Microfluidic Formation of Honeycomb-Patterned Droplets Bounded by Interface Bilayers via Bimodal Molecular Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
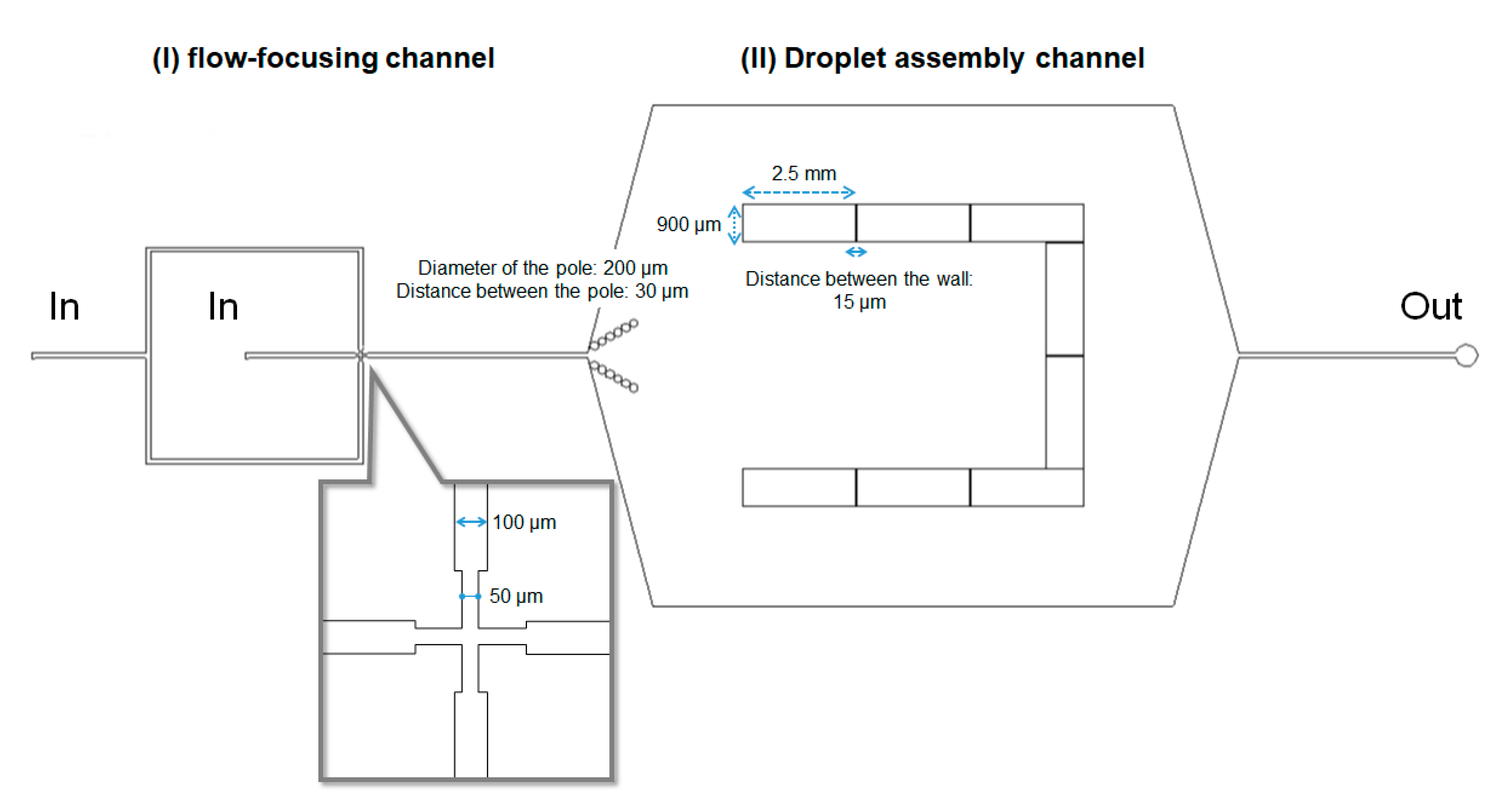
2.2. Microfluidic Device for Droplet Formation and Droplet Assembly
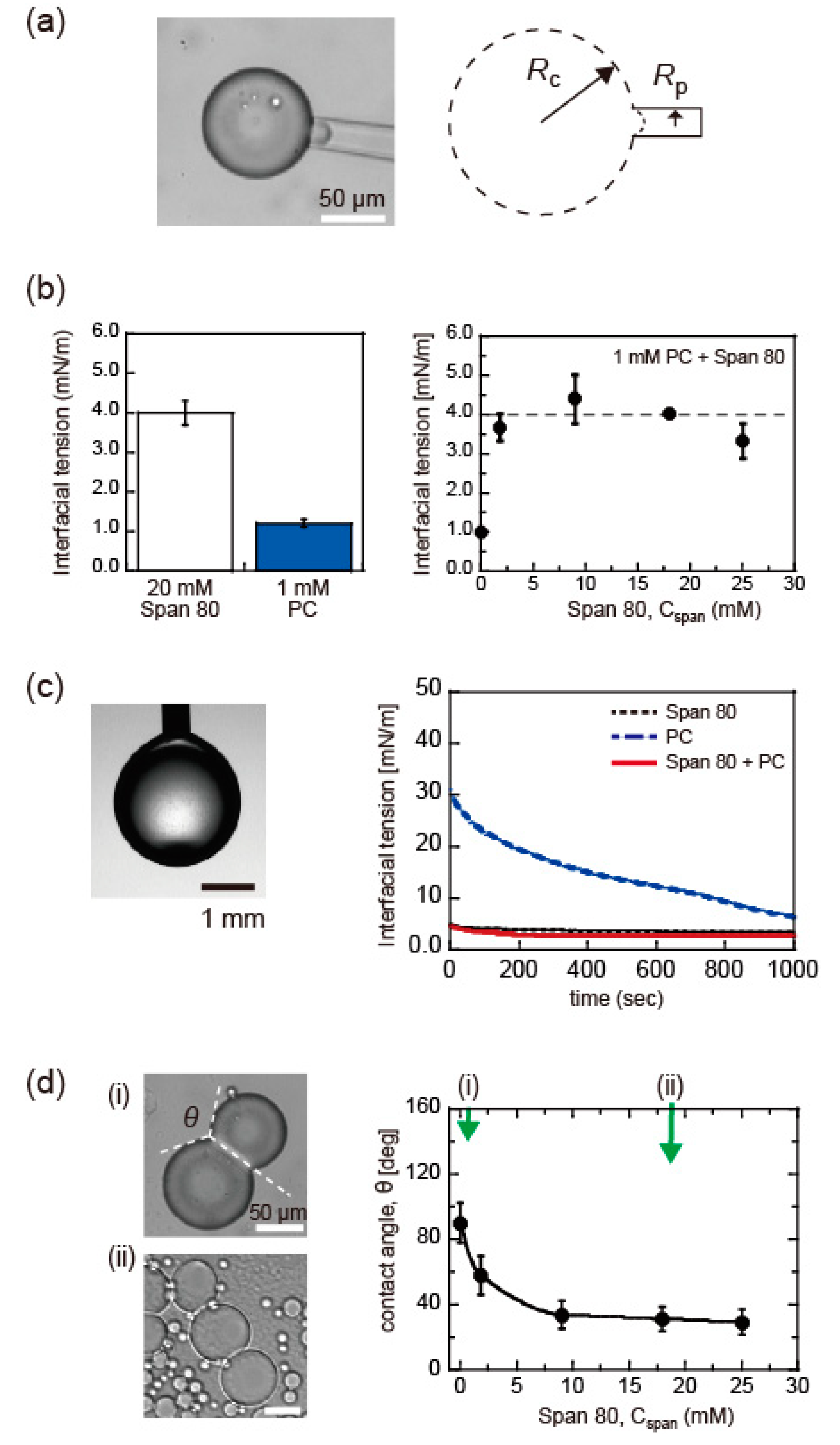
2.3. Surface Tension Measurement
2.4. Microscopic Observation of Droplets
2.5. Fluorescence Recovery after Photobleaching Experiment
3. Results and Discussion
3.1. Microfluidic Device for Formation of Honeycomb-Patterned Droplets
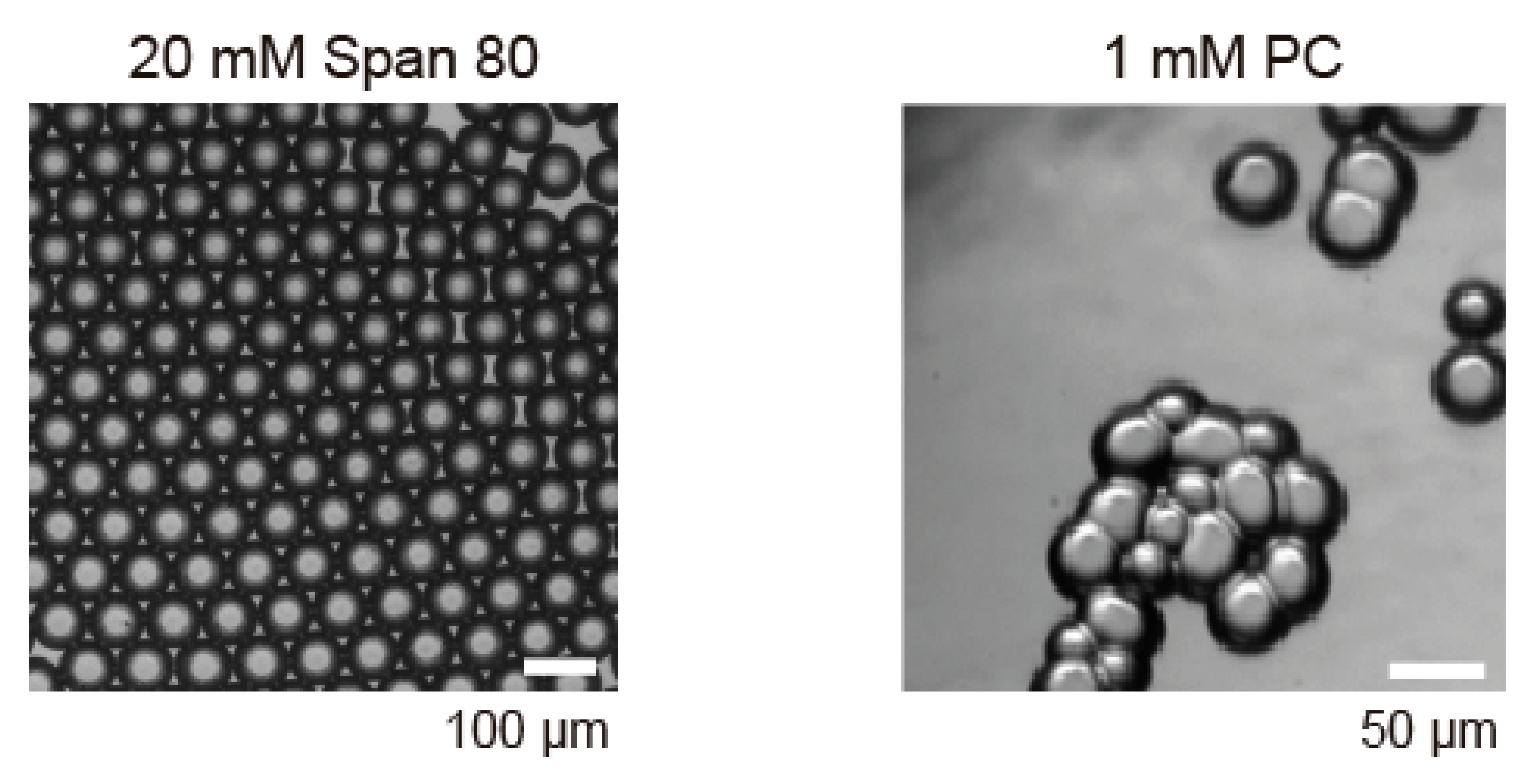
3.2. Surfactant Condition for Honeycomb Pattern Formation of DIB-Bounded Droplets
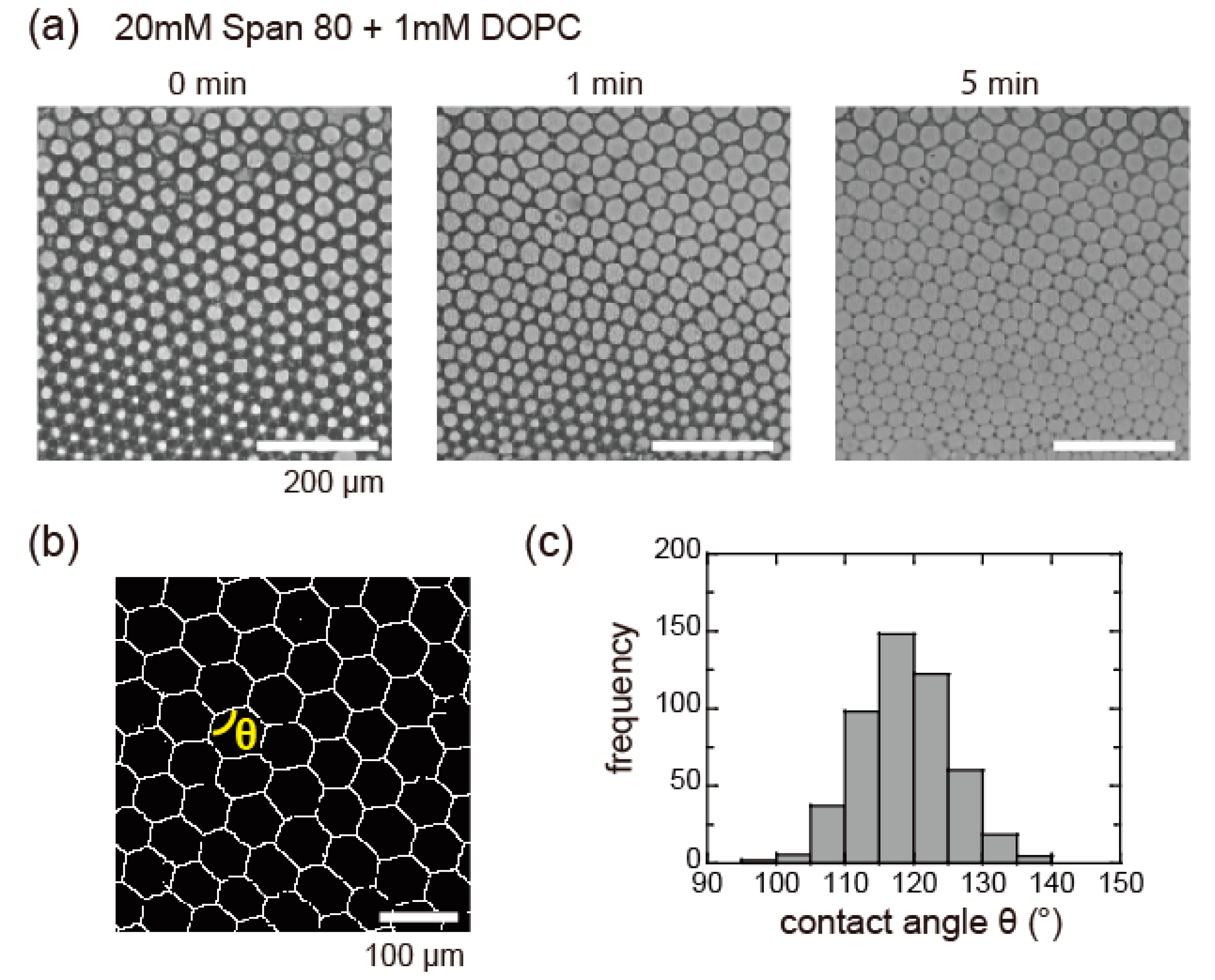
3.3. Honeycomb Pattern Formation of DIB-Bounded Droplets in Microfluidic Device
3.4. Molecular Transport through DIB-Bounded Droplets via Reconstituted Nanopores
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yanagisawa, M.; Yoshida, T.-A.; Furuta, M.; Nakata, S.; Tokita, M. Adhesive force between paired microdroplets coated with lipid monolayers. Soft Matter 2013, 9, 5891–5897. [Google Scholar] [CrossRef]
- Trantidou, T.; Friddin, M.S.; Salehi-Reyhani, A.; Ces, O.; Elani, Y. Droplet microfluidics for the construction of compartmentalised model membranes. Lab Chip 2018, 18, 2488–2509. [Google Scholar] [CrossRef] [Green Version]
- Schlicht, B.; Zagnoni, M. Droplet-interface-bilayer assays in microfluidic passive networks. Sci. Rep. 2015, 5, 9951. [Google Scholar] [CrossRef] [Green Version]
- Hwang, W.L.; Holden, M.A.; White, A.S.; Bayley, H. Electrical behavior of droplet interface bilayer networks: Experimental analysis and modeling. J. Am. Chem. Soc. 2007, 129, 11854–11864. [Google Scholar] [CrossRef]
- Wauer, T.; Gerlach, H.; Mantri, S.; Hill, J.; Bayley, H.; Sapra, T. Construction and Manipulation of Functional Three-Dimensional Droplet Networks. ACS Nano 2014, 8, 771–779. [Google Scholar] [CrossRef]
- Villar, G.; Heron, A.J.; Bayley, H. Formation of droplet networks that function in aqueous environments. Nat. Nanotechnol. 2011, 6, 803–808. [Google Scholar] [CrossRef] [Green Version]
- Funakoshi, K.; Suzuki, H.; Takeuchi, S. Lipid Bilayer Formation by Contacting Monolayers in a Microfluidic Device for Membrane Protein Analysis. Anal. Chem. 2006, 78, 8169–8174. [Google Scholar] [CrossRef]
- Murrell, M.P.; Voituriez, R.; Joanny, J.-F.; Nassoy, P.; Sykes, C.; Gardel, M.L. Liposome adhesion generates traction stress. Nat. Phys. 2014, 10, 163–169. [Google Scholar] [CrossRef]
- Franke, T.; Lipowsky, R.; Helfrich, W. Adhesion of lipid membranes induced by CrCl 3. EPL Europhys. Lett. 2006, 76, 339–345. [Google Scholar] [CrossRef]
- Egawa, H.; Furusawa, K. Liposome Adhesion on Mica Surface Studied by Atomic Force Microscopy. Langmuir 1999, 15, 1660–1666. [Google Scholar] [CrossRef]
- Akashi, K.-I.; Miyata, H.; Itoh, H.; Kinosita, K. Formation of giant liposomes promoted by divalent cations: Critical role of electrostatic repulsion. Biophys. J. 1998, 74, 2973–2982. [Google Scholar] [CrossRef] [Green Version]
- Bolognesi, G.; Friddin, M.S.; Salehi-Reyhani, A.; Barlow, N.E.; Brooks, N.J.; Ces, O.; Elani, Y. Sculpting and fusing biomimetic vesicle networks using optical tweezers. Nat. Commun. 2018, 9, 1882. [Google Scholar] [CrossRef]
- Oda, A.; Watanabe, C.; Aoki, N.; Yanagisawa, M. Liposomal adhesion via electrostatic interactions and osmotic deflation increase membrane tension and lipid diffusion coefficient. Soft Matter 2020, 16, 4549–4554. [Google Scholar] [CrossRef]
- Shimobayashi, S.F.; Mognetti, B.M.; Parolini, L.; Orsi, D.; Cicuta, P.; Di Michele, L. Direct measurement of DNA-mediated adhesion between lipid bilayers. Phys. Chem. Chem. Phys. 2015, 17, 15615–15628. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, E.; Vache, M.; Kliesch, T.-T.; Janshoff, A. Mechanical response of adherent giant liposomes to indentation with a conical AFM-tip. Soft Matter 2015, 11, 4487–4495. [Google Scholar] [CrossRef] [Green Version]
- Sugimura, K.; Ishihara, S. The mechanical anisotropy in a tissue promotes ordering in hexagonal cell packing. Development 2013, 140, 4091–4101. [Google Scholar] [CrossRef] [Green Version]
- Theberge, A.B.; Courtois, F.; Schaerli, Y.; Fischlechner, M.; Abell, C.; Hollfelder, F.; Huck, W. Microdroplets in Microfluidics: An Evolving Platform for Discoveries in Chemistry and Biology. Angew. Chem. Int. Ed. 2010, 49, 5846–5868. [Google Scholar] [CrossRef]
- Mantri, S.; Sapra, K.T.; Cheley, S.; Sharp, T.H.; Bayley, H. An engineered dimeric protein pore that spans adjacent lipid bilayers. Nat. Commun. 2013, 4, 1725. [Google Scholar] [CrossRef] [Green Version]
- Villar, G.; Graham, A.D.; Bayley, H. A Tissue-Like Printed Material. Science 2013, 340, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Graham, A.D.; Olof, S.N.; Burke, M.J.; Armstrong, J.P.; Mikhailova, E.A.; Nicholson, J.G.; Box, S.J.; Szele, F.G.; Perriman, A.W.; Bayley, H. High-Resolution Patterned Cellular Constructs by Droplet-Based 3D Printing. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Gai, Y.; Leong, C.M.; Cai, W.; Tang, S.K. Spatiotemporal periodicity of dislocation dynamics in a two-dimensional microfluidic crystal flowing in a tapered channel. Proc. Natl. Acad. Sci. USA 2016, 113, 12082–12087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatch, A.C.; Fisher, J.S.; Pentoney, S.L.; Yang, D.L.; Lee, A.P. Tunable 3D droplet self-assembly for ultra-high-density digital micro-reactor arrays. Lab Chip 2011, 11, 2509. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, M.; He, T.; Zhou, G.; Shui, L. Microfluidic Induced Controllable Microdroplets Assembly in Confined Channels. Micromachines 2015, 6, 1331–1345. [Google Scholar] [CrossRef]
- Morimoto, Y.; Takeuchi, S. Three-dimensional cell culture based on microfluidic techniques to mimic living tissues. Biomater. Sci. 2013, 1, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, M.; Umeyama, S.; Nagai, K.H.; Onoe, H.; Takinoue, M. Controlled Construction of Stable Network Structure Composed of Honeycomb-Shaped Microhydrogels. Life 2018, 8, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrandt, E.; Nirschl, H.; Kok, R.J.; Leneweit, G. Adsorption of phospholipids at oil/water interfaces during emulsification is controlled by stress relaxation and diffusion. Soft Matter 2018, 14, 3730–3737. [Google Scholar] [CrossRef]
- Abkarian, M.; Loiseau, E.; Massiera, G. Continuous droplet interface crossing encapsulation (cDICE) for high throughput monodisperse vesicle design. Soft Matter 2011, 7, 4610. [Google Scholar] [CrossRef]
- Shoji, K.; Kawano, R. Microfluidic Formation of Double-Stacked Planar Bilayer Lipid Membranes by Controlling the Water-Oil Interface. Micromachines 2018, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Mijatović, D.; Eijkel, J.C.T.; Berg, A.V.D. Technologies for nanofluidic systems: Top-down vs. bottom-up—a review. Lab Chip 2005, 5, 492. [Google Scholar] [CrossRef]
- Matsumoto, S.; Kohda, M.; Murata, S.-I. Preparation of lipid vesicles on the basis of a technique for providing W/O/W emulsions. J. Colloid Interface Sci. 1977, 62, 149–157. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, Y.; Chen, Q.; Li, G.; Yao, S. A facile microfluidic strategy for measuring interfacial tension. Appl. Phys. Lett. 2013, 103, 234102. [Google Scholar] [CrossRef]
- Jeong, D.-W.; Jang, H.; Choi, S.Q.; Choi, M.C. Enhanced stability of freestanding lipid bilayer and its stability criteria. Sci. Rep. 2016, 6, 38158. [Google Scholar] [CrossRef] [Green Version]
- Baret, J.-C. Surfactants in droplet-based microfluidics. Lab Chip 2012, 12, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.; Kuroiwa, T.; Kagota, T.; Nakajima, M.; Sato, S.; Mukataka, S.; Walde, A.P.; Ichikawa, S. Novel Method for Obtaining Homogeneous Giant Vesicles from a Monodisperse Water-in-Oil Emulsion Prepared with a Microfluidic Device. Langmuir 2008, 24, 4581–4588. [Google Scholar] [CrossRef]
- Vazquez, R.F.; Maté, S.M.; Bakás, L.S.; Fernández, M.M.; Malchiodi, E.L.; Herlax, V. Novel evidence for the specific interaction between cholesterol and α-haemolysin of Escherichia coli. Biochem. J. 2014, 458, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Hilburger, C.E.; Jacobs, M.L.; Lewis, K.R.; Peruzzi, J.A.; Kamat, N.P. Controlling Secretion in Artificial Cells with a Membrane AND Gate. ACS Synth. Boil. 2019, 8, 1224–1230. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujiwara, S.; Shoji, K.; Watanabe, C.; Kawano, R.; Yanagisawa, M. Microfluidic Formation of Honeycomb-Patterned Droplets Bounded by Interface Bilayers via Bimodal Molecular Adsorption. Micromachines 2020, 11, 701. https://doi.org/10.3390/mi11070701
Fujiwara S, Shoji K, Watanabe C, Kawano R, Yanagisawa M. Microfluidic Formation of Honeycomb-Patterned Droplets Bounded by Interface Bilayers via Bimodal Molecular Adsorption. Micromachines. 2020; 11(7):701. https://doi.org/10.3390/mi11070701
Chicago/Turabian StyleFujiwara, Shougo, Kan Shoji, Chiho Watanabe, Ryuji Kawano, and Miho Yanagisawa. 2020. "Microfluidic Formation of Honeycomb-Patterned Droplets Bounded by Interface Bilayers via Bimodal Molecular Adsorption" Micromachines 11, no. 7: 701. https://doi.org/10.3390/mi11070701
APA StyleFujiwara, S., Shoji, K., Watanabe, C., Kawano, R., & Yanagisawa, M. (2020). Microfluidic Formation of Honeycomb-Patterned Droplets Bounded by Interface Bilayers via Bimodal Molecular Adsorption. Micromachines, 11(7), 701. https://doi.org/10.3390/mi11070701




