Comprehensive Understanding of Silicon-Nanowire Field-Effect Transistor Impedimetric Readout for Biomolecular Sensing
Abstract
1. Introduction
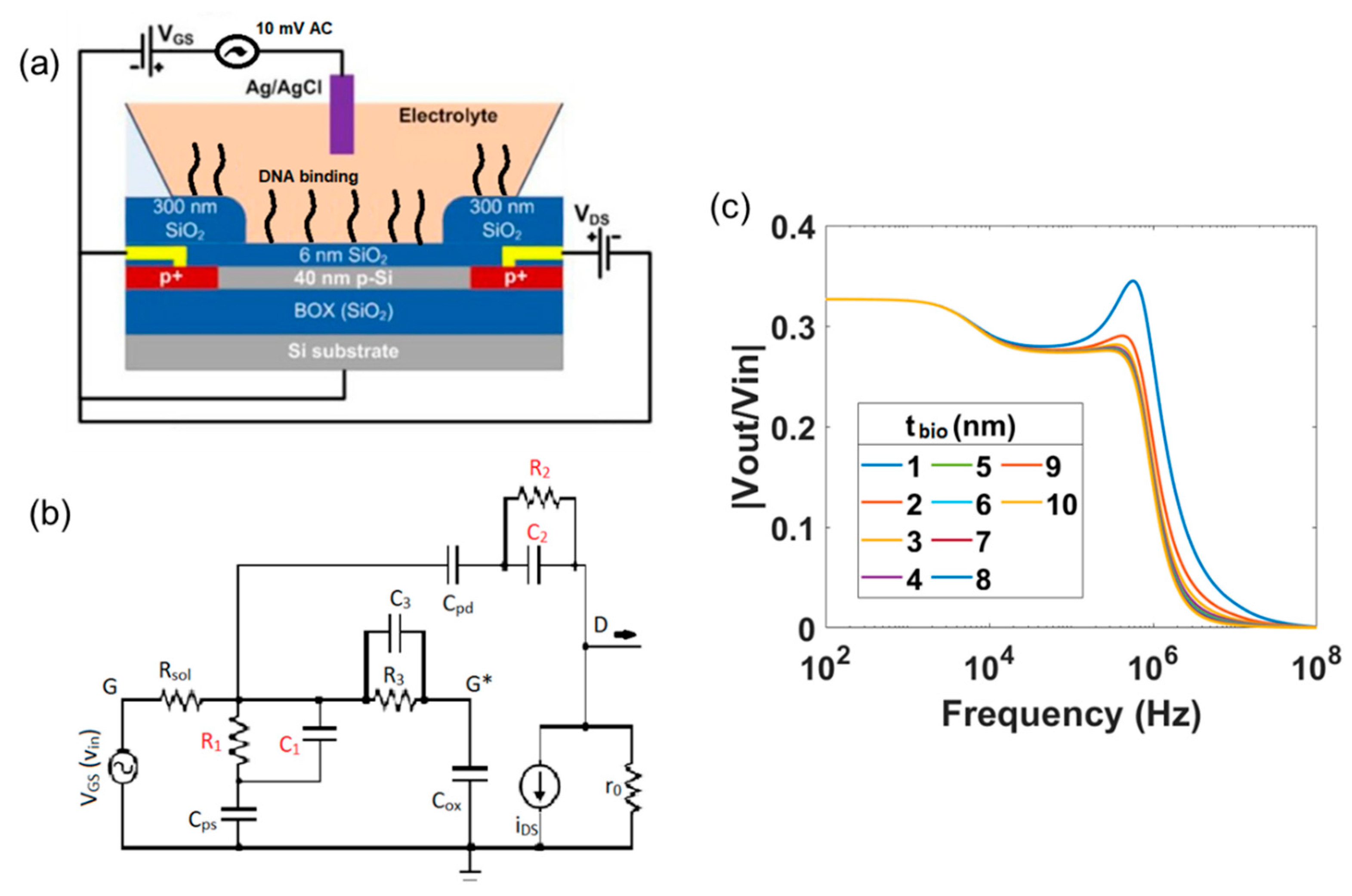
2. Electronic Circuit Model for SiNW-FET and Readout Apparatus
3. Mathematical Formulation of Transfer Function
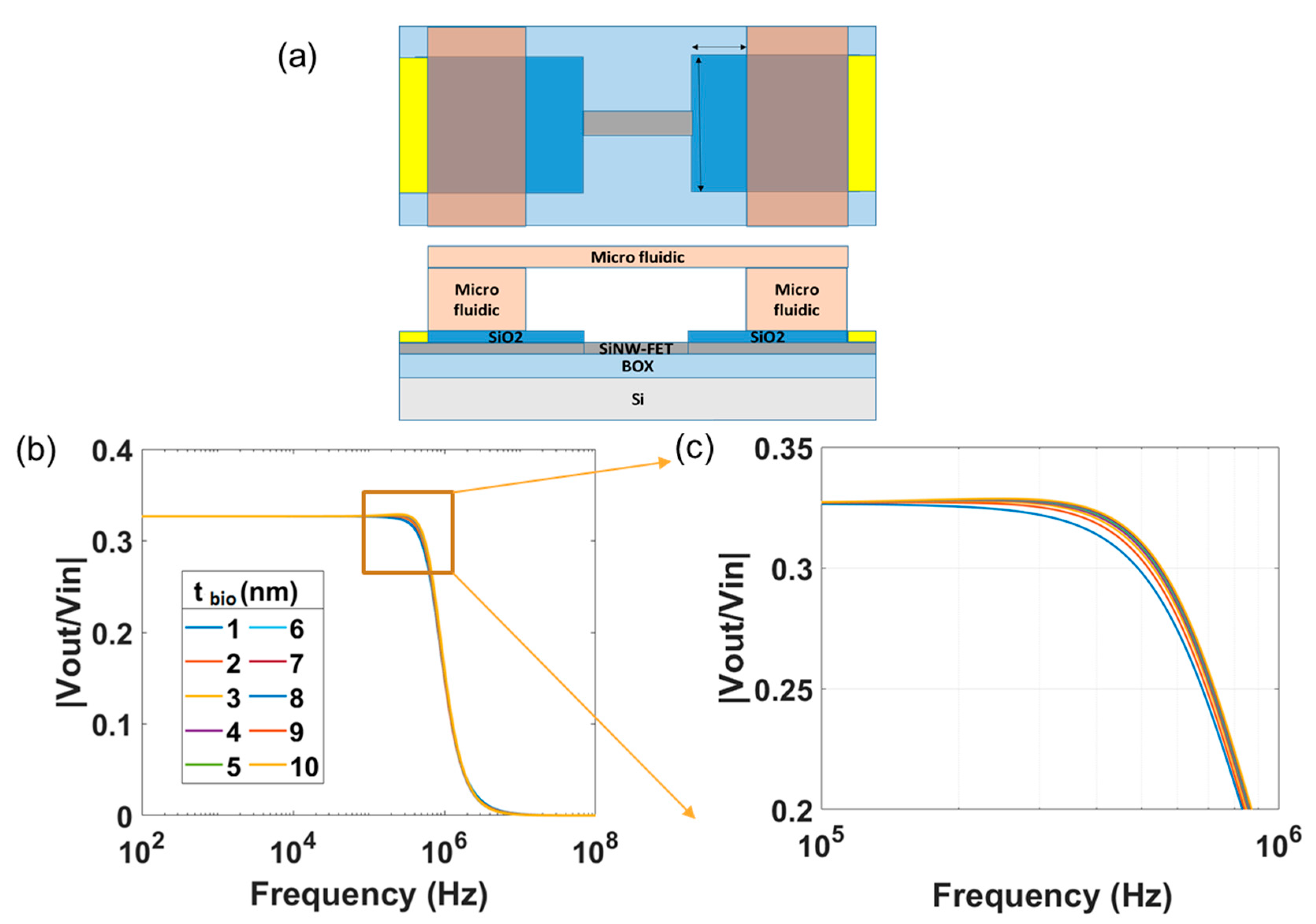
4. Investigation of the Effect of the Device Geometries and of the Electrolyte Solution on the Transfer Function
5. Impedimetric Detection of Biomolecules Using SiNW
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Xj | Junction depth |
| Vsat | Saturation velocity |
| U0 | Mobility |
| Nch | Doping concentration near channel interface |
| Vth0 | Threshold voltage |
| A0 | Bulk charge coefficient |
| A2 | Second non-saturation factor |
| Cpd | Parasitic capacitance at drain contact |
| Cps | Parasitic capacitance at source contact |
| Rsol | Resistance of electrolyte |
| tox | Oxide thickness |
| Cox | Oxide capacitance |
| Aox | Gate-oxide interface area |
| εox | Permittivity of oxide |
| gm | Transconductance parameter |
| Cfb | Feedback capacitance |
| Rfb | Feedback resistance |
| Cbio | Capacitance due to biomolecules |
| Rbio | Resistance due to biomolecules |
| tbio | Thickness of biomolecular layer |
| κbio | Relative permittivity of biomolecules |
| εbio | Permittivity of biomolecules |
| γ | Capacitance per unit area of biomolecules |
| λ | Resistance per unit area of biomolecules |
| A | Area of contact with source or drain |
| d | Thickness of passivation layer |
References
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, 17, 70–71. [Google Scholar] [CrossRef]
- Mu, L.; Chang, Y.; Sawtelle, S.D.; Wipf, M.; Duan, X.; Reed, M.A. Silicon nanowire field-effect transistors—A versatile class of potentiometric nanobiosensors. IEEE Access 2015, 3, 287–302. [Google Scholar] [CrossRef]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.; Lieber, C.; Zheng, G.F.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Stern, E.; Klemic, J.; Routenberg, D.; Wyrembak, P.; Turner-Evans, D.; Hamilton, A.; LaVan, D.; Fahmy, T.; Reed, M. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-I.; Li, B.-R.; Chen, Y.-T. Silicon nanowire field-effect transistor-based biosensors for biomedical diagnosis and cellular recording investigation. Nano Today 2011, 6, 131–154. [Google Scholar] [CrossRef]
- Makowski, M.; Ivanisevic, A. Molecular analysis of blood with micro/nano scale field effect transistor biosensors. Small 2011, 7, 1863–1875. [Google Scholar] [CrossRef]
- Tran, D.; Winter, M.; Yang, C.-T.; Stockmann, R.; Offenhäusser, A.; Thierry, B. Silicon nanowires field effect transistors: A comparative sensing performance between electrical impedance and potentiometric measurement paradigms. Anal. Chem. 2019, 91, 12568–12573. [Google Scholar] [CrossRef]
- Tran, D.; Winter, M.; Wolfrum, B.; Stockmann, R.; Yang, C.-T.; Moghaddam, M.; Offenhäusser, A.; Thierry, B. Toward intraoperative detection of disseminated tumor cells in lymph nodes with silicon nanowire field effect transistors. ACS Nano 2016, 10, 2357–2364. [Google Scholar] [CrossRef]
- Gao, A.; Lu, N.; Dai, P.; Li, T.; Pei, H.; Gao, X.; Gong, Y.; Wang, Y.; Fan, C. Silicon-nanowire-based CMOS-compatible field-effect transistor nanosensors for ultrasensitive electrical detection of nucleic acids. Nano Lett. 2011, 11, 3974–3978. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.F.; Hayden, O.; Lakadamyali, M.; Zhuang, X.W.; Lieber, C.M. Electrical detection of single viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar] [CrossRef] [PubMed]
- Rani, D.; Pachauri, V.; Mueller, A.; Vu, X.T.; Nguyen, T.C.; Ingebrandt, S. On the use of scalable nanoISFET arrays of silicon with highly reproducible sensor performance for biosensor applications. ACS Omega 2016, 1, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Nguyen, T.C.; Vu, X.T.; Weil, M.; Wilhelm, J.; Wagner, P.; Thoelen, R.; Ingebrandt, S. DNA detection with top-down fabricated silicon nanowire transistor arrays in linear operation regime. Phys. Status Solidi A 2016, 213, 1510–1519. [Google Scholar] [CrossRef]
- Duy Hien, T.; Chen, S.; Wiel, W.; Carlen, E.; Van den Berg, A. Novel top-down wafer-scale fabrication of single crystal silicon nanowires. Nano Lett. 2009, 9, 1015–1022. [Google Scholar]
- Kaisti, M. Detection principles of biological and chemical FET sensors. Biosens. Bioelectron. 2017, 98, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Shinwari, M.W.; Deen, M.J.; Landheer, D. Study of the electrolyte-insulator-semiconductor field-effect transistor (EISFET) with applications in biosensor design. Microelectron. Reliab. 2007, 47, 2025–2057. [Google Scholar] [CrossRef]
- Lee, J.; Jang, J.; Choi, B.; Yoon, J.; Kim, J.Y.; Choi, Y.K.; Kim, D.M.; Kim, D.H.; Choi, S.J. A highly responsive silicon nanowire/amplifier MOSFET hybrid biosensor. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- McKinnon, W.R.; Landheer, D.; Aers, G. Sensitivity of field-effect biosensors to charge, pH, and ion concentration in a membrane model. J. Appl. Phys. 2008, 104, 124701. [Google Scholar] [CrossRef]
- Vu, X.T.; Stockmann, R.; Wolfrum, B.; Offenhaesser, A.; Ingebrandt, S. Fabrication and application of a microfluidic-embedded silicon nanowire biosensor chip. Phys. Status Solidi A 2010, 207, 850–857. [Google Scholar] [CrossRef]
- Khodadadian, A.; Stadlbauer, B.; Heitzinger, C. Bayesian inversion for nanowire field-effect sensors. J. Comput. Electron. 2020, 19, 147–159. [Google Scholar] [CrossRef]
- Mirsian, S.; Khodadadian, A.; Hedayati, M.; Manzour-ol-Ajdad, A.; Kalantarinejad, R.; Heitzinger, C. A new method for selective functionalization of silicon nanowire sensors and Bayesian inversion for its parameters. Biosens. Bioelectron. 2019, 142, 111527. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Wagner, R.; Sigworth, F.J.; Breaker, R.; Fahmy, T.M.; Reed, M.A. Importance of the Debye screening length on nanowire field effect transistor sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-J.; Zhang, G.; Chua, J.; Chee, R.-E.; Wong, E.H.; Agarwal, A.; Buddharaju, K.; Singh, N.; Gao, Z.; Balasubramanian, N. DNA Sensing by silicon nanowire: Charge layer distance dependence. Nano Lett. 2008, 8, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Landheer, D.; McKinnon, W.R.; Jiang, W.H.; Aers, G. Effect of screening on the sensitivity of field-effect devices used to detect oligonucleotides. Appl. Phys. Lett. 2008, 92, 253901. [Google Scholar] [CrossRef]
- Dill, K.; Bromberg, S. Molecular Driving Forces: Statistical Thermodynamics in Biology, Chemistry, Physics, and Nanoscience; Garland Science: New York, NY, USA, 2012. [Google Scholar]
- Nguyen, T.C.; Vu, X.T.; Freyler, M.; Ingebrandt, S. PSPICE model for silicon nanowire field-effect transistor biosensors in impedimetric measurement mode. Phys. Status Solidi A 2013, 210, 870–876. [Google Scholar] [CrossRef]
- Schwartz, M.; Nguyen, T.C.; Vu, X.T.; Wagner, P.; Thoelen, R.; Ingebrandt, S. Impedimetric sensing of DNA with silicon nanowire transistors as alternative transducer principle. Phys. Status Solidi A 2018, 215, 1700740. [Google Scholar] [CrossRef]
- Ingebrandt, S.; Han, Y.; Nakamura, F.; Poghossian, A.; Schoning, M.J.; Offenhausser, A. Label-free detection of single nucleotide polymorphisms utilizing the differential transfer function of field-effect transistors. Biosens. Bioelectron. 2007, 22, 2834–2840. [Google Scholar] [CrossRef]
- Koppenhofer, D.; Susloparova, A.; Law, J.K.Y.; Vu, X.T.; Ingebrandt, S. Electronic monitoring of single cell-substrate adhesion events with-quasi-planar field-effect transistors. Sens. Actuators B Chem. 2015, 210, 776–783. [Google Scholar] [CrossRef]
- Susloparova, A.; Koppenhofer, D.; Law, J.K.Y.; Vu, X.T.; Ingebrandt, S. Electrical cell-substrate impedance sensing with field-effect transistors is able to unravel cellular adhesion and detachment processes on a single cell level. Lab Chip 2015, 15, 668–679. [Google Scholar] [CrossRef]
- Susloparova, A.; Vu, X.T.; Koppenhofer, D.; Law, J.K.Y.; Ingebrandt, S. Investigation of ISFET device parameters to optimize for impedimetric sensing of cellular adhesion. Phys. Status Solidi A 2014, 211, 1395–1403. [Google Scholar] [CrossRef]
- Uslu, F.; Ingebrandt, S.; Mayer, D.; Bocker-Meffert, S.; Odenthal, M.; Offenhausser, A. Labelfree fully electronic nucleic acid detection system based on a field-effect transistor device. Biosens. Bioelectron. 2004, 19, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Crescentini, M.; Rossi, M.; Ashburn, P.; Lombardini, M.; Sangiorgi, E.; Morgan, H.; Tartagni, M. AC and phase sensing of nanowires for biosensing. Biosensors 2016, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.C. Readout Concepts for Label-Free Biomolecule Detection with Advanced ISFET and Silicon Nanowire Biosensors; Technische Universität Kaiserslautern: Kaiserslautern, Germany, 2018. [Google Scholar]
- Sedra, A.S.; Smith, K.C. Microelectronic Circuits; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Daniels, J.; Pourmand, N. Label-free impedance biosensors: Opportunities and challenges. Electroanalysis 2007, 19, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Rani, D. Label-Free Detection of Biomolecules Using Silicon Nanowire Ion-sensitive Field-Effect Transistor Devices. Ph.D. Thesis, Justus-Liebig-Universität Gießen, Giessen, Germany, 2017. [Google Scholar]
- Cuervo, A.; Dans, P.D.; Carrascosa, J.L.; Orozco, M.; Gomila, G.; Fumagalli, L. Direct measurement of the dielectric polarization properties of DNA. Proc. Natl. Acad. Sci. USA 2014, 111, E3624–E3630. [Google Scholar] [CrossRef] [PubMed]

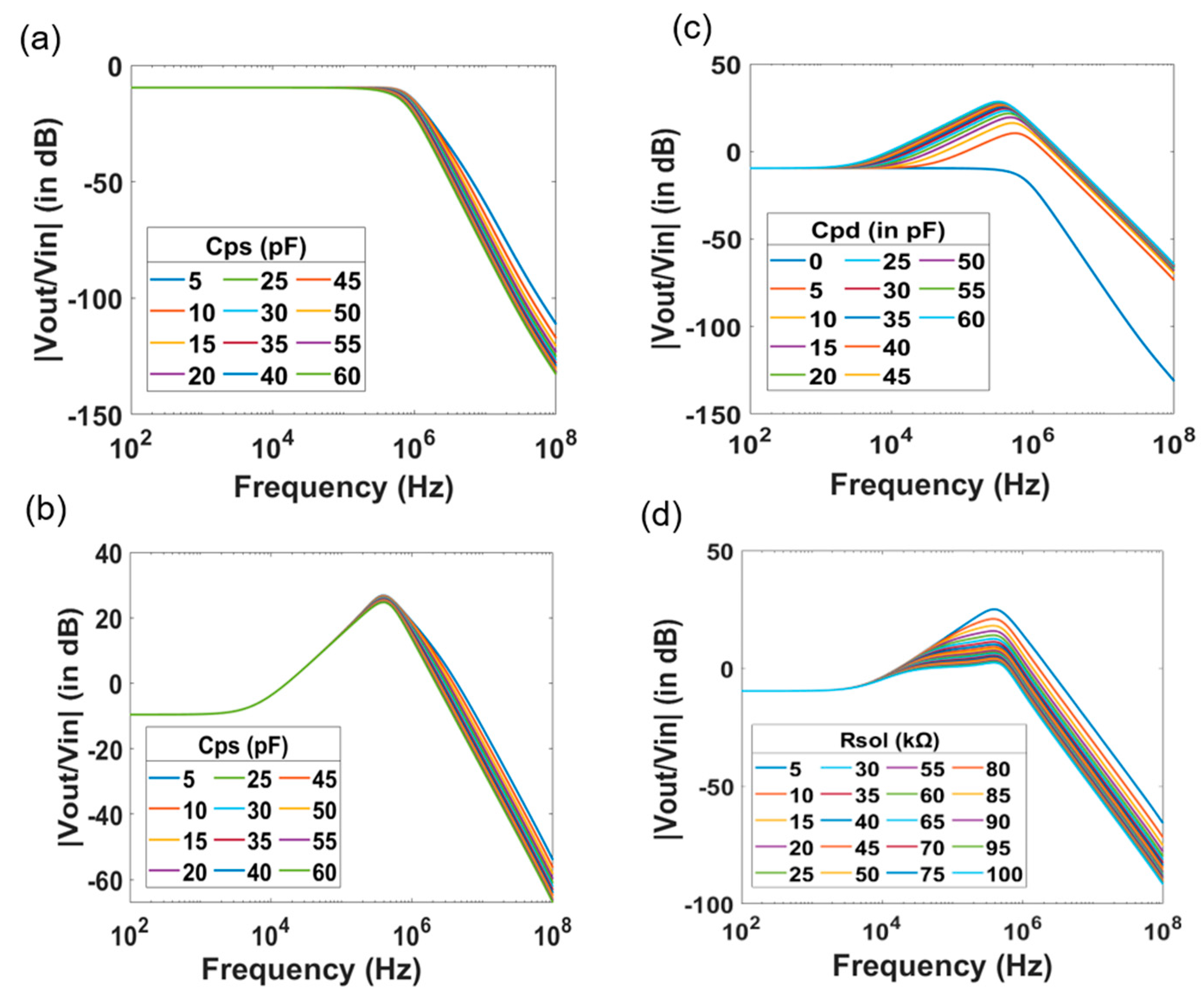
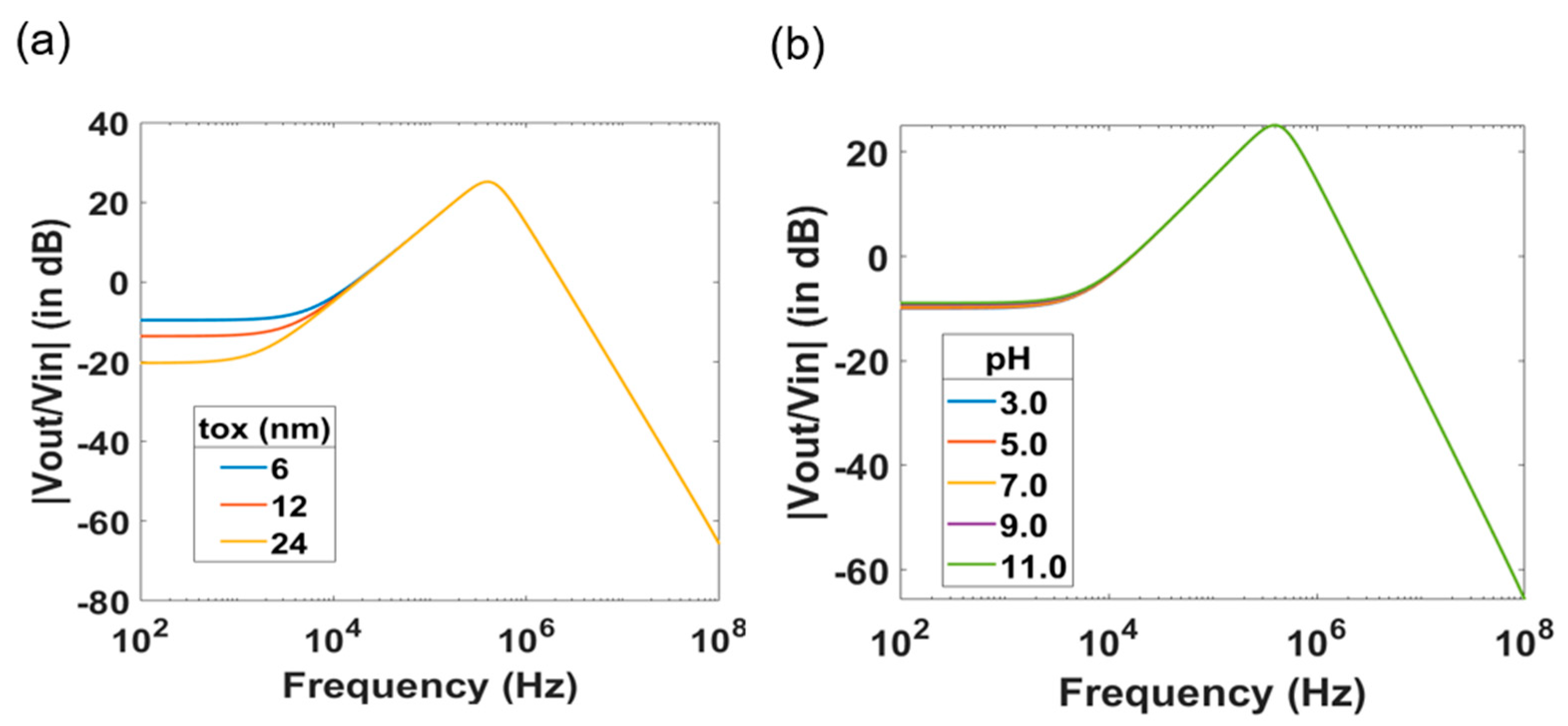
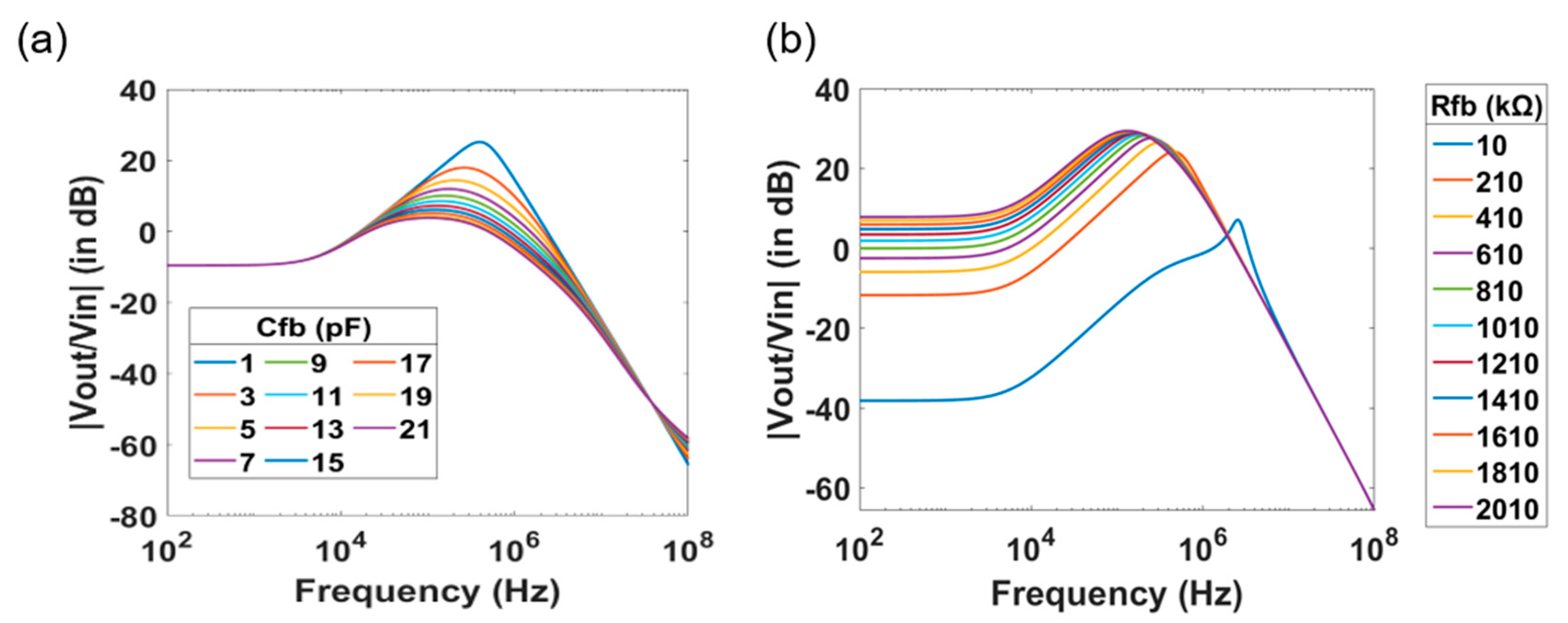

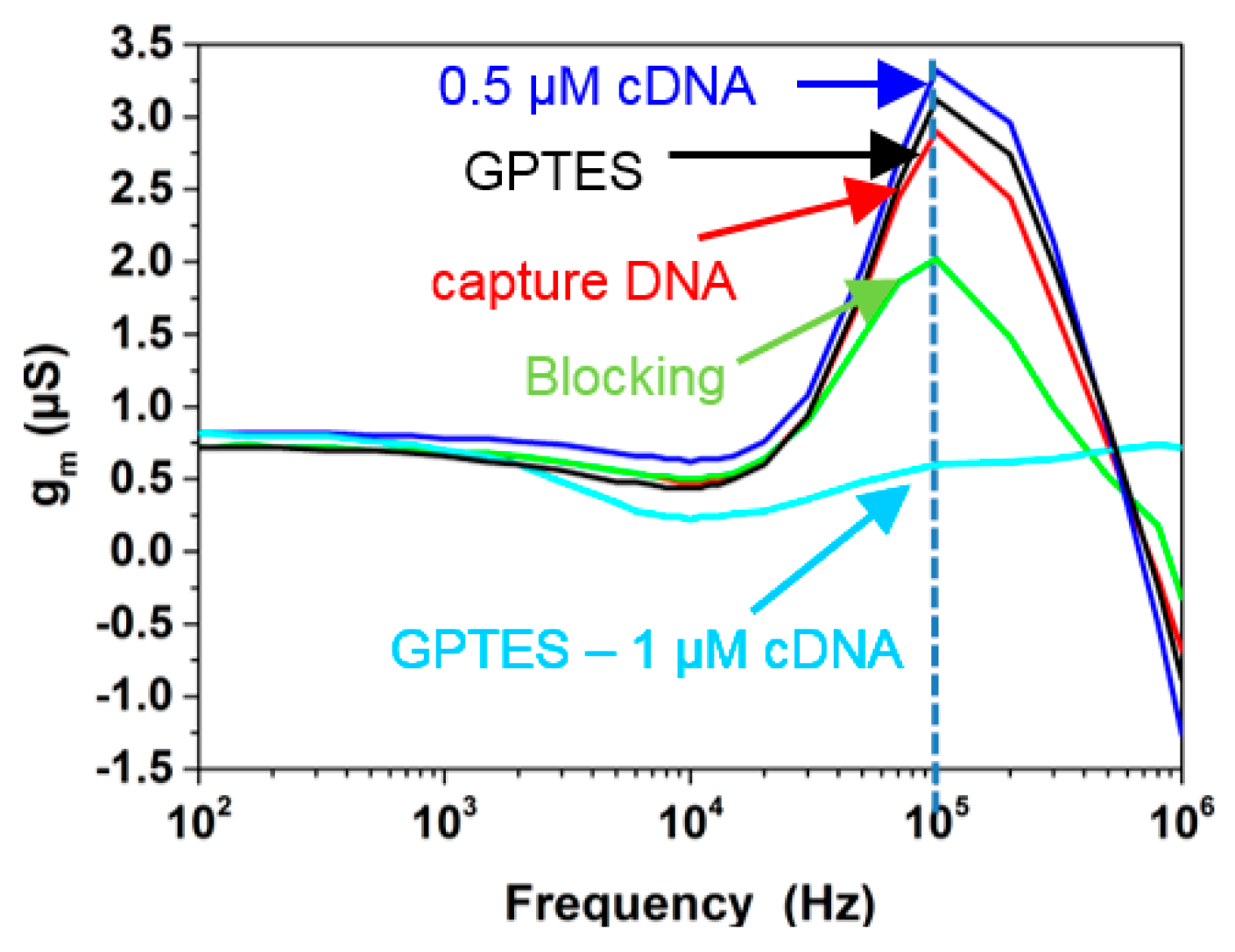
| Parameter | Value |
|---|---|
| Width | 370 nm |
| Length | 10 μm |
| tsi | 50 nm |
| tox | 6 nm |
| Vth0 | −0.4 V |
| U0 | 115 cm2/Vs |
| Vsat | 80,000 |
| Nch | 1 × 1017 cm−3 |
| A0 | −0.55 |
| A2 | 1.08 |
| Parameter | Value |
|---|---|
| 270 kΩ | |
| 1 pF | |
| 5 kΩ | |
| 50 pF | |
| 33 pF | |
| pH | 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharjee, A.; Nguyen, T.C.; Pachauri, V.; Ingebrandt, S.; Vu, X.T. Comprehensive Understanding of Silicon-Nanowire Field-Effect Transistor Impedimetric Readout for Biomolecular Sensing. Micromachines 2021, 12, 39. https://doi.org/10.3390/mi12010039
Bhattacharjee A, Nguyen TC, Pachauri V, Ingebrandt S, Vu XT. Comprehensive Understanding of Silicon-Nanowire Field-Effect Transistor Impedimetric Readout for Biomolecular Sensing. Micromachines. 2021; 12(1):39. https://doi.org/10.3390/mi12010039
Chicago/Turabian StyleBhattacharjee, Abhiroop, Thanh Chien Nguyen, Vivek Pachauri, Sven Ingebrandt, and Xuan Thang Vu. 2021. "Comprehensive Understanding of Silicon-Nanowire Field-Effect Transistor Impedimetric Readout for Biomolecular Sensing" Micromachines 12, no. 1: 39. https://doi.org/10.3390/mi12010039
APA StyleBhattacharjee, A., Nguyen, T. C., Pachauri, V., Ingebrandt, S., & Vu, X. T. (2021). Comprehensive Understanding of Silicon-Nanowire Field-Effect Transistor Impedimetric Readout for Biomolecular Sensing. Micromachines, 12(1), 39. https://doi.org/10.3390/mi12010039







