholar] [CrossRef]
- Wong, H.; Yang, B.; Cheng, Y. Chemistry of silicon oxide annealed in ammonia. Appl. Surf. Sci. 1993, 72, 49–54. [Google Scholar] [CrossRef]
- Rebib, F.; Tomasella, E.; Dubois, M.; Cellier, J.; Sauvage, T.; Jacquet, M. SiOxNy thin films deposited by reactive sputtering: Process study and structural characterisation. Thin Solid Films 2007, 515, 3480–3487. [Google Scholar] [CrossRef]
- Dasgupta, A.; Takoudis, C.G. Growth kinetics of thermal silicon oxynitridation in nitric oxide ambient. J. Appl. Phys. 2003, 93, 3615–3618. [Google Scholar] [CrossRef]
- Ramírez, J.M.; Ruiz-Caridad, A.; Wojcik, J.; Gutierrez, A.M.; Estradé, S.; Peiró, F.; Sanchís, P.; Mascher, P.; Garrido, B. Luminescence properties of Ce3+ and Tb3+ co-doped SiOxNy thin films: Prospects for color tunability in silicon-based hosts. J. Appl. Phys. 2016, 119, 113108. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, X.; Chen, P.; Liu, W.; Wong, M.; Lin, C.; Chu, P.K. Formation of silicon on plasma synthesized SiOxNy and reaction mechanism. Appl. Surf. Sci. 2005, 243, 89–95. [Google Scholar] [CrossRef]
- Lee, H.-I.; Park, J.-B.; Xianyu, W.; Kim, K.; Chung, J.G.; Kyoung, Y.K.; Byun, S.; Yang, W.Y.; Park, Y.Y.; Kim, S.M.; et al. Degradation by water vapor of hydrogenated amorphous silicon oxynitride films grown at low temperature. Sci. Rep. 2017, 7, 14146. [Google Scholar] [CrossRef]
- Dan, Y.P.; Yue, R.F.; Wang, Y.; Liu, L.T. Optical Properties of Silicon Oxynitride Films and Applications in Optical Waveguides. In Proceedings of the 1th National Conference on Nanotechnology and Applications (2008), Xiamen, China, 27–30 November 2000; pp. 98–101. [Google Scholar]
- Lin, Z.; Chen, K.; Zhang, P.; Xu, J.; Dong, H.; Li, W.; Ji, Y.; Huang, X. The Role of N-Si-O Defect States in Optical Gain from an a-SiNxOy/SiO2 Waveguide and in Light Emission from an n-a-SiNxOy/p-Si Heterojunction LED. Phys. Status Solidi (A) 2018, 215, 1700750. [Google Scholar] [CrossRef]
- Shahpanah, M.; Mehrabian, S.; Abbasi-Firouzjah, M.; Shokri, B. Improving the oxygen barrier properties of PET polymer by radio frequency plasma-polymerized SiOxNy thin film. Surf. Coat. Technol. 2019, 358, 91–97. [Google Scholar] [CrossRef]
- Madogni, V.I.; Kounouhéwa, B.; Akpo, A.; Agbomahéna, M.; Hounkpatin, S.A.; Awanou, C.N. Comparison of degradation mechanisms in organic photovoltaic devices upon exposure to a temperate and a subequatorial climate. Chem. Phys. Lett. 2015, 640, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Yang, Y.-Q.; Chen, Z.; Tao, Y.; Liu, Y.-F. Recent progress on thin-film encapsulation technologies for organic electronic devices. Opt. Commun. 2016, 362, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Oku, T.; Okumura, M.; Totsuka, M.; Shiga, T.; Takemi, M. Surface protection mechanism of insulating films at junctions in compound semiconductor devices. In Proceedings of the 15th International Workshop on Junction Technology (IWJT), Kyoto, Japan, 11–12 June 2015; pp. 71–76. [Google Scholar]
- Satoh, R.; Ro, T.; Heo, C.J.; Lee, G.H.; Xianyu, W.; Park, Y.; Park, J.; Lim, S.J.; Leem, D.S.; Bulliard, X.; et al. Bi-layered metal-oxide thin films processed at low-temperature for the encapsulation of highly stable organic photo-diode. Org. Electron. 2016, 41, 259–265. [Google Scholar] [CrossRef]
- Shim, J.; Yoon, H.G.; Na, S.-H.; Kim, I.; Kwak, S. Silicon oxynitride gas barrier coatings on poly(ether sulfone) by plasma-enhanced chemical vapor deposition. Surf. Coat. Technol. 2008, 202, 2844–2849. [Google Scholar] [CrossRef]
- Iwamori, S.; Gotoh, Y.; Moorthi, K. Characterization of silicon oxynitride gas barrier films. Vacuum 2002, 68, 113–117. [Google Scholar] [CrossRef]
- Liu, C.C.; Chang, L.S. Gas permeation properties of silicon oxynitride thin films deposited on polyether sulfone by radio frequency magnetron reactive sputtering in various N2 contents in atmosphere. Thin Solid Films 2015, 594, 35–39. [Google Scholar] [CrossRef]
- Wen, S.; Xie, X.; Yan, Z.; Huang, T.; Zeng, Z. General memristor with applications in multilayer neural networks. Neural Netw. 2018, 103, 142–149. [Google Scholar] [CrossRef]
- Wang, B.; Zou, F.; Cheng, J. A memristor-based chaotic system and its application in image encryption. Optik 2018, 154, 538–544. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Fardad, M.; Varshney, P.K. A Memristor-Based Optimization Framework for Artificial Intelligence Applications. IEEE Circuits Syst. Mag. 2018, 18, 29–44. [Google Scholar] [CrossRef]
- Wrazien, S.J.; Zhao, Y.; Krayer, J.D.; White, M.H. Characterization of SONOS oxynitride nonvolatile semiconductor memory devices. Solid State Electron. 2003, 47, 885–891. [Google Scholar] [CrossRef]
- Ielmini, D. Resistive switching memories based on metal oxides: Mechanisms, reliability and scaling. Semicond. Sci. Technol. 2016, 31, 063002. [Google Scholar] [CrossRef]
- Chua, L. Memristor-The missing circuit element. IEEE Trans. Circuit Theory 1971, 18, 507–519. [Google Scholar] [CrossRef]
- Chen, D.; Huang, S.; He, L. Effect of oxygen concentration on resistive switching behavior in silicon oxynitride film. J. Semicond. 2017, 38, 043002. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.; Zhang, X.; Sun, Y.; Liu, J.; Xu, L.; Li, W.; Chen, K.; Feng, D. The Ultra-Low Power Performance of a-SiNxOy:H Resistive Switching Memory. Phys. Status Solidi (A) 2018, 215, 1700753. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, S.; Savel’Ev, S.E.; Jiang, H.; Midya, R.; Lin, P.; Hu, M.; Ge, N.; Strachan, J.P.; Li, Z.; et al. Memristors with diffusive dynamics as synaptic emulators for neuromorphic computing. Nat. Mater. 2016, 16, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Baudzus, L.; Krummrich, P.M. Low Loss Electro-Optic Polymer Based Fast Adaptive Phase Shifters Realized in Silicon Nitride and Oxynitride Waveguide Technology. Photonics 2016, 3, 49. [Google Scholar] [CrossRef]
- Wang, J.Q.; Cheng, Z.; Shu, C.; Tsang, H.K. Optical Absorption in Graphene-on-Silicon Nitride Microring Resonator. IEEE Photon. Technol. Lett. 2015, 27, 1765–1767. [Google Scholar] [CrossRef]
- Jia, Y.; Dai, X.; Xiang, Y.; Fan, D. High quality factor silicon oxynitride optical waveguide ring resonators. Opt. Mater. 2018, 85, 138–142. [Google Scholar] [CrossRef]
- De Ridder, R.; Warhoff, K.; Driessen, A.; Lambeck, P.; Albers, H. Silicon oxynitride planar waveguiding structures for application in optical communication. IEEE J. Sel. Top. Quantum Electron. 1998, 4, 930–937. [Google Scholar] [CrossRef]
- Trenti, A.; Borghi, M.; Biasi, S.; Ghulinyan, M.; Ramiro-Manzano, F.; Pucker, G.; Pavesi, L. Thermo-optic coefficient and nonlinear refractive index of silicon oxynitride waveguides. AIP Adv. 2018, 8, 025311. [Google Scholar] [CrossRef]
- Loka, C.; Lee, K.; Moon, S.W.; Choi, Y.; Lee, K.-S. Enhanced transmittance of sapphire by silicon oxynitride thin films annealed at high temperatures. Mater. Lett. 2018, 213, 354–357. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Balaji, N.; Park, C.; Triet, N.M.; Le, A.H.T.; Lee, S.; Jeon, M.; Oh, D.; Dao, V.A.; Yi, J. Al2O3 /SiON stack layers for effective surface passivation and anti-reflection of high efficiency n-type c-Si solar cells. Semicond. Sci. Technol. 2017, 32, 25005. [Google Scholar] [CrossRef]
- Sahouane, N.; Necaibia, A.; Ziane, A.; Dabou, R.; Bouraiou, A.; Mostefaoui, M.; Rouabhia, A.; Mostefaoui, M. Realization and modeling of multilayer antireflection coatings for solar cells application. Mater. Res. Express 2018, 5, 065515. [Google Scholar] [CrossRef]
- Parashar, P.K.; Komarala, V.K. Engineered optical properties of silver-aluminum alloy nanoparticles embedded in SiON matrix for maximizing light confinement in plasmonic silicon solar cells. Sci. Rep. 2017, 7, 12520. [Google Scholar] [CrossRef]
- Qu, L.; Tang, L.; Bei, R.; Zhao, J.; Chi, Z.; Liu, S.; Chen, X.; Aldred, M.P.; Zhang, Y.; Xu, J. Flexible Multifunctional Aromatic Polyimide Film: Highly Efficient Photoluminescence, Resistive Switching Characteristic, and Electroluminescence. ACS Appl. Mater. Interfaces 2018, 10, 11430–11435. [Google Scholar] [CrossRef]
- Lam, J.-Y.; Shih, C.-C.; Lee, W.-Y.; Chueh, C.-C.; Jang, G.-W.; Huang, C.-J.; Tung, S.-H.; Chen, W.-C. Bio-Based Transparent Conductive Film Consisting of Polyethylene Furanoate and Silver Nanowires for Flexible Optoelectronic Devices. Macromol. Rapid Commun. 2018, 39, 1800271. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Liao, Y.-H.; Hu, C.-H. Effects of N2 addition on enhanced scratch resistance of flexible polycarbonate substrates by low temperature plasma-polymerized organo-silicon oxynitride. J. Non-Cryst. Solids 2009, 355, 182–192. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lai, Y.C.; Chen, J.H. Low temperature atmospheric pressure plasma-polymerized organosilicon oxynitride films enhance scratch resistance of flexible carbon fiber-reinforced polymer composites. In Proceedings of the International Symposium on Material Science and Engineering (ISMSE 2018), Seoul, South Korea, 19–21 January 2018; p. 020002. [Google Scholar]
- Zhang, Z.B.; Shao, Z.H.; Luo, Y.M.; An, P.Y.; Zhang, M.Y.; Xu, C.H. Hydrophobic, transparent and hard silicon oxynitride coating from perhydropolysilazane. Polym. Int. 2015, 64, 971–978. [Google Scholar] [CrossRef]



| Deposition Method | Precursor Gases | Ratio of Precursor Gases | Deposition Condition | Reference |
|---|---|---|---|---|
| PECVD | SiH4, N2O | SiH4/N2O = 0.05–0.125 | 200 °C, 97.09 Pa | [82] |
| RF-PECVD | SiH4, N2O, NH3 | (NH3 + SiH4)/N2O = 0.64–3.22 | 120 Pa | [76] |
| ECR-PECVD | N2, O2, SiH4 | O2/N2 = 0.03–0.1 | - | [83] |
| IC-PECVD | N2, Ar, SiH4 | N2/Ar = 0.0625–0.5 | 90–250 °C, 1–6 Pa | [84] |
| LPCVD | N2O, NH3 SiH2Cl2 | N2O/NH3 = 4.8- | 860 °C, 53.2 Pa | [85] |
| Method | Advantages | Disadvantages |
|---|---|---|
| PECVD | Flexible operation method, High process repeatability, High step coverage, Low deposition temperature (<400 °C) [92] | High cost, High H content in film |
| LPCVD | Uniform film, Complete structure, Less pinhole defects, High deposition speed, Large-area preparation [80] | Low heating rate, Long reaction time, High deposition temperature (generally >550 °C) |
| HTCVD | Simple operation and operation, High reaction rate, Low H content in film and dense structure [80] | High deformation, Impaired interface performance |
| Photo-CVD | Low reaction temperature (≤250 °C), Smooth film surface, Less by-products [89] | High cost, Low film stability |
| Method | Advantages | Disadvantages |
|---|---|---|
| CVD | High deposition rate Low deposition temperature Uniform film | Hydrogen content has an effect on electrical conductivity [111] |
| PVD | Low hydrogen content | Low deposition rate [112] Target poisoning is common |
| Oxynitridation | Relatively simple operation, large-scale preparation | Film thickness is difficult to control Toxicity of raw gas [110] Low N2 nitriding degree [113] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; He, L.; Guang, F.; Li, L.; Xin, Z.; Liu, R. A Review: Preparation, Performance, and Applications of Silicon Oxynitride Film. Micromachines 2019, 10, 552. https://doi.org/10.3390/mi10080552
Shi Y, He L, Guang F, Li L, Xin Z, Liu R. A Review: Preparation, Performance, and Applications of Silicon Oxynitride Film. Micromachines. 2019; 10(8):552. https://doi.org/10.3390/mi10080552
Chicago/Turabian StyleShi, Yue, Liang He, Fangcao Guang, Luhai Li, Zhiqing Xin, and Ruping Liu. 2019. "A Review: Preparation, Performance, and Applications of Silicon Oxynitride Film" Micromachines 10, no. 8: 552. https://doi.org/10.3390/mi10080552
APA StyleShi, Y., He, L., Guang, F., Li, L., Xin, Z., & Liu, R. (2019). A Review: Preparation, Performance, and Applications of Silicon Oxynitride Film. Micromachines, 10(8), 552. https://doi.org/10.3390/mi10080552





