DNA Microsystems for Biodiagnosis
Abstract
:1. Introduction
2. Current Challenges in Disease Detection and Treatment
2.1. Current Challenges in Alzheimer’s Disease
2.2. Current Challenges in Autoimmune Hepatitis (AIH)
2.3. Current Challenges in Disease-Causing Mutations
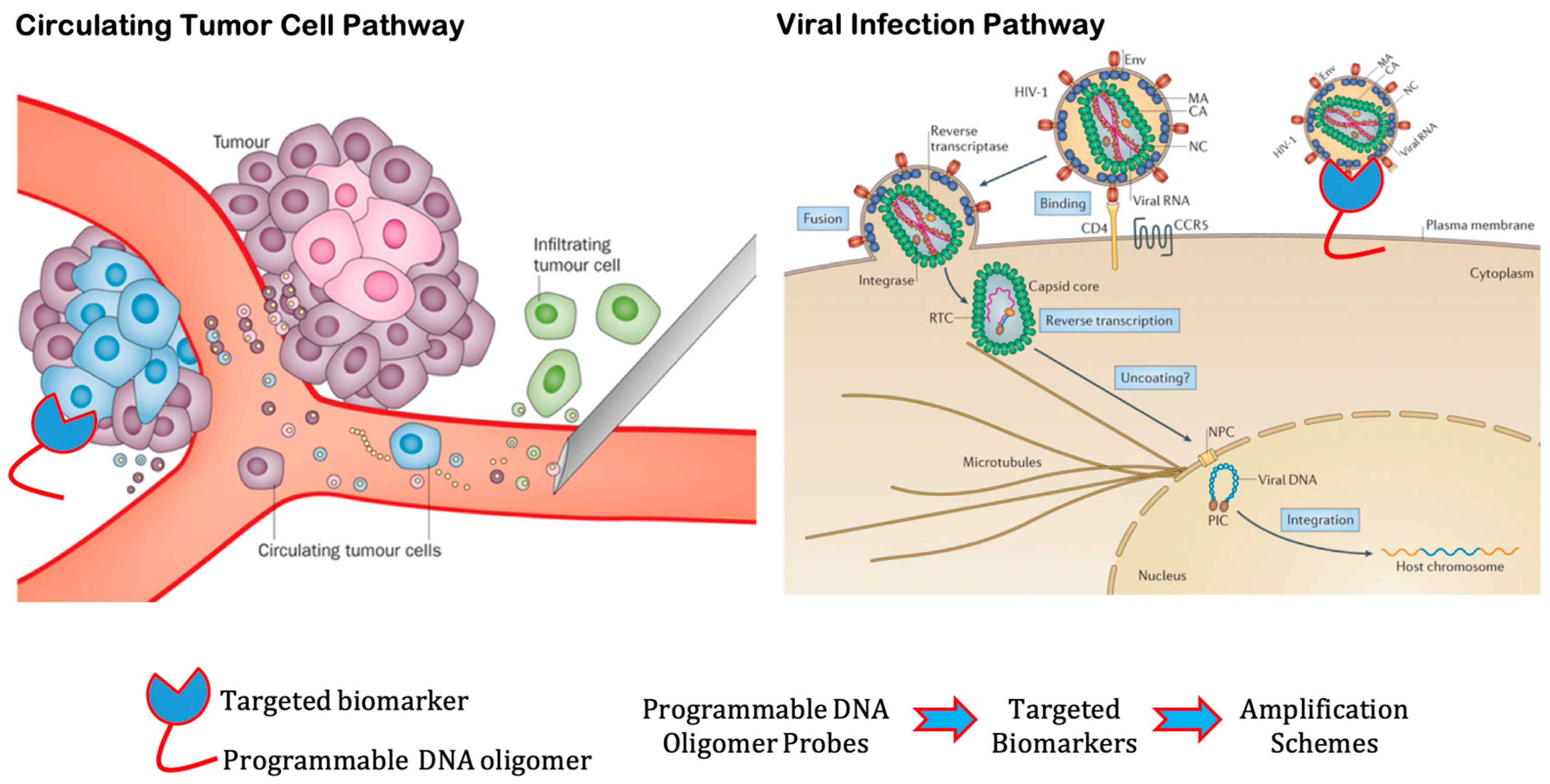
2.4. Current Challenges in Detection of Circulating Tumor Cells
2.5. Current Challenges in Viral Infections
3. Current Methods for Diagnosis
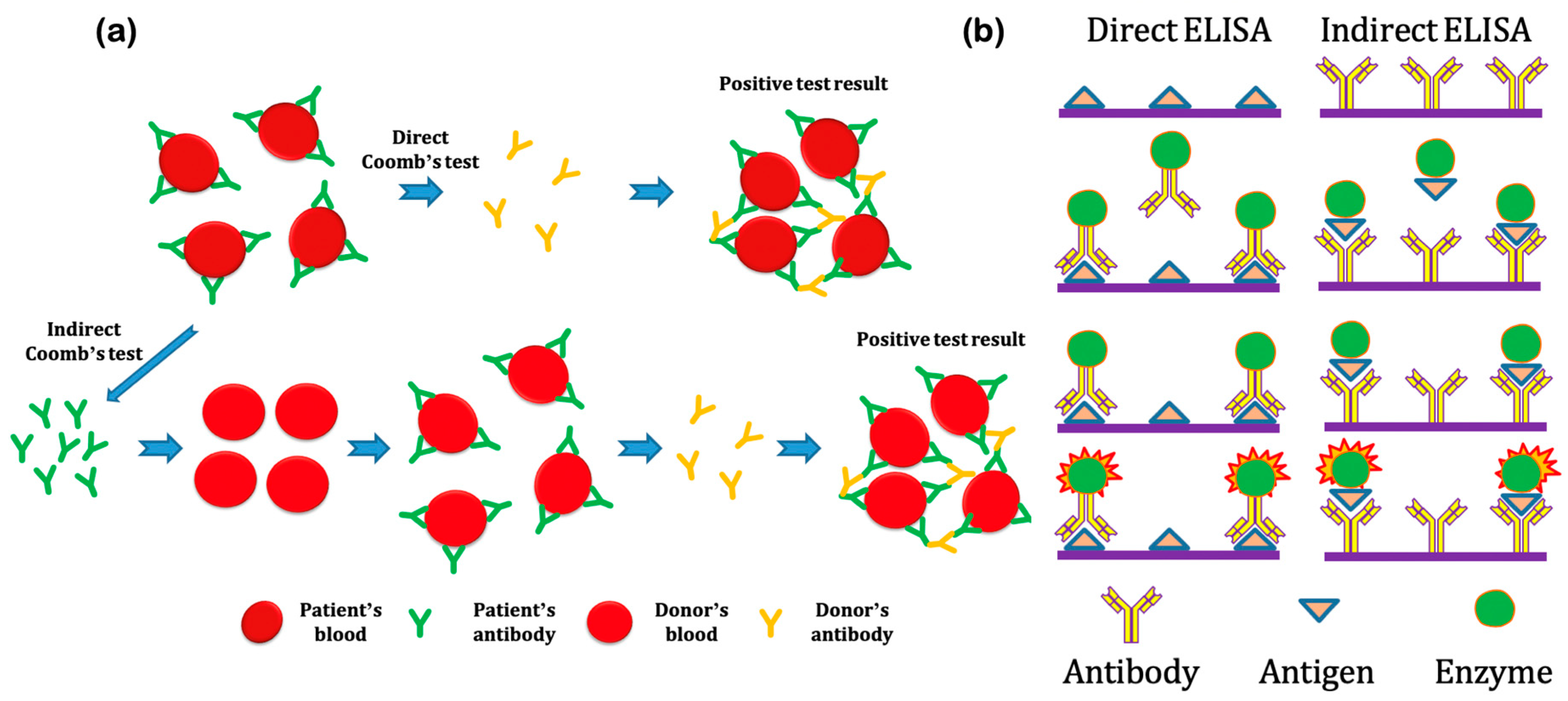
3.1. Microscopic Agglutination Test (MAT)
3.2. Enzyme-Linked Immunosorbent Assay (ELISA)
3.3. Polymerase Chain Reaction (PCR)
3.4. Multiplex Methods for Diagnosis
3.5. Loop-Mediated Isothermal Amplification (LAMP)
3.6. Next-Generation Sequencing (NGS)
4. Overview DNA Nanotechnology
4.1. Structural DNA Nanotechnology
4.1.1. Active and Passive DNA Nanostructures
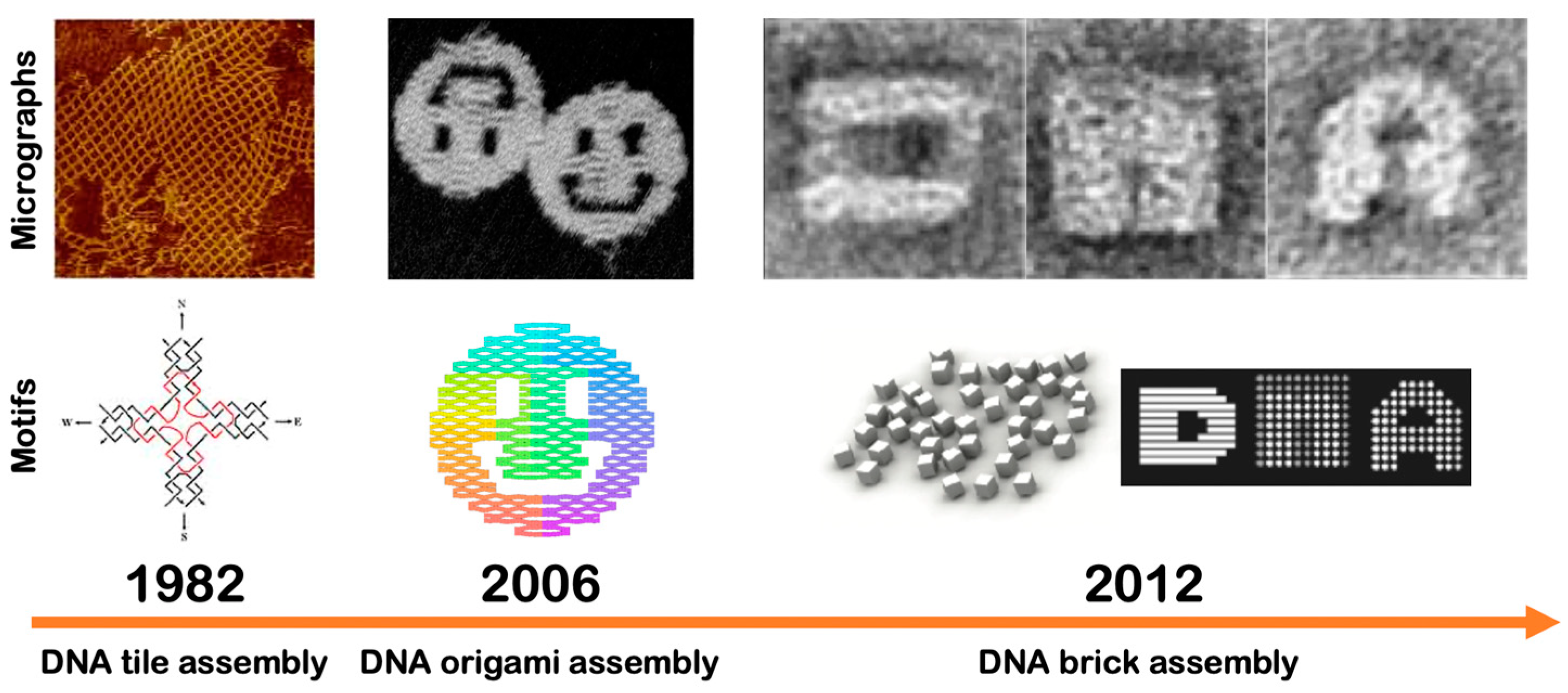
4.1.2. DNA Tiles
4.1.3. DNA Origamis
4.1.4. DNA Bricks
4.2. Dynamic DNA Systems
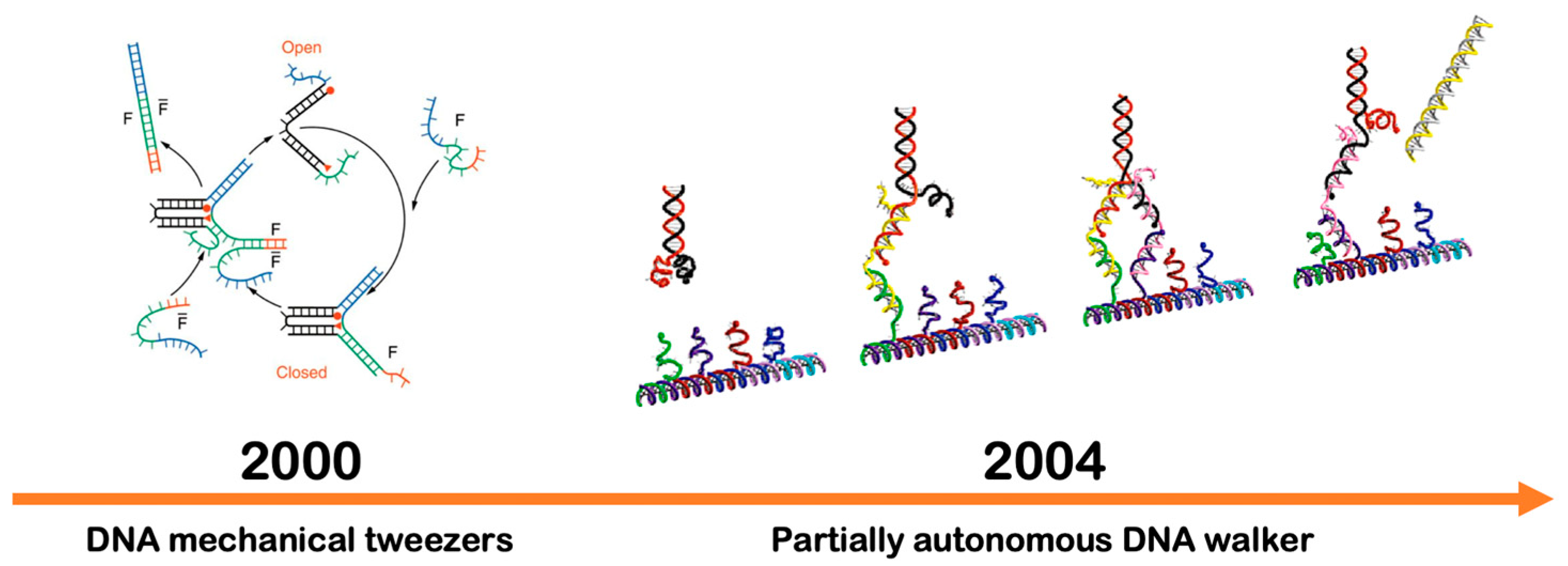
DNA Strand Displacement
4.3. Major Advantages and Limitations
4.4. Current DNA-Based Approaches Being Developed towards Diagnosis
5. DNA Biosensors
5.1. DNA Nanostructures for Biomarker Diagnosis
5.2. Hybrid Enzyme-DNA Biosensors
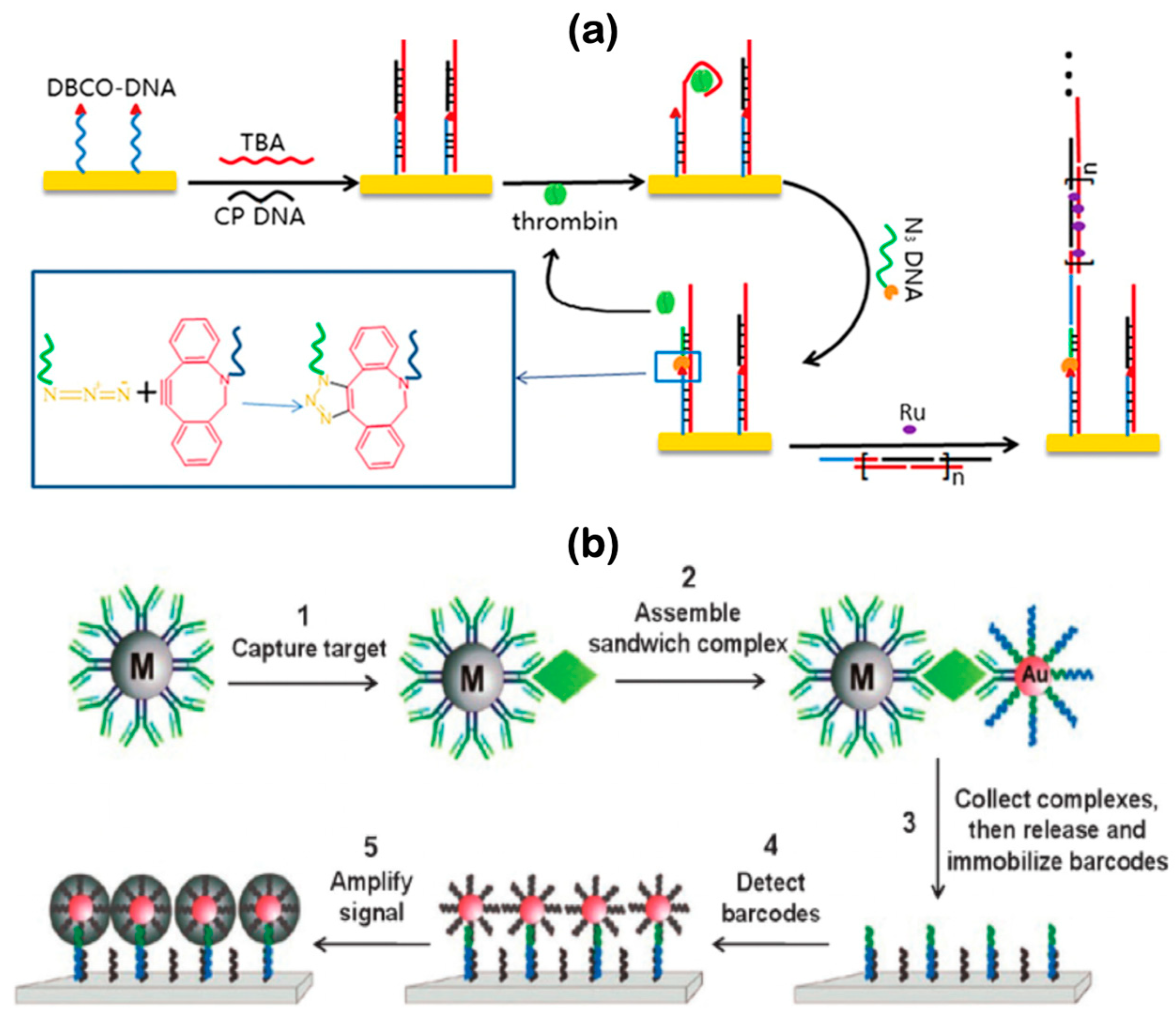
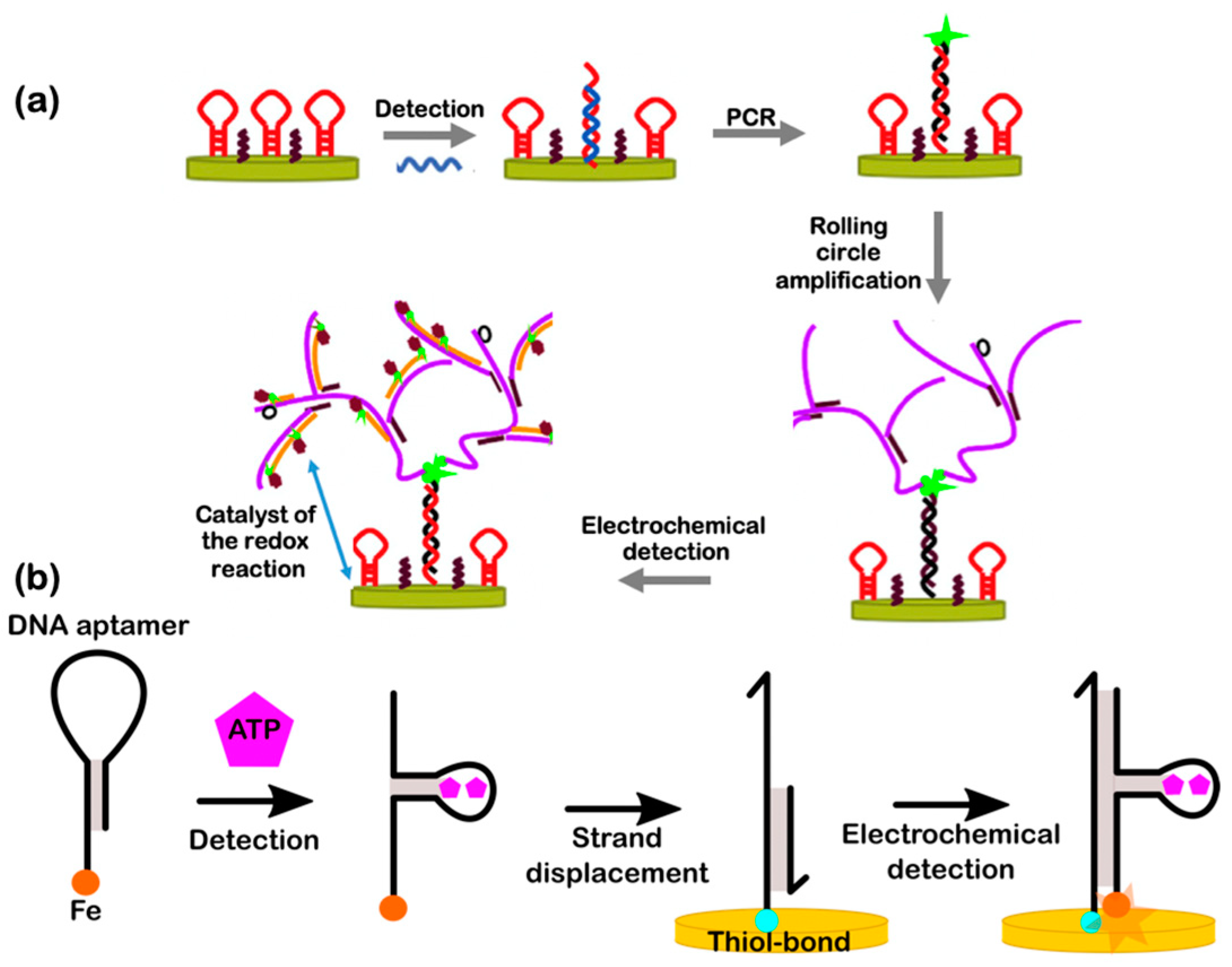
5.3. Hybrid DNA Electrochemical Biosensors
6. DNA-based Medicine
6.1. DNA Nanostructures as an In Vivo Drug Delivery Vehicle
6.2. DNA Nanostructures as Personalized Therapy
7. DNA Amplification Systems
7.1. DNA Hybridization Chain Reaction (HCR)
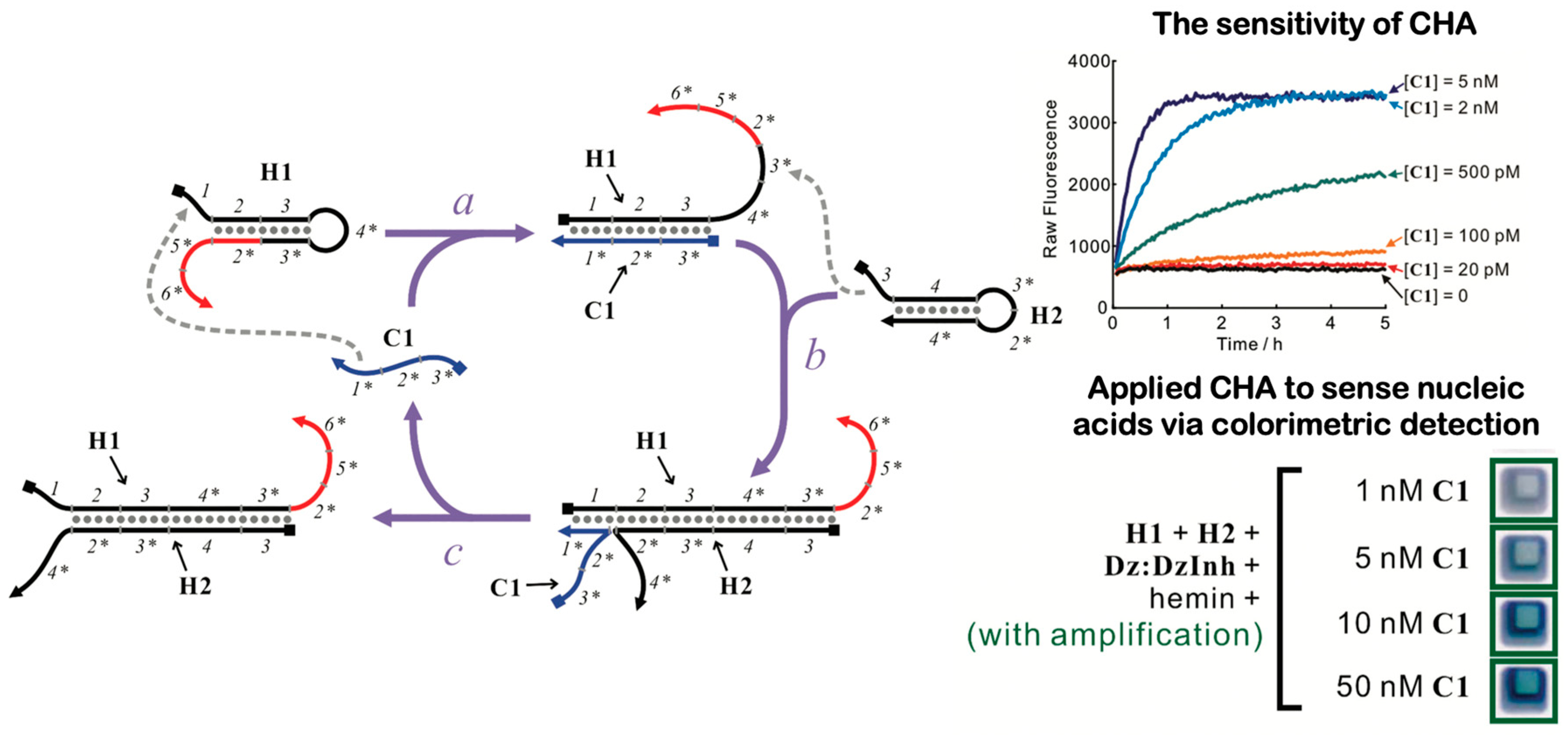
7.2. DNA Catalytic Hairpin Assembly (CHA)
7.3. DNA Rolling Circle Amplification (RCA)
8. DNA Kinesin-Like Motors
8.1. DNA Walkers
8.2. DNA Bipedal Motors
9. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sasson, A. Medical biotechnology: Achievements, prospects and perceptions; United Nations University Press: Tokyo, Japan, 2005. [Google Scholar]
- Amexo, M.; Tolhurst, R.; Barnish, G.; Bates, I. Malaria misdiagnosis: Effects on the poor and vulnerable. Lancet 2004, 364, 1896–1898. [Google Scholar] [CrossRef]
- Livermore, D.M. Bacterial resistance: Origins, epidemiology, and impact. Clin. Infect. Dis. 2003, 36, S11–S23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef]
- Syedmoradi, L.; Daneshpour, M.; Alvandipour, M.; Gomez, F.A.; Hajghassem, H.; Omidfar, K. Point of care testing: The impact of nanotechnology. Biosens. Bioelectron. 2017, 87, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Hauck, T.S.; Giri, S.; Gao, Y.; Chan, W.C. Nanotechnology diagnostics for infectious diseases prevalent in developing countries. Adv. Drug Delivery Rev. 2010, 62, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Walper, S.A.; Aragones, G.L.; Sapsford, K.E.; Brown, C.W.; Rowland, C.E.; Breger, J.C.; Medintz, I.L. Detecting biothreat agents: From current diagnostics to developing sensor technologies. ACS Sens. 2018, 3, 1894–2024. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.J.; Castro, C.M.; Im, H.; Lee, H.; Weissleder, R. A magneto-DNA nanoparticle system for rapid detection and phenotyping of bacteria. Nat. Nanotechnol. 2013, 8, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, A.V.; Han, D.R.; Shih, W.M.; Yan, H. Challenges and opportunities for structural DNA nanotechnology. Nat. Nanotechnol. 2011, 6, 763–772. [Google Scholar] [CrossRef]
- Condon, A. Designed DNA molecules: Principles and applications of molecular nanotechnology. Nat. Rev. Genet. 2006, 7, 565–575. [Google Scholar] [CrossRef]
- Wu, H.; Liao, J.; Li, Q.; Yang, M.; Zhao, M.; Lu, Q. Epigenetics as biomarkers in autoimmune diseases. Clin. Immunol. 2018, 196, 34–39. [Google Scholar] [CrossRef]
- Yordanova, A.; Eppard, E.; Kürpig, S.; Bundschuh, R.A.; Schönberger, S.; Gonzalez-Carmona, M.; Feldmann, G.; Ahmadzadehfar, H.; Essler, M. Theranostics in nuclear medicine practice. OncoTargets Ther. 2017, 10, 4821–4828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phata, J. Nanotechnology in agricultural diseases and food safety. J. Phytol. 2010, 2, 83–92. [Google Scholar]
- Scott, N.R. Nanotechnology and animal health. Rev. Sci. Tech.-Off. Int. Epizoot. 2005, 24, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medintz, I. Universal tools for biomolecular attachment to surfaces. Nat. Mater. 2006, 5, 842. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G. A century of alzheimer’s disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Belloy, M.E.; Napolioni, V.; Greicius, M.D. A quarter century of apoe and alzheimer’s disease: Progress to date and the path forward. Neuron 2019, 101, 820–838. [Google Scholar] [CrossRef] [Green Version]
- Dube, U.; Del-Aguila, J.L.; Li, Z.; Budde, J.P.; Jiang, S.; Hsu, S.; Ibanez, L.; Fernandez, M.V.; Farias, F.; Norton, J.; et al. An atlas of cortical circular rna expression in alzheimer disease brains demonstrates clinical and pathological associations. Nat. Neurosci. 2019, 22, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Santoso, M.R.; Rezaee, F.; Aghaverdi, H.; Mahmoudi, M.; Perry, G. Advances in alzheimer’s diagnosis and therapy: The implications of nanotechnology. Trends Biotechnol. 2017, 35, 937–953. [Google Scholar] [CrossRef]
- Sragovich, S.; Ziv, Y.; Vaisvaser, S.; Shomron, N.; Hendler, T.; Gozes, I. The autism-mutated adnp plays a key role in stress response. Transl. Psychiatry 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Faghihi, M.A.; Modarresi, F.; Khalil, A.M.; Wood, D.E.; Sahagan, B.G.; Morgan, T.E.; Finch, C.E.; Laurent, G.S., III; Kenny, P.J.; Wahlestedt, C. Expression of a noncoding rna is elevated in alzheimer’s disease and drives rapid feed-forward regulation of β-secretase. Nat. Med. 2008, 14, 723. [Google Scholar] [CrossRef] [Green Version]
- Marrack, P.; Kappler, J.; Kotzin, B.L. Autoimmune disease: Why and where it occurs. Nat. Med. 2001, 7, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, Y.; Hokari, A. Autoimmune hepatitis: Current challenges and future prospects. Clin. Exp. Gastroenterol. 2017, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisetsky, D.S. Antinuclear antibodies in healthy people: The tip of autoimmunity’s iceberg? Arthritis Res. Ther. 2011, 13, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grygiel-Gorniak, B.; Rogacka, N.; Puszczewicz, M. Antinuclear antibodies in healthy people and non-rheumatic diseases - diagnostic and clinical implications. Reumatologia 2018, 56, 243–248. [Google Scholar] [CrossRef]
- Antonarakis, S.E.; Krawczak, M.; Cooper, D.N. Disease-causing mutations in the human genome. Eur. J. Pediatr. 2000, 159, S173–S178. [Google Scholar] [CrossRef]
- Lee, J.Y.; Nagano, Y.; Taylor, J.P.; Lim, K.L.; Yao, T.P. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and hdac6-dependent mitophagy. J. Cell. Biol. 2010, 189, 671–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emrich, C.A.; Tian, H.; Medintz, I.L.; Mathies, R.A. Microfabricated 384-lane capillary array electrophoresis bioanalyzer for ultrahigh-throughput genetic analysis. Anal. Chem. 2002, 74, 5076–5083. [Google Scholar] [CrossRef]
- Medintz, I.L.; Paegel, B.M.; Blazej, R.G.; Emrich, C.A.; Berti, L.; Scherer, J.R.; Mathies, R.A. High-performance genetic analysis using microfabricated capillary array electrophoresis microplates. Electrophoresis 2001, 22, 3845–3856. [Google Scholar] [CrossRef]
- Lyon, G.J.; Wang, K. Identifying disease mutations in genomic medicine settings: Current challenges and how to accelerate progress. Genome Med. 2012, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Lemke, J.; Altmueller, J.; Thiele, H.; Glaus, E.; Fleischhauer, J.; Nurnberg, P.; Neidhardt, J.; Berger, W. Identification of novel and recurrent disease-causing mutations in retinal dystrophies using whole exome sequencing (wes): Benefits and limitations. PLoS ONE 2016, 11, e0158692. [Google Scholar] [CrossRef] [Green Version]
- Bryant, L.; Lozynska, O.; Han, G.; Morgan, J.I.W.; Gai, X.; Maguire, A.M.; Aleman, T.; Bennett, J. On variants and disease-causing mutations: Case studies of a sema4a variant identified in inherited blindness. Ophthalmic Genet. 2018, 39, 144–146. [Google Scholar] [CrossRef] [PubMed]
- Eran, P.; Almogit, A.; David, Z.; Wolf, H.R.; Hana, G.; Yaniv, B.; Elon, P.; Isaac, A. The d144e substitution in the vsx1 gene: A non-pathogenic variant or a disease causing mutation? Ophthalmic Genet. 2008, 29, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Hess, K.L.; Medintz, I.L.; Jewell, C.M. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today 2019, 27, 73–98. [Google Scholar] [CrossRef]
- Deriy, L.V.; Gomez, E.A.; Zhang, G.; Beacham, D.W.; Hopson, J.A.; Gallan, A.J.; Shevchenko, P.D.; Bindokas, V.P.; Nelson, D.J. Disease-causing mutations in the cystic fibrosis transmembrane conductance regulator determine the functional responses of alveolar macrophages. J. Biol. Chem. 2009, 284, 35926–35938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.S.; Zhang, D.Y. Simulation-guided DNA probe design for consistently ultraspecific hybridization. Nat. Chem. 2015, 7, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterlini-Brechot, P.; Benali, N.L. Circulating tumor cells (ctc) detection: Clinical impact and future directions. Cancer Lett. 2007, 253, 180–204. [Google Scholar] [CrossRef] [PubMed]
- Lowes, L.E.; Lock, M.; Rodrigues, G.; D’Souza, D.; Bauman, G.; Ahmad, B.; Venkatesan, V.; Allan, A.L.; Sexton, T. The significance of circulating tumor cells in prostate cancer patients undergoing adjuvant or salvage radiation therapy. Prostate Cancer Prostatic Dis. 2015, 18, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Westphal, M.; Lamszus, K. Circulating biomarkers for gliomas. Nat. Rev. Neurol. 2015, 11, 556–566. [Google Scholar] [CrossRef]
- Hong, B.; Zu, Y. Detecting circulating tumor cells: Current challenges and new trends. Theranostics 2013, 3, 377–394. [Google Scholar] [CrossRef] [Green Version]
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038. [Google Scholar] [CrossRef]
- Liu, X.; Wu, L.; Wang, L.; Jiang, W. A dual-targeting DNA tetrahedron nanocarrier for breast cancer cell imaging and drug delivery. Talanta 2018, 179, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Huang, W.; Chan, L.; Zhou, B.W.; Chen, T.F. A multifunctional DNA origami as carrier of metal complexes to achieve enhanced tumoral delivery and nullified systemic toxicity. Biomaterials 2016, 103, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Mathur, D.; Samanta, A.; Oh, E.; Diaz, S.A.; Susumu, K.; Ancona, M.G.; Medintz, I.L. Quantum dot encapsulation using a peptide-modified tetrahedral DNA cage. Chem. Mater. 2017, 29, 5762–5766. [Google Scholar] [CrossRef]
- Campbell, E.M.; Hope, T.J. Hiv-1 capsid: The multifaceted key player in hiv-1 infection. Nat. Rev. Microbiol. 2015, 13, 471–483. [Google Scholar] [CrossRef] [Green Version]
- Easterbrook, P.J.; Roberts, T.; Sands, A.; Peeling, R. Diagnosis of viral hepatitis. Curr. Opin. HIV AIDS 2017, 12, 302. [Google Scholar] [CrossRef]
- Bray, M. Highly pathogenic rna viral infections: Challenges for antiviral research. Antiviral Res. 2008, 78, 1–8. [Google Scholar] [CrossRef]
- Parvin, R.; Begum, J.A.; Chowdhury, E.H.; Islam, M.R.; Beer, M.; Harder, T. Co-subsistence of avian influenza virus subtypes of low and high pathogenicity in bangladesh: Challenges for diagnosis, risk assessment and control. Sci. Rep. 2019, 9, 8306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollack, A.; Kontorovich, A.R.; Fuster, V.; Dec, G.W. Viral myocarditis--diagnosis, treatment options, and current controversies. Nat. Rev. Cardiol. 2015, 12, 670–680. [Google Scholar] [CrossRef]
- Taubert, R.; Diestelhorst, J.; Junge, N.; Kirstein, M.M.; Pischke, S.; Vogel, A.; Bantel, H.; Baumann, U.; Manns, M.P.; Wedemeyer, H.; et al. Increased seroprevalence of hav and parvovirus b19 in children and of hev in adults at diagnosis of autoimmune hepatitis. Sci. Rep. 2018, 8, 17452. [Google Scholar] [CrossRef]
- Cole, J.R., Jr.; Sulzer, C.R.; Pursell, A.R. Improved microtechnique for the leptospiral microscopic agglutination test. Appl. Microbiol. 1973, 25, 976–980. [Google Scholar] [CrossRef] [Green Version]
- Niloofa, R.; Fernando, N.; de Silva, N.L.; Karunanayake, L.; Wickramasinghe, H.; Dikmadugoda, N.; Premawansa, G.; Wickramasinghe, R.; de Silva, H.J.; Premawansa, S.; et al. Diagnosis of leptospirosis: Comparison between microscopic agglutination test, igm-elisa and igm rapid immunochromatography test. PLoS ONE 2015, 10, e0129236. [Google Scholar] [CrossRef] [Green Version]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay, elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J. Immunol. 1972, 109, 129–135. [Google Scholar] [PubMed]
- Lequin, R.M. Enzyme immunoassay (eia)/enzyme-linked immunosorbent assay (elisa). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vranish, J.N.; Ancona, M.G.; Oh, E.; Susumu, K.; Medintz, I.L. Enhancing coupled enzymatic activity by conjugating one enzyme to a nanoparticle. Nanoscale 2017, 9, 5172–5187. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Tsumoto, K.; Kubota, K.; Suzuki, E.; Nagamune, T.; Nishimura, H.; Schueler, P.A.; Winter, G.; Kumagai, I.; Mohoney, W.C. Open sandwich elisa: A novel immunoassay based on the interchain interaction of antibody variable region. Nat. Biotechnol. 1996, 14, 1714–1718. [Google Scholar] [CrossRef]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Correction to: Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Nat. Med. 2018, 72, 43. [Google Scholar] [CrossRef] [Green Version]
- Ferrier, C.M.; de Witte, H.H.; Straatman, H.; van Tienoven, D.H.; van Geloof, W.L.; Rietveld, F.J.; Sweep, C.G.; Ruiter, D.J.; van Muijen, G.N. Comparison of immunohistochemistry with immunoassay (elisa) for the detection of components of the plasminogen activation system in human tumour tissue. Br. J. Cancer 1999, 79, 1534–1541. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Tokeshi, M.; Kimura, H.; Kitamori, T. Determination of carcinoembryonic antigen in human sera by integrated bead bed immunoasay in a microchip for cancer diagnosis. Anal. Chem. 2001, 73, 1213–1218. [Google Scholar] [CrossRef]
- Chirathaworn, C.; Inwattana, R.; Poovorawan, Y.; Suwancharoen, D. Interpretation of microscopic agglutination test for leptospirosis diagnosis and seroprevalence. Asian Pac. J. Trop. Biomed. 2014, 4, S162–S164. [Google Scholar] [CrossRef] [Green Version]
- Ochman, H.; Gerber, A.S.; Hartl, D.L. Genetic applications of an inverse polymerase chain reaction. Genetics 1988, 120, 621–623. [Google Scholar]
- Kubista, M.; Andrade, J.M.; Bengtsson, M.; Forootan, A.; Jonak, J.; Lind, K.; Sindelka, R.; Sjoback, R.; Sjogreen, B.; Strombom, L.; et al. The real-time polymerase chain reaction. Mol. Aspects Med. 2006, 27, 95–125. [Google Scholar] [CrossRef] [PubMed]
- Garibyan, L.; Avashia, N. Polymerase chain reaction. J. Investig. Dermatol. 2013, 133, E1–E4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valones, M.A.A.; Guimaraes, R.L.; Brandao, L.A.C.; de Souza, P.R.E.; Carvalho, A.D.T.; Crovela, S. Principles and applications of polymerase chain reaction in medical diagnostic fields: A review. Braz. J. Microbiol. 2009, 40, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molloy, T.J.; Devriese, L.A.; Helgason, H.H.; Bosma, A.J.; Hauptmann, M.; Voest, E.E.; Schellens, J.H.M.; van’t Veer, L.J. A multimarker qpcr-based platform for the detection of circulating tumour cells in patients with early-stage breast cancer. Br. J. Cancer 2011, 104, 1913–1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutteridge, A.; Rathbone, V.M.; Gibbons, R.; Amary, F.; Archard, N.; Davies, K.; Brown, J.; Jorgensen, M.; Gupta, M.; Flanagan, A.M.; et al. Digital pcr analysis of circulating tumour DNA in a broad cohort of sarcoma patients. J. Pathol. 2016, 240, 22. [Google Scholar]
- Roux, G.; Ravel, C.; Varlet-Marie, E.; Jendrowiak, R.; Bastien, P.; Sterkers, Y. Inhibition of polymerase chain reaction: Pathogen-specific controls are better than human gene amplification. PLoS ONE 2019, 14, e0219276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elnifro, E.M.; Ashshi, A.M.; Cooper, R.J.; Klapper, P.E. Multiplex pcr: Optimization and application in diagnostic virology. Clin. Microbiol. Rev. 2000, 13, 559–570. [Google Scholar] [CrossRef]
- Medintz, I.; Wong, W.W.; Berti, L.; Shiow, L.; Tom, J.; Scherer, J.; Sensabaugh, G.; Mathies, R.A. High-performance multiplex snp analysis of three hemochromatosis-related mutations with capillary array electrophoresis microplates. Genome Res. 2001, 11, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Edwards, M.C.; Gibbs, R.A. Multiplex pcr - advantages, development, and applications. Genome Res. 1994, 3, S65–S75. [Google Scholar] [CrossRef] [Green Version]
- Shum, J.; Paul, N. Chemically modified primers for improved multiplex polymerase chain reaction. Anal. Biochem. 2009, 388, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.; Meena, R.K.; Aggarwal, A.; Chhabra, R. Multiplex pcr as a novel method in the diagnosis of spinal tuberculosis-a pilot study. Acta Neurochir. 2017, 159, 503–507. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Bose, M.E.; Beck, E.T.; Fan, J.; Tiwari, S.; Metallo, J.; Jurgens, L.A.; Kehl, S.C.; Ledeboer, N.; Kumar, S.; et al. Rapid multiplex reverse transcription-pcr typing of influenza a and b virus, and subtyping of influenza a virus into h1, 2, 3, 5, 7, 9, n1 (human), n1 (animal), n2, and n7, including typing of novel swine origin influenza a (h1n1) virus, during the 2009 outbreak in milwaukee, wisconsin. J. Clin. Microbiol. 2009, 47, 2772–2778. [Google Scholar] [PubMed] [Green Version]
- Wei, B.; Cha, S.Y.; Kang, M.; Park, I.J.; Moon, O.K.; Park, C.K.; Jang, H.K. Development and application of a multiplex pcr assay for rapid detection of 4 major bacterial pathogens in ducks. Poultry Sci. 2013, 92, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Frasca, S., Jr.; Sunila, I.; French, R.A. Application of a multiplex pcr for the detection of protozoan pathogens of the eastern oyster crassostrea virginica in field samples. Dis. Aquat. Organ. 2004, 59, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Riyaz-Ul-Hassan, S.; Syed, S.; Johri, S.; Verma, V.; Qazi, G.N. Application of a multiplex pcr assay for the detection of shigella, escherichia coli and shiga toxin-producing esch. Coli in milk. J. Dairy Res. 2009, 76, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [Green Version]
- Bui, H.; Shah, S.; Mokhtar, R.; Song, T.; Garg, S.; Reif, J. Localized DNA hybridization chain reactions on DNA origami. ACS Nano 2018, 12, 1146–1155. [Google Scholar] [CrossRef]
- Bui, H.; Miao, V.; Garg, S.; Mokhtar, R.; Song, T.; Reif, J. Design and analysis of localized DNA hybridization chain reactions. Small 2017, 13, 1602983. [Google Scholar] [CrossRef]
- Wong, Y.P.; Othman, S.; Lau, Y.L.; Radu, S.; Chee, H.Y. Loop-mediated isothermal amplification (lamp): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018, 124, 626–643. [Google Scholar] [CrossRef] [Green Version]
- Velders, A.H.; Schoen, C.; Saggiomo, V. Loop-mediated isothermal amplification (lamp) shield for arduino DNA detection. BMC Res. Notes 2018, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Waterfield, T.; Fairley, D.; Blackwood, B.; McKenna, J.; Shields, M.D. A systematic review of the diagnostic accuracy of loop-mediated-isothermal amplification (lamp) in thediagnosis of invasive meningococcal disease in children. BMC Pediatr. 2019, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Salamin, O.; Kuuranne, T.; Saugy, M.; Leuenberger, N. Loop-mediated isothermal amplification (lamp) as an alternative to pcr: A rapid on-site detection of gene doping. Drug Test. Anal. 2017, 9, 1731–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheu, S.C.; Tsou, P.C.; Lien, Y.Y.; Lee, M.S. Development of loop-mediated isothermal amplification (lamp) assays for the rapid detection of allergic peanut in processed food. Food Chem. 2018, 257, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.C.; Wang, C.M.; Cai, Y.Y. Loop-mediated isothermal amplification for rapid detection of acute viral necrobiotic virus in scallop chlamys farreii. Acta Virol. 2009, 53, 161–167. [Google Scholar] [CrossRef]
- Diribe, O.; North, S.; Sawyer, J.; Roberts, L.; Fitzpatrick, N.; La Ragione, R. Design and application of a loop-mediated isothermal amplification assay for the rapid detection of staphylococcus pseudintermedius. J. Vet. Diagn. Investig. 2014, 26, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Behjati, S.; Tarpey, P.S. What is next generation sequencing? Arch. Dis. Child. Educ. Pract. Ed. 2013, 98, 236–238. [Google Scholar] [CrossRef]
- Medintz, I.L.; Paegel, B.M.; Mathies, R.A. Microfabricated capillary array electrophoresis DNA analysis systems. J. Chromatogr. A 2001, 924, 265–270. [Google Scholar] [CrossRef]
- Slatko, B.E.; Gardner, A.F.; Ausubel, F.M. Overview of next-generation sequencing technologies. Curr. Protoc. Mol. Biol. 2018, 122, e59. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Steinberg, K.M.; Larson, D.E.; Wilson, R.K.; Mardis, E.R. The next-generation sequencing revolution and its impact on genomics. Cell 2013, 155, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Seleman, M.; Hoyos-Bachiloglu, R.; Geha, R.S.; Chou, J. Uses of next-generation sequencing technologies for the diagnosis of primary immunodeficiencies. Front Immunol. 2017, 8, 847. [Google Scholar] [CrossRef] [Green Version]
- Boonham, N.; Kreuze, J.; Winter, S.; van der Vlugt, R.; Bergervoet, J.; Tomlinson, J.; Mumford, R. Methods in virus diagnostics: From elisa to next generation sequencing. Virus Res. 2014, 186, 20–31. [Google Scholar] [CrossRef]
- Head, S.R.; Komori, H.K.; LaMere, S.A.; Whisenant, T.; Van Nieuwerburgh, F.; Salomon, D.R.; Ordoukhanian, P. Library construction for next-generation sequencing: Overviews and challenges. Biotechniques 2014, 56, 61. [Google Scholar] [CrossRef] [Green Version]
- Seeman, N.C.; Sleiman, H.F. DNA nanotechnology. Nat. Rev. Mater. 2018, 3, 1–23. [Google Scholar] [CrossRef]
- Church, G.M.; Gao, Y.; Kosuri, S. Next-generation digital information storage in DNA. Science 2012, 337, 1628. [Google Scholar] [CrossRef] [Green Version]
- Seeman, N.C. DNA nanotechnology: From the pub to information-based chemistry. Methods Mol. Biol. 2018, 1811, 1–9. [Google Scholar] [PubMed]
- Zhang, D.Y.; Seelig, G. Dynamic DNA nanotechnology using strand-displacement reactions. Nat. Chem. 2011, 3, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Mathur, D.; Medintz, I.L. The growing development of DNA nanostructures for potential healthcare-related applications. Adv. Healthc. Mater. 2019, 8, 1801546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Nangreave, J.; Liu, Y.; Yan, H. Structural DNA nanotechnology: State of the art and future perspective. J. Am. Chem. Soc. 2014, 136, 11198–11211. [Google Scholar] [CrossRef] [Green Version]
- Douglas, S.M.; Dietz, H.; Liedl, T.; Högberg, B.; Graf, F.; Shih, W.M. Self-assembly of DNA into nanoscale three-dimensional shapes. Nature 2009, 459, 414–418. [Google Scholar] [CrossRef]
- Jiang, Q.; Song, C.; Nangreave, J.; Liu, X.; Lin, L.; Qiu, D.; Wang, Z.-G.; Zou, G.; Liang, X.; Yan, H. DNA origami as a carrier for circumvention of drug resistance. J. Am. Chem. Soc. 2012, 134, 13396–13403. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Turberfield, A.J.; Yurke, B.; Winfree, E. Engineering entropy-driven reactions and networks catalyzed by DNA. Science 2007, 318, 1121–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, N.V.; Torring, T.; Rotaru, A.; Jacobsen, M.F.; Ravnsbaek, J.B.; Subramani, R.; Mamdouh, W.; Kjems, J.; Mokhir, A.; Besenbacher, F.; et al. Single-molecule chemical reactions on DNA origami. Nat. Nanotechnol. 2010, 5, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Dirks, R.M.; Pierce, N.A. Triggered amplification by hybridization chain reaction. Proc. Natl. Acad. Sci. USA 2004, 101, 15275–15278. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Park, S.H.; Feng, L.; Finklestein, G.; Reif, J.; LaBean, T.H. 4x4 DNA tile and lattices: Characterization, self-assembly, and metallization of a novel DNA nanostructure motif. In Proceedings of the Ninth International Meeting on DNA Based Computers (DNA9), Madison, WI, USA, 1–3 June 2003. [Google Scholar]
- Rothemund, P.W. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, Y.; Ong, L.L.; Shih, W.M.; Yin, P. Three-dimensional structures self-assembled from DNA bricks. Science 2012, 338, 1177–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeman, N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982, 99, 237–247. [Google Scholar] [CrossRef]
- Simmel, F.C.; Yurke, B.; Singh, H.R. Principles and applications of nucleic acid strand displacement reactions. Chem. Rev. 2019, 119, 6326–6369. [Google Scholar] [CrossRef]
- Yurke, B.; Turberfield, A.J.; Mills, A.P.; Simmel, F.C.; Neumann, J.L. A DNA-fuelled molecular machine made of DNA. Nature 2000, 406, 605–608. [Google Scholar] [CrossRef]
- Yan, H.; LaBean, T.H.; Feng, L.; Reif, J.H. Directed nucleation assembly of DNA tile complexes for barcode-patterned lattices. Proc. Natl. Acad. Sci. USA 2003, 100, 8103–8108. [Google Scholar] [CrossRef] [Green Version]
- Tikhomirov, G.; Petersen, P.; Qian, L. Fractal assembly of micrometre-scale DNA origami arrays with arbitrary patterns. Nature 2017, 552, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Shih, W.M.; Lin, C.X. Knitting complex weaves with DNA origami. Curr. Opin. Struct. Biol. 2010, 20, 276–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nangreave, J.; Han, D.R.; Liu, Y.; Yan, H. DNA origami: A history and current perspective. Curr. Opin. Chem. Biol. 2010, 14, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Gothelf, K.V. Lego-like DNA structures. Science 2012, 338, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Dai, M.; Yin, P. Complex shapes self-assembled from single-stranded DNA tiles. Nature 2012, 485, 623–626. [Google Scholar] [CrossRef] [Green Version]
- Ong, L.L.; Hanikel, N.; Yaghi, O.K.; Grun, C.; Strauss, M.T.; Bron, P.; Lai-Kee-Him, J.; Schueder, F.; Wang, B.; Wang, P.; et al. Programmable self-assembly of three-dimensional nanostructures from 10,000 unique components. Nature 2017, 552, 72–77. [Google Scholar] [CrossRef]
- Bui, H.; Garg, S.; Miao, V.; Song, T.; Mokhtar, R.; Reif, J. Design and analysis of linear cascade DNA hybridization chain reactions using DNA hairpins. New J. Phys. 2017, 19, 015006. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.L.; Hou, M.F.; Xia, Y.K.; He, W.H.; Yan, A.; Weng, Y.P.; Zeng, L.P.; Chen, J.H. A ratiometric electrochemical biosensor for the exosomal micrornas detection based on bipedal DNA walkers propelled by locked nucleic acid modified toehold mediate strand displacement reaction. Biosens. Bioelectron. 2018, 102, 33–40. [Google Scholar] [CrossRef]
- Shin, J.S.; Pierce, N.A. A synthetic DNA walker for molecular transport. J. Am. Chem. Soc. 2004, 126, 10834–10835. [Google Scholar] [CrossRef] [Green Version]
- Song, T.; Eshra, A.; Shah, S.; Bui, H.; Fu, D.; Yang, M.; Mokhtar, R.; Reif, J. Fast and compact DNA logic circuits based on single-stranded gates using strand-displacing polymerase. Nat. Nanotechnol. 2019, 14, 1075–1081. [Google Scholar] [CrossRef]
- Song, T.; Garg, S.; Mokhtar, R.; Bui, H.; Reif, J. Design and analysis of compact DNA strand displacement circuits for analog computation using autocatalytic amplifiers. ACS Synth. Biol. 2018, 7, 46–53. [Google Scholar] [CrossRef]
- Zadeh, J.N.; Steenberg, C.D.; Bois, J.S.; Wolfe, B.R.; Pierce, M.B.; Khan, A.R.; Dirks, R.M.; Pierce, N.A. Nupack: Analysis and design of nucleic acid systems. J. Comput. Chem. 2011, 32, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Lakin, M.R.; Youssef, S.; Polo, F.; Emmott, S.; Phillips, A. Visual dsd: A design and analysis tool for DNA strand displacement systems. Bioinformatics 2011, 27, 3211–3213. [Google Scholar] [CrossRef] [PubMed]
- Chidchob, P.; Sleiman, H.F. Recent advances in DNA nanotechnology. Curr. Opin. Chem. Biol. 2018, 46, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Kim, B.; Hussain, R.; Amin, R.; Park, S.H. DNA nanotechnology: A future perspective. Nanoscale Res. Lett. 2013, 8, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, T.; Shah, S.; Bui, H.; Garg, S.; Eshra, A.; Fu, D.; Yang, M.; Mokhtar, R.; Reif, J. Programming DNA-based biomolecular reaction networks on cancer cell membranes. J. Am. Chem. Soc. 2019, 141, 16539–16543. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.; Díaz, S.A.; Fontana, J.; Chiriboga, M.; Veneziano, R.; Medintz, I.L. Utilizing the organizational power of DNA scaffolds for new nanophotonic applications. Adv. Opt. Mater. 2019, 7, 1900562. [Google Scholar] [CrossRef] [Green Version]
- Thakur, M.S.; Ragavan, K.V. Biosensors in food processing. J. Food Sci. Tech. 2013, 50, 625–641. [Google Scholar] [CrossRef] [Green Version]
- Spillmann, C.M.; Medintz, I.L. Use of biomolecular scaffolds for assembling multistep light harvesting and energy transfer devices. J. Photochem. Photobiol. C 2015, 23, 1–24. [Google Scholar] [CrossRef]
- Wen, Y.L.; Pei, H.; Shen, Y.; Xi, J.J.; Lin, M.H.; Lu, N.; Shen, X.Z.; Li, J.; Fan, C.H. DNA nanostructure-based interfacial engineering for pcr-free ultrasensitive electrochemical analysis of microrna (vol 2, pg 267, 2012). Sci. Rep. 2013, 3, 867. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: Rna ligands to bacteriophage t4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, J.W. Functional DNA nanotechnology: Emerging applications of dnazymes and aptamers. Curr. Opin. Biotechnol. 2006, 17, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Torabi, S.-F.; Lu, Y. Small-molecule diagnostics based on functional DNA nanotechnology: A dipstick test for mercury. Faraday Discuss. R. Soc. Chem. 2011, 149, 125–157. [Google Scholar] [CrossRef] [Green Version]
- Zharov, V.P.; Mercer, K.E.; Galitovskaya, E.N.; Smeltzer, M.S. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys. J. 2006, 90, 619–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, F.; Lillehoj, P.B.; Ho, C.-M. DNA diagnostics: Nanotechnology-enhanced electrochemical detection of nucleic acids. Pediatr. Res. 2010, 67, 458–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.S.; Ren, L.J.; Liu, X.H.; Zhou, M.R.; Li, L.L.; Xu, J.J.; Zhu, X.L. DNA nanotechnology for cancer diagnosis and therapy. Int. J. Mol. Sci. 2018, 19, 1671. [Google Scholar] [CrossRef] [Green Version]
- Field, L.D.; Walper, S.A.; Susumu, K.; Lasarte-Aragones, G.; Oh, E.; Medintz, I.L.; Delehanty, J.B. A quantum dot-protein bioconjugate that provides for extracellular control of intracellular drug release. Bioconjug. Chem. 2018, 29, 2455–2467. [Google Scholar] [CrossRef]
- Sangtani, A.; Petryayeva, E.; Wu, M.; Susumu, K.; Oh, E.; Huston, A.L.; Lasarte-Aragones, G.; Medintz, I.L.; Algar, W.R.; Delehanty, J.B. Intracellularly actuated quantum dot-peptide-doxorubicin nanobioconjugates for controlled drug delivery via the endocytic pathway. Bioconjug. Chem. 2018, 29, 136–148. [Google Scholar] [CrossRef]
- Sangtani, A.; Petryayeva, E.; Susumu, K.; Oh, E.; Huston, A.L.; Lasarte-Aragones, G.; Medintz, I.L.; Algar, W.R.; Delehanty, J.B. Nanoparticle-peptide-drug bioconjugates for unassisted defeat of multidrug resistance in a model cancer cell line. Bioconjug. Chem. 2019, 30, 525–530. [Google Scholar] [CrossRef]
- Ahmad, J.; Akhter, S.; Rizwanullah, M.; Khan, M.A.; Pigeon, L.; Addo, R.T.; Greig, N.H.; Midoux, P.; Pichon, C.; Kamal, M.A. Nanotechnology based theranostic approaches in alzheimer’s disease management: Current status and future perspective. Curr. Alzheimer Res. 2017, 14, 1164–1181. [Google Scholar] [CrossRef] [Green Version]
- Cagnin, S.; Caraballo, M.; Guiducci, C.; Martini, P.; Ross, M.; SantaAna, M.; Danley, D.; West, T.; Lanfranchi, G. Overview of electrochemical DNA biosensors: New approaches to detect the expression of life. Sensors 2009, 9, 3122–3148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowland, C.E.; Brown III, C.W.; Delehanty, J.B.; Medintz, I.L. Nanomaterial-based sensors for the detection of biological threat agents. Mater. Today 2016, 19, 464–477. [Google Scholar] [CrossRef]
- Bui, H.; Brown III, C.W.; Buckhout-White, S.; Díaz, S.A.; Stewart, M.H.; Susumu, K.; Oh, E.; Ancona, M.G.; Goldman, E.R.; Medintz, I.L. Transducing protease activity into DNA output for developing smart bionanosensors. Small 2019, 15, 1805384. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Overview of piezoelectric biosensors, immunosensors and DNA sensors and their applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jensen, M.K.; Keasling, J.D. Development of biosensors and their application in metabolic engineering. Curr. Opin. Chem. Biol. 2015, 28, 1–8. [Google Scholar] [CrossRef]
- Tamura, T.; Hamachi, I. Recent progress in design of protein-based fluorescent biosensors and their cellular applications. ACS Chem. Biol. 2014, 9, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and their applications–a review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Wang, J. From DNA biosensors to gene chips. Nucleic Acids Res. 2000, 28, 3011–3016. [Google Scholar] [CrossRef]
- Yang, D.; Ning, L.; Gao, T.; Ye, Z.; Li, G. Enzyme-free dual amplification strategy for protein assay by coupling toehold-mediated DNA strand displacement reaction with hybridization chain reaction. Electrochem. Commun. 2015, 58, 33–36. [Google Scholar] [CrossRef]
- Algar, W.R.; Hildebrandt, N.; Vogel, S.S.; Medintz, I.L. Fret as a biomolecular research tool—understanding its potential while avoiding pitfalls. Nat. Methods 2019, 16, 815–829. [Google Scholar] [CrossRef]
- Chen, R.P.; Blackstock, D.; Sun, Q.; Chen, W. Dynamic protein assembly by programmable DNA strand displacement. Nat. Chem. 2018, 10, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Roh, Y.H.; Ruiz, R.C.H.; Peng, S.; Lee, J.B.; Luo, D. Engineering DNA-based functional materials. Chem. Soc. Rev. 2011, 40, 5730–5744. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Liu, M.; Liu, Y.; Woodbury, N.W.; Yan, H. Interenzyme substrate diffusion for an enzyme cascade organized on spatially addressable DNA nanostructures. J. Am. Chem. Soc. 2012, 134, 5516–5519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, W.P.; Thomsen, R.P.; Turner, K.B.; Walper, S.A.; Vranish, J.; Kjems, J.; Ancona, M.G.; Medintz, I.L. Enhanced catalysis from multienzyme cascades assembled on a DNA origami triangle. ACS Nano 2019, 13, 13677–13689. [Google Scholar] [CrossRef]
- Ellis, G.A.; Klein, W.P.; Lasarte-Aragonés, G.; Thakur, M.; Walper, S.A.; Medintz, I.L. Artificial multienzyme scaffolds: Pursuing in vitro substrate channeling with an overview of current progress. ACS Catal. 2019, 9, 10812–10869. [Google Scholar] [CrossRef] [Green Version]
- Linko, V.; Eerikäinen, M.; Kostiainen, M.A. A modular DNA origami-based enzyme cascade nanoreactor. Chem. Commun. 2015, 51, 5351–5354. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.C.; Mastali, M.; Gau, V.; Suchard, M.A.; Moller, A.K.; Bruckner, D.A.; Babbitt, J.T.; Li, Y.; Gornbein, J.; Landaw, E.M.; et al. Use of electrochemical DNA biosensors for rapid molecular identification of uropathogens in clinical urine specimens. J. Clin. Microbiol. 2006, 44, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Guo, J.; Zhai, Q.; Xia, J.; Yi, G. Ultrasensitive electrochemical biosensor for specific detection of DNA based on molecular beacon mediated circular strand displacement polymerization and hyperbranched rolling circle amplification. Anal. Chim. Acta 2016, 934, 52–58. [Google Scholar] [CrossRef]
- Wang, L.; Fang, L.; Liu, S. Responsive hairpin DNA aptamer switch to program the strand displacement reaction for the enhanced electrochemical assay of atp. Analyst 2015, 140, 5877–5880. [Google Scholar] [CrossRef]
- Sagadevan, S.; Periasamy, M. A review on role of nanostructures in drug delivery system. Rev. Adv. Mater. Sci. 2014, 36, 112–117. [Google Scholar]
- Kim, K.R.; Kang, S.J.; Lee, A.Y.; Hwang, D.; Park, M.; Park, H.; Kim, S.; Hur, K.; Chung, H.S.; Mao, C.D.; et al. Highly tumor-specific DNA nanostructures discovered by in vivo screening of a nucleic acid cage library and their applications in tumor-targeted drug delivery. Biomaterials 2019, 195, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fong, W.K.; Hanley, T.; Boyd, B.J. Stimuli responsive liquid crystals provide ’on-demand’ drug delivery in vitro and in vivo. J. Control. Release 2009, 135, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Espargaro, A.; Medina, A.; Di Pietro, O.; Munoz-Torrero, D.; Sabate, R. Ultra rapid in vivo screening for anti-alzheimer anti-amyloid drugs. Sci. Rep. 2016, 6, 23349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Jiang, Q.; Li, N.; Dai, L.; Liu, Q.; Song, L.; Wang, J.; Li, Y.; Tian, J.; Ding, B.; et al. DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano 2014, 8, 6633–6643. [Google Scholar] [CrossRef]
- Douglas, S.M.; Bachelet, I.; Church, G.M. A logic-gated nanorobot for targeted transport of molecular payloads. Science 2012, 335, 831–834. [Google Scholar] [CrossRef]
- Sakamoto, J.H.; van de Ven, A.L.; Godin, B.; Blanco, E.; Serda, R.E.; Grattoni, A.; Ziemys, A.; Bouamrani, A.; Hu, T.; Ranganathan, S.I.; et al. Enabling individualized therapy through nanotechnology. Pharmacol. Res. 2010, 62, 57–89. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.X.; Asghar, W.; Demirci, U.; Wan, Y. Nanostructured substrates for isolation of circulating tumor cells. Nano Today 2013, 8, 374–387. [Google Scholar] [CrossRef] [Green Version]
- Fornaguera, C.; Garcia-Celma, M.J. Personalized nanomedicine: A revolution at the nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Dowsett, M.; Dunbier, A.K. Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clin. Cancer Res. 2008, 14, 8019–8026. [Google Scholar] [CrossRef] [Green Version]
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discovery Today 2010, 15, 842–850. [Google Scholar] [CrossRef]
- Cai, W.; Gao, T.; Hong, H.; Sun, J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 2008, 1, 17–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, T.; Kawakami, S.; Hatamoto, M.; Imachi, H.; Takahashi, M.; Araki, N.; Yamaguchi, T.; Kubota, K. In situ DNA-hybridization chain reaction (hcr): A facilitated in situ hcr system for the detection of environmental microorganisms. Environ. Microbiol. 2015, 17, 2532–2541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.S.; Li, B.; Milligan, J.N.; Bhadra, S.; Ellington, A.D. Real-time detection of isothermal amplification reactions with thermostable catalytic hairpin assembly. J. Am. Chem. Soc. 2013, 135, 7430–7433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Ellington, A.D.; Chen, X. Rational, modular adaptation of enzyme-free DNA circuits to multiple detection methods. Nucleic Acids Res. 2011, 39, e110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Zhou, X.; Zhao, J.; Xu, W. A cascade amplification strategy of catalytic hairpin assembly and hybridization chain reaction for the sensitive fluorescent assay of the model protein carcinoembryonic antigen. Microchim. Acta 2018, 185, 100. [Google Scholar] [CrossRef]
- Lizardi, P.M.; Huang, X.H.; Zhu, Z.R.; Bray-Ward, P.; Thomas, D.C.; Ward, D.C. Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat. Genet. 1998, 19, 225–232. [Google Scholar] [CrossRef]
- Zhou, H.P.; Bouwman, K.; Schotanus, M.; Verweij, C.; Marrero, J.A.; Dillon, D.; Costa, J.; Lizardi, P.; Haab, B.B. Two-color, rolling-circle amplification on antibody microarrays for sensitive, multiplexed serum-protein measurements. Genome Biol. 2004, 5, R28. [Google Scholar] [CrossRef] [Green Version]
- Schweitzer, B.; Wiltshire, S.; Lambert, J.; O’Malley, S.; Kukanskis, K.; Zhu, Z.R.; Kingsmore, S.F.; Lizardi, P.M.; Ward, D.C. Immunoassays with rolling circle DNA amplification: A versatile platform for ultrasensitive antigen detection. Proc. Natl. Acad. Sci. USA 2000, 97, 10113–10119. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhu, S.; Huang, P.; Chen, Y. Highly specific quantification of microrna by coupling probe–rolling circle amplification and förster resonance energy transfer. Anal. Biochem. 2016, 502, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Guittet, O.; Mingoes, C.; El Banna, N.; Huang, M.E.; Lepoivre, M.; Hildebrandt, N. Quantification of cellular deoxyribonucleoside triphosphates by rolling circle amplification and forster resonance energy transfer. Anal. Chem. 2019, 91, 14561–14568. [Google Scholar] [CrossRef]
- Bui, H.; Onodera, C.; Kidwell, C.; Tan, Y.; Graugnard, E.; Kuang, W.; Lee, J.; Knowlton, W.B.; Yurke, B.; Hughes, W.L. Programmable periodicity of quantum dot arrays with DNA origami nanotubes. Nano Lett. 2010, 10, 3367–3372. [Google Scholar] [CrossRef]
- Mathur, D.; Klein, W.P.; Chiriboga, M.; Bui, H.; Oh, E.; Nita, R.; Naciri, J.; Johns, P.; Fontana, J.; Diaz, S.A.; et al. Analyzing fidelity and reproducibility of DNA templated plasmonic nanostructures. Nanoscale 2019, 11, 20693–20706. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Bui, H.; Eshra, A.; Shah, S.; Reif, J. Nucleic acid hairpins: A robust and powerful motif for molecular devices. In World Scientific Series in Nanoscience and Nanotechnology; Zhang, Y., Xu, B., Eds.; World Scientific Publishing Co: Singapore, 2019; Volume 19, p. 175. [Google Scholar]
- Pan, J.; Li, F.R.; Cha, T.G.; Chen, H.R.; Choi, J.H. Recent progress on DNA based walkers. Curr. Opin. Biotechnol. 2015, 34, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Xu, L.L.; Liu, Z.; Zou, L.N.; Li, G.P.; Ye, B.X. An enzyme-free and label-free signal-on aptasensor based on dnazyme-driven DNA walker strategy. Anal. Chim. Acta 2019, 1081, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Omabegho, T.; Sha, R.; Seeman, N.C. A bipedal DNA brownian motor with coordinated legs. Science 2009, 324, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Tomov, T.E.; Tsukanov, R.; Liber, M.; Masoud, R.; Plavner, N.; Nir, E. Rational design of DNA motors: Fuel optimization through single-molecule fluorescence. J. Am. Chem. Soc. 2013, 135, 11935–11941. [Google Scholar] [CrossRef]
- Praetorius, F.; Kick, B.; Behler, K.L.; Honemann, M.N.; Weuster-Botz, D.; Dietz, H. Biotechnological mass production of DNA origami. Nature 2017, 552, 84–87. [Google Scholar] [CrossRef]
- Mei, Q.; Wei, X.; Su, F.; Liu, Y.; Youngbull, C.; Johnson, R.; Lindsay, S.; Yan, H.; Meldrum, D. Stability of DNA origami nanoarrays in cell lysate. Nano Lett. 2011, 11, 1477–1482. [Google Scholar] [CrossRef] [Green Version]
- Green, C.M.; Mathur, D.; Medintz, I.L. Understanding the fate of DNA nanostructures inside the cell. J. Mater. Chem. B 2020. [Google Scholar] [CrossRef]
- Rowland, C.E.; Brown, C.W.; Medintz, I.L.; Delehanty, J.B. Intracellular fret-based probes: A review. Methods Appl. Fluoresc. 2015, 3, 042006. [Google Scholar] [CrossRef]
- Claussen, J.C.; Hildebrandt, N.; Susumu, K.; Ancona, M.G.; Medintz, I.L. Complex logic functions implemented with quantum dot bionanophotonic circuits. ACS Appl. Mater. Interfaces 2014, 6, 3771–3778. [Google Scholar] [CrossRef] [PubMed]
- Claussen, J.C.; Algar, W.R.; Hildebrandt, N.; Susumu, K.; Ancona, M.G.; Medintz, I.L. Biophotonic logic devices based on quantum dots and temporally-staggered forster energy transfer relays. Nanoscale 2013, 5, 12156–12170. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.D.; Kim, Y.C.; Diaz, S.A.; Buckhout-White, S.; Mathur, D.; Medintz, I.L.; Melinger, J.S. Optical properties of vibronically coupled cy3 dimers on DNA scaffolds. J. Phys. Chem. B 2018, 122, 5020–5029. [Google Scholar] [CrossRef] [PubMed]
- Buckhout-White, S.; Spillmann, C.M.; Algar, W.R.; Khachatrian, A.; Melinger, J.S.; Goldman, E.R.; Ancona, M.G.; Medintz, I.L. Assembling programmable fret-based photonic networks using designer DNA scaffolds. Nat. Commun. 2014, 5, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapsford, K.E.; Tyner, K.M.; Dair, B.J.; Deschamps, J.R.; Medintz, I.L. Analyzing nanomaterial bioconjugates: A review of current and emerging purification and characterization techniques. Anal. Chem. 2011, 83, 4453–4488. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres Vidal, A.; Medintz, I.L.; Bui, H. DNA Microsystems for Biodiagnosis. Micromachines 2020, 11, 445. https://doi.org/10.3390/mi11040445
Torres Vidal A, Medintz IL, Bui H. DNA Microsystems for Biodiagnosis. Micromachines. 2020; 11(4):445. https://doi.org/10.3390/mi11040445
Chicago/Turabian StyleTorres Vidal, Alana, Igor L. Medintz, and Hieu Bui. 2020. "DNA Microsystems for Biodiagnosis" Micromachines 11, no. 4: 445. https://doi.org/10.3390/mi11040445
APA StyleTorres Vidal, A., Medintz, I. L., & Bui, H. (2020). DNA Microsystems for Biodiagnosis. Micromachines, 11(4), 445. https://doi.org/10.3390/mi11040445





