The Effect of the Layered Internal Structure of Fibrous Beds on the Hydrodynamic Diffusive Behavior of Microparticles
Abstract
:1. Introduction
2. Calculation Methods
2.1. Stokesian Dynamics
2.2. Repulsive Force Acting on Microparticles and Fiber Particles
2.3. Calculation Conditions
3. Results
3.1. Permeation and Capture of Microparticles through Fibrous Bed
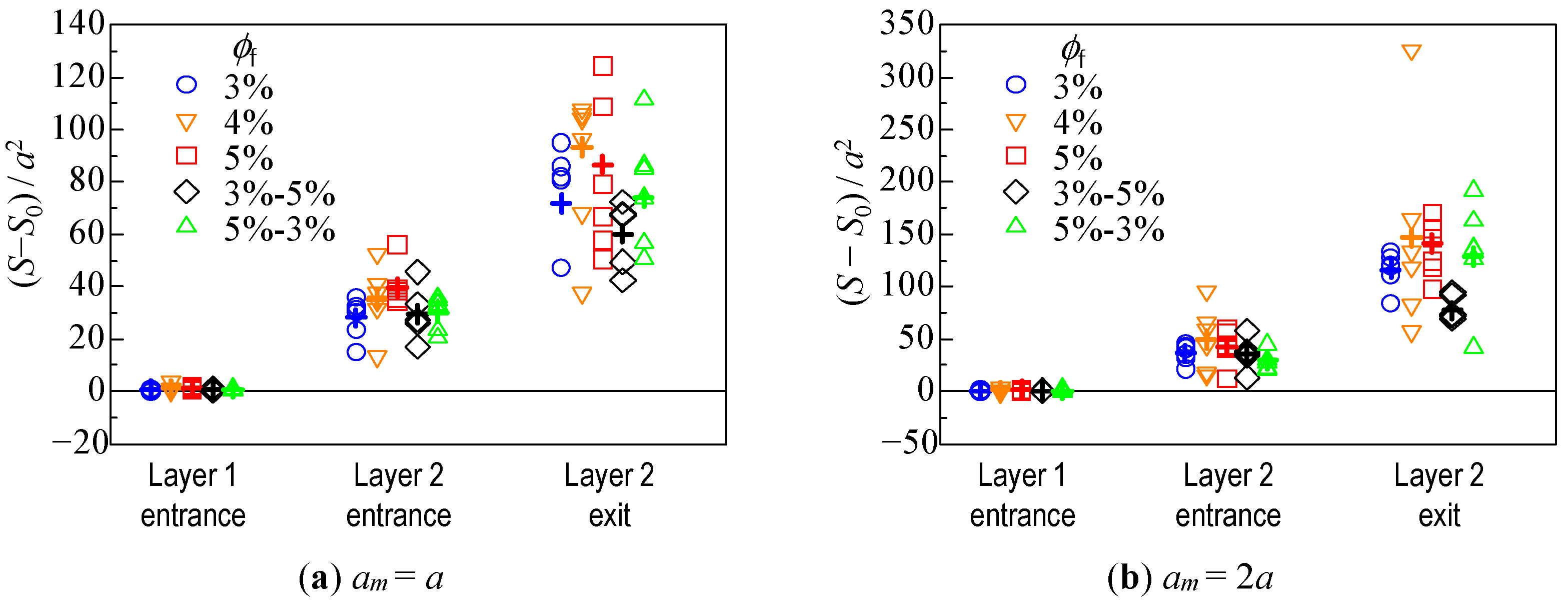
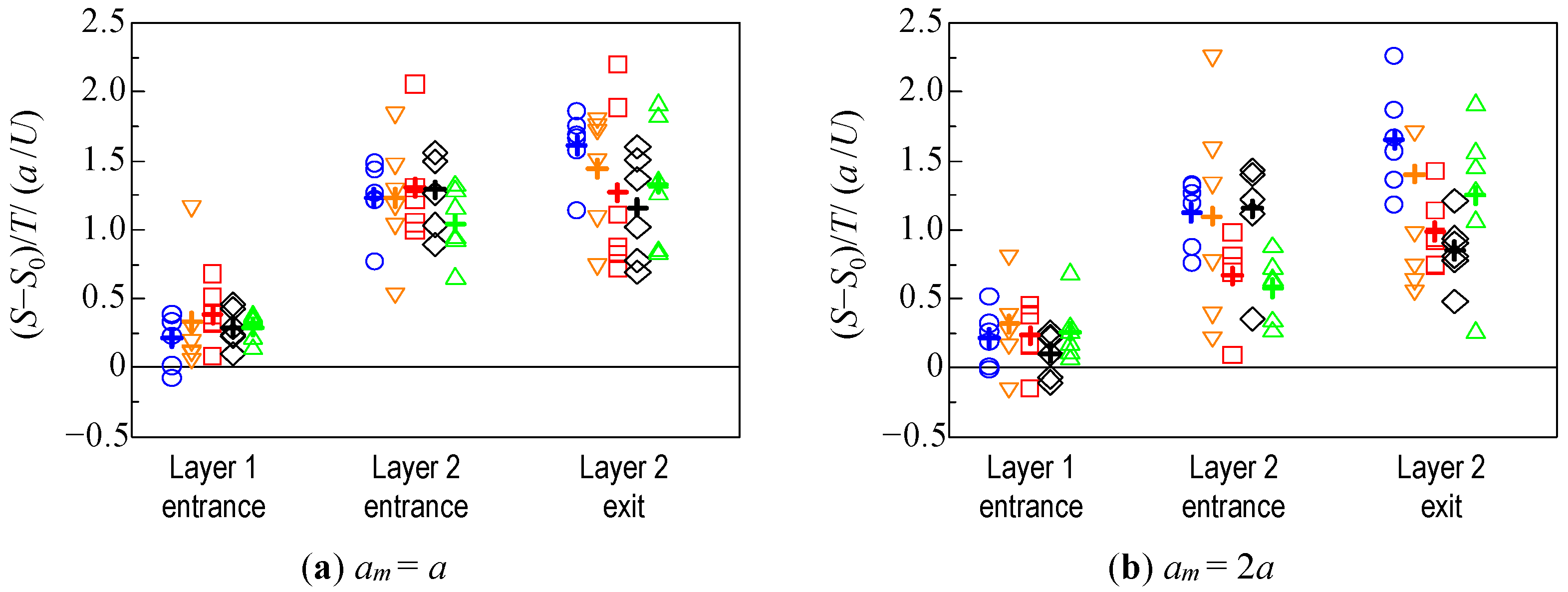
3.2. Hydrodynamic Diffusion of Microparticles
3.2.1. Permeation Time and Distance Traveled by Particles
3.2.2. Velocity in the Travel Direction
3.2.3. Change in Dispersion of Particles Due to Hydrodynamic Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lenshof, A.; Laurell, T. Continuous separation of cells and particles in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.R.; Cox, E.C.; Austin, R.H.; Sturm, J.C. Continuous particle separation through deterministic lateral displacement. Science 2004, 304, 987–990. [Google Scholar] [CrossRef]
- Damit, B.; Lee, C.; Wu, C.-Y. Flash infrared radiation disinfection of fibrous filters contaminated with bioaerosols. J. Appl. Microbiol. 2011, 110, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Javadpour, F.; Jeje, A. Modeling filtration of platelet-rich plasma in fibrous filters. Transp. Porous Media 2012, 91, 677–696. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Martino, A.D.; Marcacci, M. Platelet-rich plasma (PRP) to treat sports injuries: Evidence to support its use. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 516–527. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Orive, G.; Andia, I. The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 2007, 28, 4551–4560. [Google Scholar] [CrossRef]
- Erikson, A.; Andersen, H.N.; Naess, S.N.; Sikorski, P.; Davies, C.D.L. Physical and chemical modifications of collagen gels: Impact on diffusion. Biopolymers 2007, 89, 135–143. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Frimpong, B.D.; Munn, L.L.; Jain, R.K. Diffusion anisotropy in collagen gels and tumors: The effect of fiber network orientation. Biophys. J. 2010, 99, 3119–3128. [Google Scholar] [CrossRef] [Green Version]
- Phillips, R.J.; Deen, W.M.; Brady, J.F. Hindered transport in fibrous membranes and gels: Effect of solute size and fiber configuration. J. Colloid Interface Sci. 1990, 139, 363–373. [Google Scholar] [CrossRef]
- Nicolai, H.; Herzhaft, B.; Hinch, E.J.; Oger, L.; Guazzelli, E. Particle velocity fluctuations and hydrodynamic self-diffusion of sedimenting non-Brownian spheres. Phys. Fluids 1995, 7, 12–23. [Google Scholar] [CrossRef]
- Guazzelli, E.; Hinch, J. Fluctuations and Instability in Sedimentation. Annu. Rev. Fluid Mech. 2011, 43, 97–116. [Google Scholar] [CrossRef] [Green Version]
- Harada, S.; Otomo, R. Diffusive behavior of a thin particle layer in fluid by hydrodynamic interaction. Phys. Rev. E 2009, 80, 066311. [Google Scholar] [CrossRef] [Green Version]
- Jackson, G.W.; James, D.F. The permeability of fibrous porous media. Can. J. Chem. Eng. 1986, 64, 364–374. [Google Scholar] [CrossRef]
- Sangani, A.S.; Acrivos, A. Slow flow past periodic arrays of cylinders with application to heat transfer. Int. J. Multiph. Flow 1982, 8, 193–206. [Google Scholar] [CrossRef]
- Drummond, J.E.; Tahir, M.I. Laminar viscous flow through regular arrays of parallel solid cylinders. Int. J. Multiph. Flow 1984, 10, 515–540. [Google Scholar] [CrossRef]
- Clague, D.S.; Phillips, R.J. A numerical calculation of the hydraulic permeability of three-dimensional disordered fibrous media. Phys. Fluids 1997, 9, 1562–1572. [Google Scholar] [CrossRef]
- Otomo, R.; Mori, K. Numerical analysis on motion of microparticles passing through straight and tortuous fibrous layers. Fluid Dyn. Res. 2021, 53, 025503. [Google Scholar] [CrossRef]
- Shou, D.; Fan, J.; Zhang, H.; Qian, X.; Zhang, Z. Inverse problem of air filtration of nanoparticles: Optimal quality factors of fibrous filters. J. Nanomater. 2015, 16, 351. [Google Scholar] [CrossRef]
- Durlofsky, L.; Brady, J.F.; Bossis, G. Dynamic simulation of hydrodynamically interacting particles. J. Fluid Mech. 1987, 180, 21–49. [Google Scholar] [CrossRef] [Green Version]
- Brady, J.F.; Phillips, R.J.; Lester, J.C.; Bossis, G. Dynamic simulation of hydrodynamically interacting suspensions. J. Fluid Mech. 1988, 195, 257–280. [Google Scholar] [CrossRef] [Green Version]
- Brady, J.F.; Bossis, G. Stokesian dynamics. Annu. Rev. Fluid Mech. 1988, 20, 111–157. [Google Scholar] [CrossRef]
- Phillips, R.J.; Brady, J.F. Hydrodynamic transport properties of hard-sphere dispersions. I. Suspensions of freely mobile particles. Phys. Fluids 1988, 31, 3462–3472. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Karrila, S.J. Microhydrodynamics; Butterworth-Heinemann: Boston, MA, USA; London, UK, 1991. [Google Scholar]
- Jeffrey, D.J.; Onishi, Y. Calculation of the resistance and mobility functions for two unequal rigid spheres in low−Reynolds−number flow. J. Fluid Mech. 1984, 139, 261–290. [Google Scholar] [CrossRef]
- Nott, P.R.; Brady, J.F. Pressure-driven flow of suspension and theory. J. Fluid Mech. 1994, 275, 157–199. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Nott, P.R. Normal stress and microstructure in bounded sheared suspensions via Stokesian dynamics simulations. J. Fluid Mech. 2000, 412, 279–301. [Google Scholar] [CrossRef]

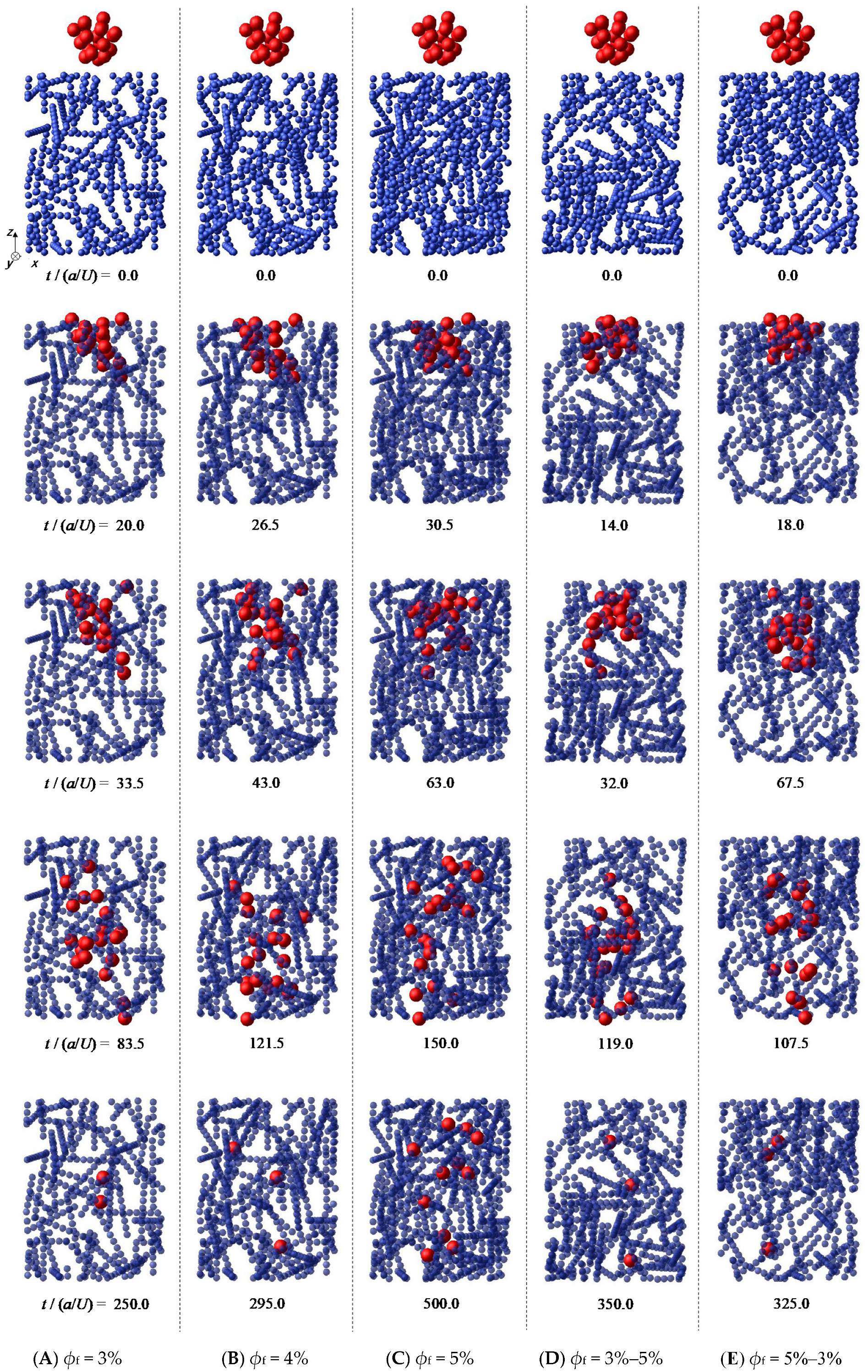
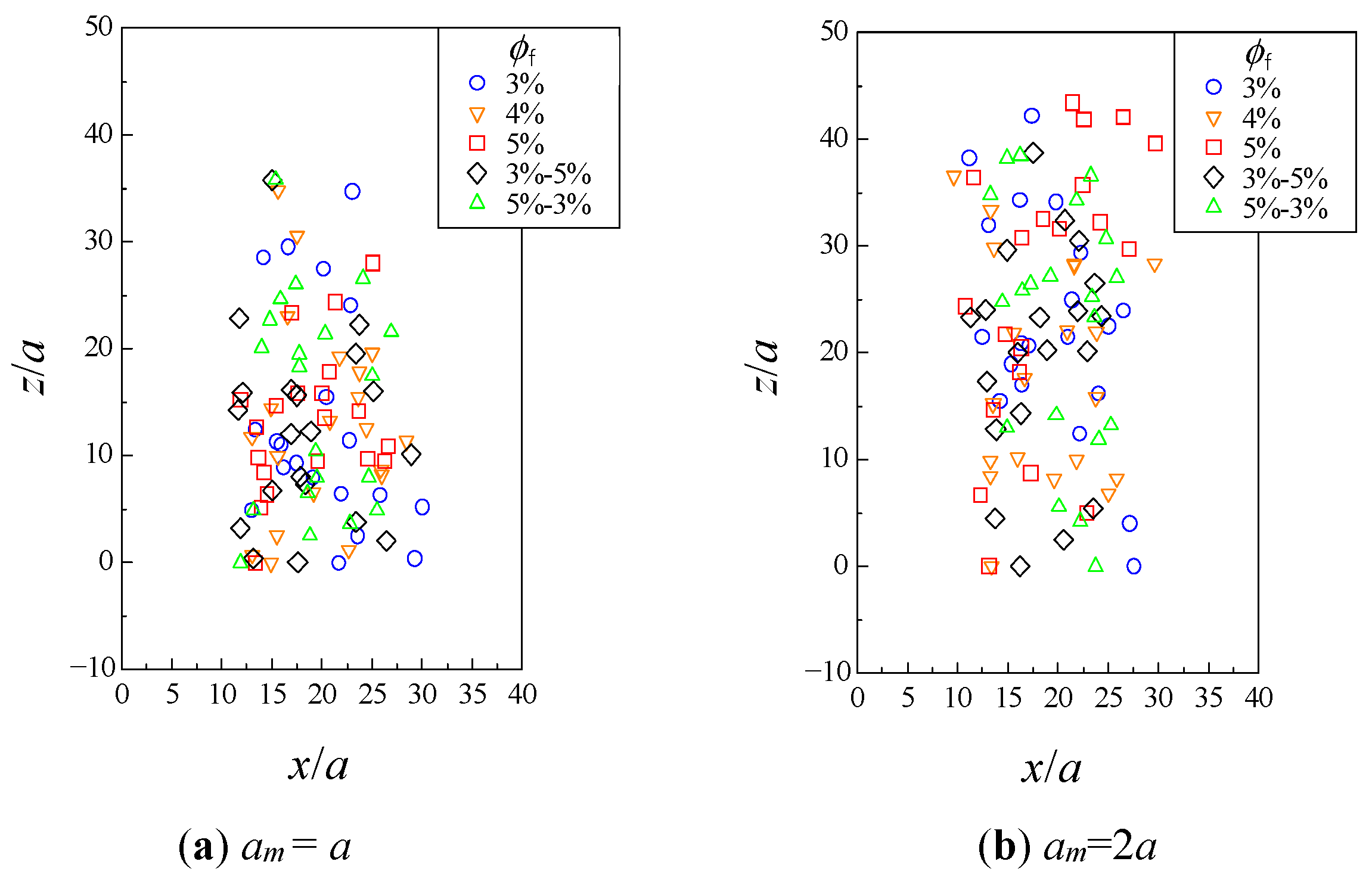


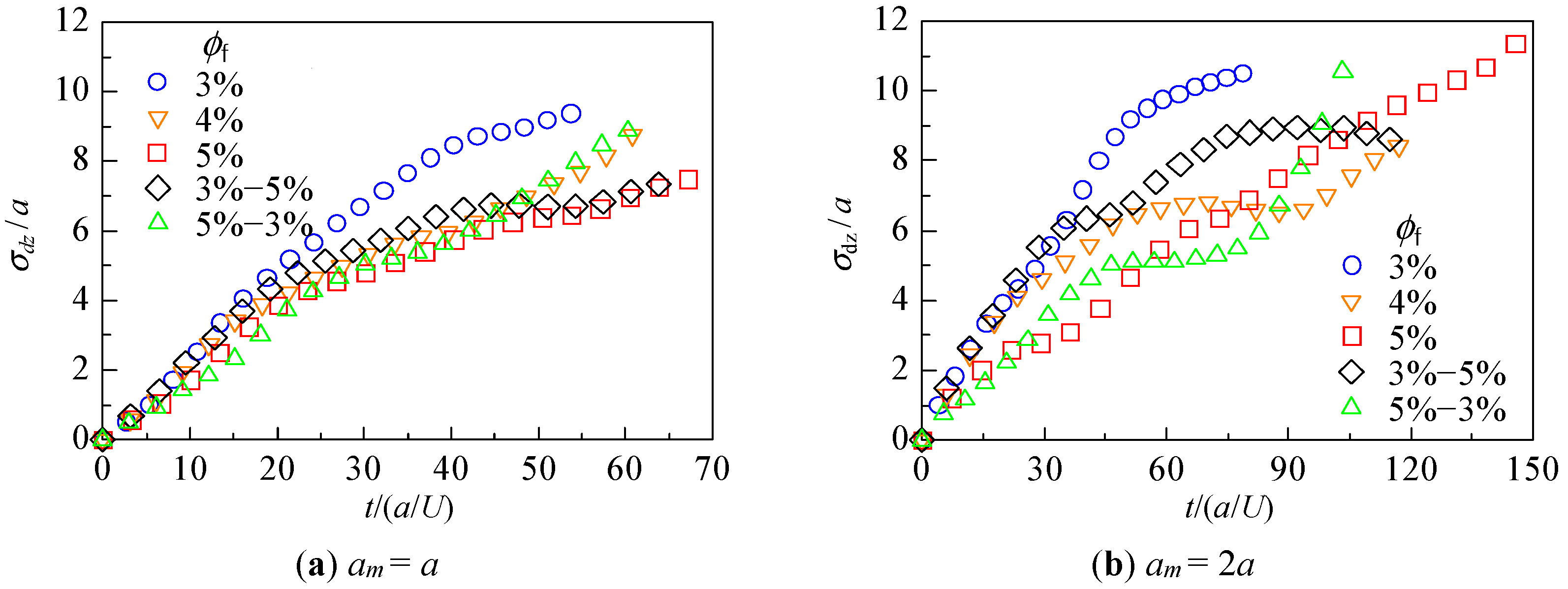
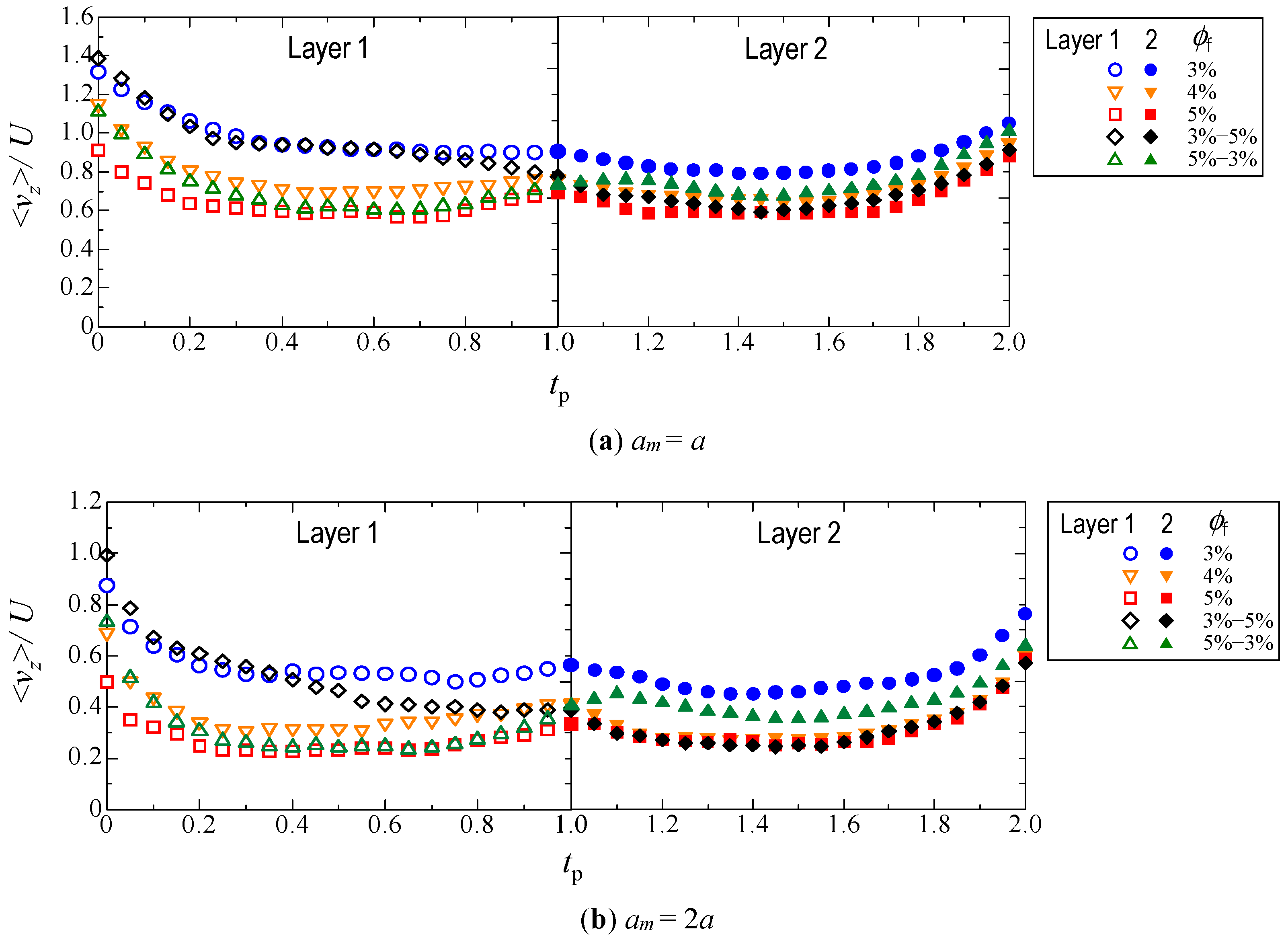
| Adding # | Fiber Volume Fraction ϕf | Number of Fiber Particles | ||
|---|---|---|---|---|
| Layer 1 | Layer 2 | Layer 1 | Layer 2 | |
| (A) | 3% | 520 | ||
| (B) | 4% | 700 | ||
| (C) | 5% | 880 | ||
| (D) | 3% | 5% | 260 | 440 |
| (E) | 5% | 3% | 440 | 260 |
| # | ϕf | am = a | am = 2a | ||||
|---|---|---|---|---|---|---|---|
| Layer 1 | Layer 2 | Total | Layer 1 | Layer 2 | Total | ||
| (A) | 3% | 0.3 | 0.0 | 0.3 | 1.3 | 1.0 | 2.3 |
| (B) | 4% | 1.3 | 0.5 | 1.8 | 3.0 | 2.5 | 5.5 |
| (C) | 5% | 0.5 | 0.8 | 1.3 | 4.8 | 3.8 | 8.6 |
| (D) | 3%–5% | 0.5 | 0.3 | 0.8 | 1.7 | 3.5 | 5.2 |
| (E) | 5%–3% | 1.2 | 0.2 | 1.4 | 3.5 | 1.3 | 4.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otomo, R.; Kira, R. The Effect of the Layered Internal Structure of Fibrous Beds on the Hydrodynamic Diffusive Behavior of Microparticles. Micromachines 2021, 12, 1241. https://doi.org/10.3390/mi12101241
Otomo R, Kira R. The Effect of the Layered Internal Structure of Fibrous Beds on the Hydrodynamic Diffusive Behavior of Microparticles. Micromachines. 2021; 12(10):1241. https://doi.org/10.3390/mi12101241
Chicago/Turabian StyleOtomo, Ryoko, and Ryosuke Kira. 2021. "The Effect of the Layered Internal Structure of Fibrous Beds on the Hydrodynamic Diffusive Behavior of Microparticles" Micromachines 12, no. 10: 1241. https://doi.org/10.3390/mi12101241





