Fabricating Silicon Resonators for Analysing Biological Samples
Abstract
:1. Introduction
2. MEMS Resonators and Actuators for Biological Measurements
2.1. Common Means of Actuation
2.2. Common Sensing Techniques
2.3. Biological and Biomedical Use
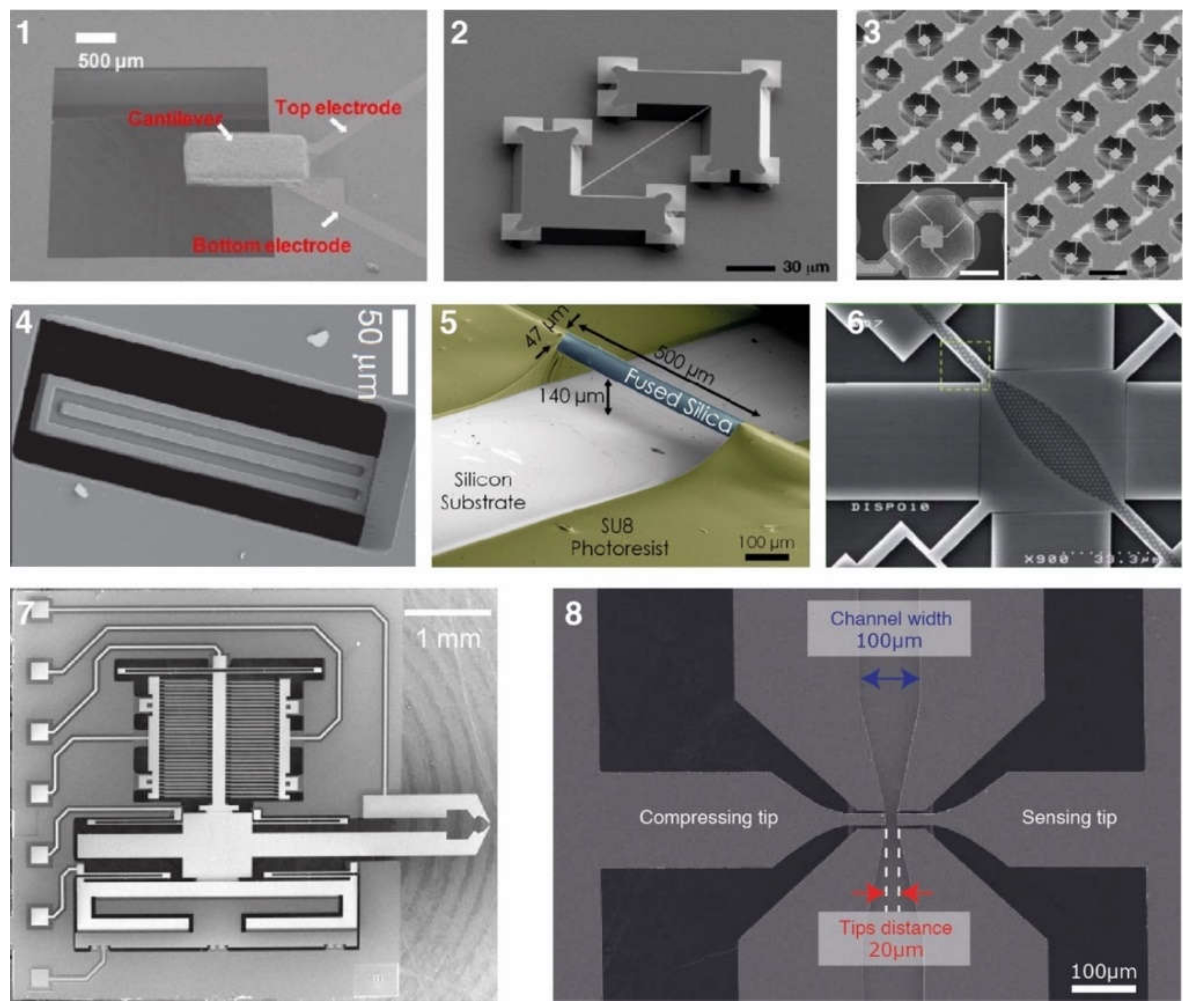
3. Fabricating MEMS Devices
3.1. Common Device Structures
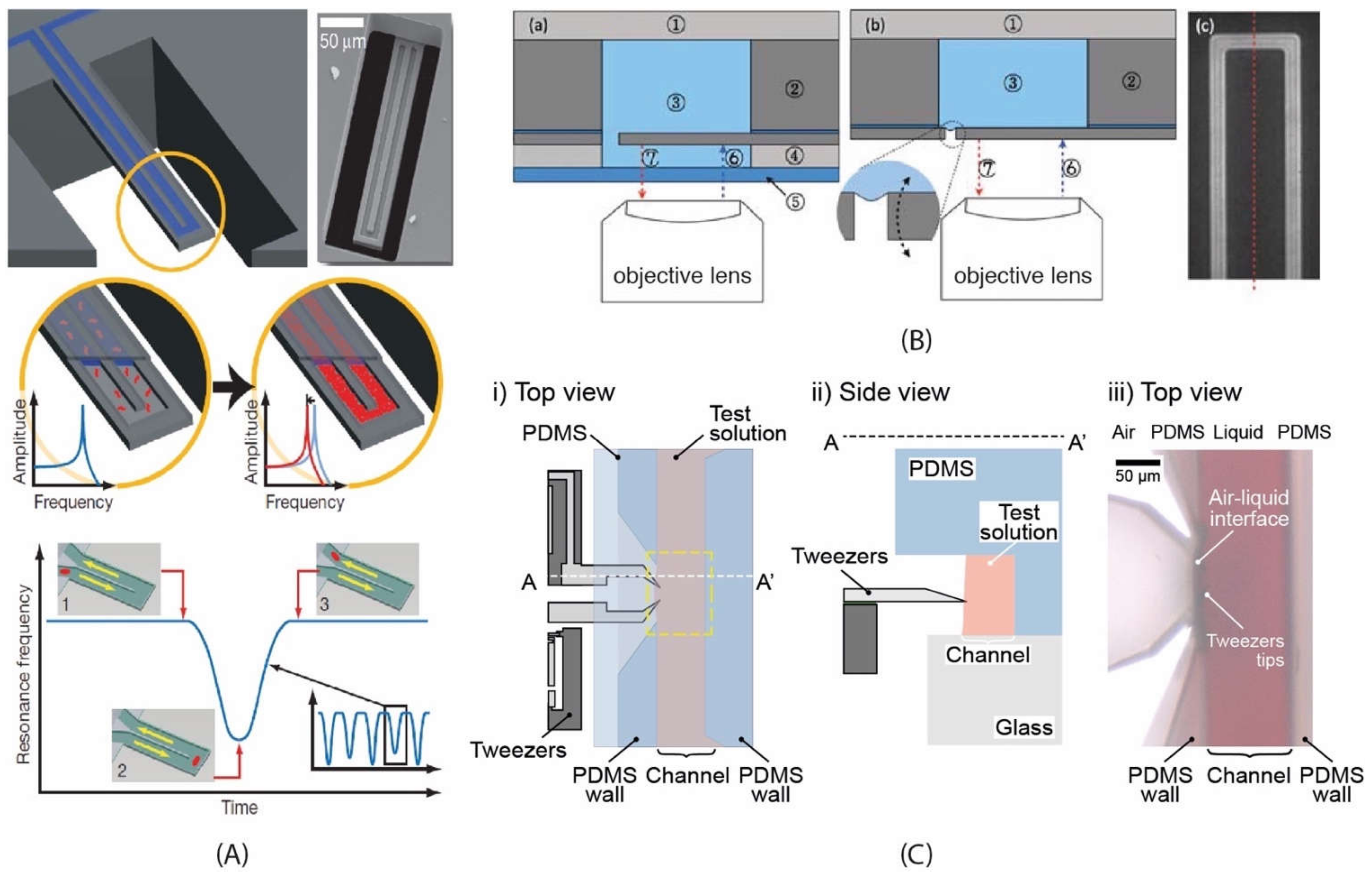
3.1.1. Suspended Structures
3.1.2. Suspended Channel Structures
3.1.3. MEMS Squeezers
3.2. Fundamental Fabrication Processes
4. Biological Applications
4.1. Working at the Molecular/Subcellular Level
4.1.1. Targets
4.1.2. Applications and Perspectives
4.2. Working with Whole Cells
4.2.1. Targets
4.2.2. Applications and Perspectives
4.3. Working with Cellular Aggregates, Tissue, and Whole Organisms
4.3.1. Targets
4.3.2. Applications and Perspectives
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, P.; Wang, W.; Ru, C.; Sun, Y.; Liu, X. MEMS-based platforms for mechanical manipulation and characterization of cells. J. Micromech. Microeng. 2017, 27, 123003. [Google Scholar] [CrossRef]
- Sun, Y.; Nelson, B.J. MEMS for cellular force measurements and molecular detection. Int. J. Inf. Acquis. 2012, 1, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Z.; Walter, B.; Mairiaux, E.; Faucher, M.; Buchaillot, L.; Legrand, B. MEMS piezoresistive ring resonator for AFM imaging with pico-Newton force resolution. J. Micromech. Microeng. 2013, 23, 035016. [Google Scholar] [CrossRef]
- Kim, D.-H.; Wong, P.K.; Park, J.; Levchenko, A.; Sun, Y. Microengineered Platforms for Cell Mechanobiology. Annu. Rev. Biomed. Eng. 2009, 11, 203–233. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.J.; Dufrêne, Y.F. Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology. Nat. Nanotechnol. 2008, 3, 261–269. [Google Scholar] [CrossRef]
- Wu, G.; Ji, H.; Hansen, K.; Thundat, T.; Datar, R.; Cote, R.; Hagan, M.F.; Chakraborty, A.K.; Majumdar, A. Origin of nanomechanical cantilever motion generated from biomolecular interactions. Proc. Natl. Acad. Sci. USA 2001, 98, 1560–1564. [Google Scholar] [CrossRef]
- Yamahata, C.; Collard, D.; Legrand, B.; Takekawa, T.; Kumemura, M.; Hashiguchi, G.; Fujita, H. Silicon Nanotweezers With Subnanometer Resolution for the Micromanipulation of Biomolecules. J. Microelectromech. Syst. 2008, 17, 623–631. [Google Scholar] [CrossRef]
- Gupta, A.; Akin, D.; Bashir, R. Single virus particle mass detection using microresonators with nanoscale thickness. Appl. Phys. Lett. 2004, 84, 1976–1978. [Google Scholar] [CrossRef]
- Ilic, B.; Yang, Y.; Craighead, H.G. Virus detection using nanoelectromechanical devices. Appl. Phys. Lett. 2004, 85, 2604–2606. [Google Scholar] [CrossRef] [Green Version]
- Ilic, B.; Czaplewski, D.; Craighead, H.G.; Neuzil, P.; Campagnolo, C.; Batt, C. Mechanical resonant immunospecific biological detector. Appl. Phys. Lett. 2000, 77, 450–452. [Google Scholar] [CrossRef]
- Burg, T.P.; Godin, M.; Knudsen, S.M.; Shen, W.; Carlson, G.; Foster, J.S.; Babcock, K.; Manalis, S.R. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature 2007, 446, 1066–1069. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Millet, L.J.; Kim, N.; Li, H.; Jin, X.; Popescu, G.; Aluru, N.R.; Hsia, K.J.; Bashir, R. Measurement of adherent cell mass and growth. Proc. Natl. Acad. Sci. USA 2010, 107, 20691–20696. [Google Scholar] [CrossRef] [Green Version]
- Bryan, A.K.; Goranov, A.; Amon, A.; Manalis, S.R. Measurement of mass, density, and volume during the cell cycle of yeast. Proc. Natl. Acad. Sci. USA 2010, 107, 999–1004. [Google Scholar] [CrossRef] [Green Version]
- Sakuma, S.; Nakahara, K.; Arai, F. Continuous Mechanical Indexing of Single-Cell Spheroids Using a Robot-Integrated Microfluidic Chip. IEEE Robot. Autom. Lett. 2019, 4, 2973–2980. [Google Scholar] [CrossRef]
- Sun, Y.; Fry, S.N.; Potasek, D.P.; Bell, D.J.; Nelson, B.J. Characterizing fruit fly flight behavior using a microforce sensor with a new comb-drive configuration. J. Microelectromech. Syst. 2005, 14, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Naik, A.K.; Hanay, M.S.; Hiebert, W.K.; Feng, X.L.; Roukes, M.L. Towards single-molecule nanomechanical mass spectrometry. Nat. Nanotechnol. 2009, 4, 445–450. [Google Scholar] [CrossRef]
- Su, M.; Li, S.; Dravid, V.P. Microcantilever resonance-based DNA detection with nanoparticle probes. Appl. Phys. Lett. 2003, 82, 3562–3564. [Google Scholar] [CrossRef] [Green Version]
- Popescu, G.; Park, K.; Mir, M.; Bashir, R. New technologies for measuring single cell mass. Lab Chip 2014, 14, 646–652. [Google Scholar] [CrossRef]
- Zangle, T.A.; Teitell, M.A. Live-cell mass profiling: An emerging approach in quantitative biophysics. Nat. Methods 2014, 11, 1221–1228. [Google Scholar] [CrossRef] [Green Version]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Mater. 2007, 55, 3989–4014. [Google Scholar] [CrossRef] [Green Version]
- Cross, S.E.; Jin, Y.-S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef]
- Zheng, Y.; Wen, J.; Nguyen, J.; Cachia, M.A.; Wang, C.; Sun, Y. Decreased deformability of lymphocytes in chronic lymphocytic leukemia. Sci. Rep. 2015, 5, 7613. [Google Scholar] [CrossRef] [Green Version]
- Shelby, J.P.; White, J.; Ganesan, K.; Rathod, P.K.; Chiu, D.T. A microfluidic model for single-cell capillary obstruction by Plasmodium falciparum-infected erythrocytes. Proc. Natl. Acad. Sci. USA 2003, 100, 14618–14622. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.Y.H.; Lim, C.T. Biomechanics approaches to studying human diseases. Trends Biotechnol. 2007, 25, 111–118. [Google Scholar] [CrossRef]
- Rajagopalan, J.; Saif, M.T.A. MEMS Sensors and Microsystems for Cell Mechanobiology. J. Micromech. Microeng. 2011, 21, 54002–54012. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.P. Biophysical Tools for Cellular and Subcellular Mechanical Actuation of Cell Signaling. Biophys. J. 2016, 111, 1112–1118. [Google Scholar] [CrossRef] [Green Version]
- Alessandrini, A.; Facci, P. AFM: A versatile tool in biophysics. Meas. Sci. Technol. 2005, 16, R65–R92. [Google Scholar] [CrossRef]
- Strick, T.R.; Allemand, J.F.; Bensimon, D.; Bensimon, A.; Croquette, V. The elasticity of a single supercoiled DNA molecule. Science 1996, 271, 1835–1837. [Google Scholar] [CrossRef]
- Wang, M.D.; Yin, H.; Landick, R.; Gelles, J.; Block, S.M. Stretching DNA with optical tweezers. Biophys. J. 1997, 72, 1335–1346. [Google Scholar] [CrossRef] [Green Version]
- Chu, Y.-S.; Dufour, S.; Thiery, J.P.; Perez, E.; Pincet, F. Johnson-Kendall-Roberts theory applied to living cells. Phys. Rev. Lett. 2005, 94, 028102. [Google Scholar] [CrossRef]
- Suresh, S.; Spatz, J.; Mills, J.P.; Micoulet, A.; Dao, M.; Lim, C.T.; Beil, M.; Seufferlein, T. Connections between single-cell biomechanics and human disease states: Gastrointestinal cancer and malaria. Acta Biomater. 2005, 1, 15–30. [Google Scholar] [CrossRef]
- Nguyen, B.; Tanious, F.A.; Wilson, W.D. Biosensor-surface plasmon resonance: Quantitative analysis of small molecule–nucleic acid interactions. Methods 2007, 42, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Kim, D.; Tse, H.T.; Tseng, P.; Peng, L.; Dhar, M.; Karumbayaram, S.; Di Carlo, D. High-throughput physical phenotyping of cell differentiation. Microsyst. Nanoeng. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiura, H.; Sakuma, S.; Kaneko, M.; Arai, F. On-Chip Method to Measure Mechanical Characteristics of a Single Cell by Using Moiré Fringe. Micromachines 2015, 6, 660–673. [Google Scholar] [CrossRef] [Green Version]
- Byun, S.; Son, S.; Amodei, D.; Cermak, N.; Shaw, J.; Kang, J.H.; Hecht, V.C.; Winslow, M.M.; Jacks, T.; Mallick, P.; et al. Characterizing deformability and surface friction of cancer cells. Proc. Natl. Acad. Sci. USA 2013, 110, 7580–7585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, L.; Kang, J.H.; Olcum, S.; Payer, K.R.; Calistri, N.L.; Kimmerling, R.J.; Manalis, S.R.; Miettinen, T.P. Mass measurements during lymphocytic leukemia cell polyploidization decouple cell cycle- and cell size-dependent growth. Proc. Natl. Acad. Sci. USA 2020, 117, 15659–15665. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Y.-K.; Yang, M.T.; Desai, R.A.; Yu, X.; Liu, Z.; Chen, C.S. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat. Methods 2010, 7, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M.; Maire, C.L.; Chou, N.; Murakami, M.A.; Knoff, D.S.; Kikuchi, Y.; Kimmerling, R.J.; Liu, H.; Haidar, S.; Calistri, N.L.; et al. Drug sensitivity of single cancer cells is predicted by changes in mass accumulation rate. Nat. Biotechnol. 2016, 34, 1161–1167. [Google Scholar] [CrossRef] [Green Version]
- Cermak, N.; Olcum, S.; Delgado, F.F.; Wasserman, S.C.; Payer, K.R.; A Murakami, M.; Knudsen, S.M.; Kimmerling, R.J.; Stevens, M.M.; Kikuchi, Y.; et al. High-throughput measurement of single-cell growth rates using serial microfluidic mass sensor arrays. Nat. Biotechnol. 2016, 34, 1052–1059. [Google Scholar] [CrossRef] [Green Version]
- Cetin, A.E.; Stevens, M.M.; Calistri, N.L.; Fulciniti, M.; Olcum, S.; Kimmerling, R.J.; Munshi, N.C.; Manalis, S.R. Determining therapeutic susceptibility in multiple myeloma by single-cell mass accumulation. Nat. Commun. 2017, 8, 1613. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Miettinen, T.P.; Chen, L.; Olcum, S.; Katsikis, G.; Doyle, P.S.; Manalis, S.R. Noninvasive monitoring of single-cell mechanics by acoustic scattering. Nat. Methods 2019, 16, 263–269. [Google Scholar] [CrossRef]
- Corbin, E.A.; Dorvel, B.R.; Millet, L.J.; King, W.P.; Bashir, R. Micro-patterning of mammalian cells on suspended MEMS resonant sensors for long-term growth measurements. Lab Chip 2014, 14, 1401–1404. [Google Scholar] [CrossRef] [Green Version]
- Corbin, E.A.; Adeniba, O.O.; Cangellaris, O.V.; King, W.P.; Bashir, R. Evidence of differential mass change rates between human breast cancer cell lines in culture. Biomed. Microdevices 2017, 19, 10. [Google Scholar] [CrossRef]
- Martínez-Martín, D.; Fläschner, G.; Gaub, B.; Martin, S.; Newton, R.; Beerli, C.; Mercer, J.; Gerber, C.; Müller, D.J. Inertial picobalance reveals fast mass fluctuations in mammalian cells. Nature 2017, 550, 500–505. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.A.; Sadeghipour, E.; Engel, L.; Nelson, W.J.; Pruitt, B.L. MEMS device for applying shear and tension to an epithelium combined with fluorescent live cell imaging. J. Micromech. Microeng. 2020, 30, 125004. [Google Scholar] [CrossRef]
- Wierzbicki, R.; Houston, K.; Heerlein, H.; Barth, W.; Debski, T.; Eisinberg, A.; Menciassi, A.; Carrozza, M.C.; Dario, P. Design and fabrication of an electrostatically driven microgripper for blood vessel manipulation. Microelectron. Eng. 2006, 83, 1651–1654. [Google Scholar] [CrossRef]
- Kawahara, T.; Sugita, M.; Hagiwara, M.; Arai, F.; Kawano, H.; Shihira-Ishikawa, I.; Miyawaki, A. On-chip microrobot for investigating the response of aquatic microorganisms to mechanical stimulation. Lab Chip 2013, 13, 1070. [Google Scholar] [CrossRef]
- Sun, Y.; Wan, K.T.; Roberts, K.P.; Bischof, J.C.; Nelson, B.J. Mechanical property characterization of mouse zona pellucida. IEEE Trans. Nanobiosci. 2003, 2, 279–286. [Google Scholar] [CrossRef]
- Huang, Q.-A.; Lee, N.K.S. Analysis and design of polysilicon thermal flexure actuator. J. Micromech. Microeng. 1999, 9, 64–70. [Google Scholar] [CrossRef]
- Zhang, W.; Gnerlich, M.; Paly, J.J.; Sun, Y.; Jing, G.; Voloshin, A.; Tatic-Lucic, S. A polymer V-shaped electrothermal actuator array for biological applications. J. Micromech. Microeng. 2008, 18, 075020. [Google Scholar] [CrossRef]
- Guan, C.; Zhu, Y. An electrothermal microactuator with Z-shaped beams. J. Micromech. Microeng. 2010, 20, 085014. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Q. A review on actuation and sensing techniques for MEMS-based microgrippers. J. Micro-Bio Robot. 2017, 13, 1–14. [Google Scholar] [CrossRef]
- Devasia, S.; Eleftheriou, E.; Moheimani, S.O.R. A Survey of Control Issues in Nanopositioning. IEEE Trans. Control Syst. Technol. 2007, 15, 802–823. [Google Scholar] [CrossRef]
- Tadigadapa, S.; Mateti, K. Piezoelectric MEMS sensors: State-of-the-art and perspectives. Meas. Sci. Technol. 2009, 20, 092001. [Google Scholar] [CrossRef]
- Burg, T.P.; Manalis, S.R. Suspended microchannel resonators for biomolecular detection. Appl. Phys. Lett. 2003, 83, 2698–2700. [Google Scholar] [CrossRef] [Green Version]
- Bryan, A.K.; Hecht, V.C.; Shen, W.; Payer, K.; Grover, W.H.; Manalis, S.R. Measuring single cell mass, volume, and density with dual suspended microchannel resonators. Lab Chip 2014, 14, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Tarhan, M.C.; Lafitte, N.; Tauran, Y.; Jalabert, L.; Kumemura, M.; Perret, G.; Kim, B.; Coleman, A.W.; Fujita, H.; Collard, D. A rapid and practical technique for real-time monitoring of biomolecular interactions using mechanical responses of macromolecules. Sci. Rep. 2016, 6, 28001. [Google Scholar] [CrossRef]
- Tauran, Y.; Tarhan, M.C.; Mollet, L.; Gerves, J.B.; Kumemura, M.; Jalabert, L.; Lafitte, N.; Byun, I.; Kim, B.; Fujita, H.; et al. Elucidating the mechanism of the considerable mechanical stiffening of DNA induced by the couple Zn2+/Calix[4]arene-1,3-O-diphosphorous acid. Sci. Rep. 2018, 8, 1226. [Google Scholar] [CrossRef] [Green Version]
- Chong, C.H.; Isamoto, K.; Toshiyoshi, H. Optically modulated MEMS scanning endoscope. IEEE Photonics Technol. Lett. 2006, 18, 133–135. [Google Scholar] [CrossRef]
- Nakada, M.; Chong, C.; Morosawa, A.; Isamoto, K.; Suzuki, T.; Fujita, H.; Toshiyoshi, H. Optical coherence tomography by all-optical MEMS fiber endoscope. IEICE Electron. Exp. 2010, 7, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Kwon, T.; Eom, K.; Park, J.; Yoon, D.S.; Lee, H.L.; Kim, T.S. Micromechanical observation of the kinetics of biomolecular interactions. Appl. Phys. Lett. 2008, 93, 173901. [Google Scholar] [CrossRef]
- Fischer, L.M.; Wright, V.A.; Guthy, C.; Yang, N.; McDermott, M.T.; Buriak, J.M.; Evoy, S. Specific detection of proteins using nanomechanical resonators. Sens. Actuator B-Chem 2008, 134, 613–617. [Google Scholar] [CrossRef]
- Martín-Pérez, A.; Ramos, D.; Gil-Santos, E.; García-López, S.; Yubero, M.L.; Kosaka, P.M.; san Paulo, Á.; Tamayo, J.; Calleja, M. Mechano-Optical Analysis of Single Cells with Transparent Microcapillary Resonators. ACS Sens. 2019, 4, 3325–3332. [Google Scholar] [CrossRef]
- Agache, V.; Blanco-Gomez, G.; Baleras, F.; Caillat, P. An embedded microchannel in a MEMS plate resonator for ultrasensitive mass sensing in liquid. Lab Chip 2011, 11, 2598–2603. [Google Scholar] [CrossRef]
- Takayama, Y.; Perret, G.; Kumemura, M.; Ataka, M.; Meignan, S.; Karsten, S.; Fujita, H.; Collard, D.; Lagadec, C.; Tarhan, M. Developing a MEMS Device with Built-in Microfluidics for Biophysical Single Cell Characterization. Micromachines 2018, 9, 275. [Google Scholar] [CrossRef] [Green Version]
- Carrascosa, L.G.; Moreno, M.; Álvarez, M.; Lechuga, L.M. Nanomechanical biosensors: A new sensing tool. TrAC Trends Anal. Chem. 2006, 25, 196–206. [Google Scholar] [CrossRef]
- Yunas, J.; Mulyanti, B.; Hamidah, I.; Mohd Said, M.; Pawinanto, R.E.; Wan Ali, W.A.F.; Subandi, A.; Hamzah, A.A.; Latif, R.; Yeop Majlis, B. Polymer-Based MEMS Electromagnetic Actuator for Biomedical Application: A Review. Polymers 2020, 12, 1184. [Google Scholar] [CrossRef]
- Waggoner, P.S.; Craighead, H.G. Micro- and nanomechanical sensors for environmental, chemical, and biological detection. Lab Chip 2007, 7, 1238–1255. [Google Scholar] [CrossRef]
- Lee, J.; Shen, W.; Payer, K.; Burg, T.P.; Manalis, S.R. Toward Attogram Mass Measurements in Solution with Suspended Nanochannel Resonators. Nano Lett. 2010, 10, 2537–2542. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Chunara, R.; Shen, W.; Payer, K.; Babcock, K.; Burg, T.P.; Manalis, S.R. Suspended microchannel resonators with piezoresistive sensors. Lab Chip 2011, 11, 645–651. [Google Scholar] [CrossRef] [Green Version]
- Baek, I.-B.; Byun, S.; Lee, B.K.; Ryu, J.-H.; Kim, Y.; Yoon, Y.S.; Jang, W.I.; Lee, S.; Yu, H.Y. Attogram mass sensing based on silicon microbeam resonators. Sci. Rep. 2017, 7, 46660. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Du, R.; Cao, Y.; Mohammad, M.A.; Dew, S.K.; McDermott, M.T.; Evoy, S. Diazonium Chemistry for the Bio-Functionalization of Glassy Nanostring Resonator Arrays. Sensors 2015, 15, 18724–18741. [Google Scholar] [CrossRef] [Green Version]
- Waggoner, P.S.; Varshney, M.; Craighead, H.G. Detection of prostate specific antigen with nanomechanical resonators. Lab Chip 2009, 9, 3095–3099. [Google Scholar] [CrossRef]
- Corbin, E.A.; Kong, F.; Lim, C.T.; King, W.P.; Bashir, R. Biophysical properties of human breast cancer cells measured using silicon MEMS resonators and atomic force microscopy. Lab Chip 2015, 15, 839–847. [Google Scholar] [CrossRef]
- Corbin, E.A.; Adeniba, O.O.; Ewoldt, R.H.; Bashir, R. Dynamic mechanical measurement of the viscoelasticity of single adherent cells. Appl. Phys. Lett. 2016, 108, 093701. [Google Scholar] [CrossRef] [Green Version]
- van den Hurk, R.; Baghelani, M.; Chen, J.; Daneshmand, M.; Evoy, S. Al-Mo nanocomposite functionalization for membrane-based resonance detection of bovine Herpesvirus-1. Sens. Actuators A 2019, 296, 186–191. [Google Scholar] [CrossRef]
- van den Hurk, R.; Nelson-Fitzpatrick, N.; Evoy, S. Fabrication and characterization of aluminum-molybdenum nanocomposite membranes. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. 2014, 32, 052002. [Google Scholar] [CrossRef]
- Burg, T.P.; Mirza, A.R.; Milovic, N.; Tsau, C.; Popescu, G.A.; Foster, J.S.; Manalis, S.R. Vacuum-packaged suspended microchannel resonant mass sensor for biomolecular detection. J. Microelectromech. Syst. 2006, 15, 1466–1476. [Google Scholar] [CrossRef]
- Olcum, S.; Cermak, N.; Wasserman, S.C.; Christine, K.S.; Atsumi, H.; Payer, K.R.; Shen, W.; Lee, J.; Belcher, A.M.; Bhatia, S.N.; et al. Weighing nanoparticles in solution at the attogram scale. Proc. Natl. Acad. Sci. USA 2014, 111, 1310–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godin, M.; Delgado, F.F.; Son, S.; Grover, W.H.; Bryan, A.K.; Tzur, A.; Jorgensen, P.; Payer, K.; Grossman, A.D.; Kirschner, M.W.; et al. Using buoyant mass to measure the growth of single cells. Nat. Methods 2010, 7, 387–390. [Google Scholar] [CrossRef] [Green Version]
- Grover, W.H.; Bryan, A.K.; Diez-Silva, M.; Suresh, S.; Higgins, J.M.; Manalis, S.R. Measuring single-cell density. Proc. Natl. Acad. Sci. USA 2011, 108, 10992–10996. [Google Scholar] [CrossRef] [Green Version]
- Son, S.; Tzur, A.; Weng, Y.; Jorgensen, P.; Kim, J.; Kirschner, M.W.; Manalis, S.R. Direct observation of mammalian cell growth and size regulation. Nat. Methods 2012, 9, 910–912. [Google Scholar] [CrossRef] [Green Version]
- Keeler, E.G.; Jing, P.; Wu, J.; Zou, C.; Lin, L.Y. MEMS Resonant Mass Sensor With Integrated Optical Manipulation. IEEE Trans. Nanotechnol. 2018, 17, 714–718. [Google Scholar] [CrossRef]
- Keeler, E.G.; Zou, C.; Lin, L.Y. Optically accessible MEMS resonant mass sensor for biological applications. J. Microelectromech. Syst. 2019, 28, 494–503. [Google Scholar] [CrossRef]
- Blanco-Gomez, G.; Trioux, E.; Agache, V. Hollow square-and ring-plate MEMS oscillators embedded in a phase-locked loop for low limit of detection in liquid. Electron. Device Lett. 2012, 33, 609–611. [Google Scholar] [CrossRef]
- Tarhan, M.C.; Yokokawa, R.; Jalabert, L.; Collard, D.; Fujita, H. Pick-and-Place Assembly of Single Microtubules. Small 2017, 16, 1701136. [Google Scholar] [CrossRef]
- Kim, K.; Cheng, J.; Liu, Q.; Wu, X.Y.; Sun, Y. Investigation of mechanical properties of soft hydrogel microcapsules in relation to protein delivery using a MEMS force sensor. J. Biomed. Mater. Res. A 2010, 92, 103–113. [Google Scholar] [CrossRef]
- Perret, G.; Lacornerie, T.; Manca, F.; Giordano, S.; Kumemura, M.; Lafitte, N.; Jalabert, L.; Tarhan, M.C.; Lartigau, E.F.; Cleri, F.; et al. Real-time mechanical characterization of DNA degradation under therapeutic X-rays and its theoretical modeling. Microsyst. Nanoeng. 2016, 2, 16062. [Google Scholar] [CrossRef]
- Montasser, I.; Coleman, A.W.; Tauran, Y.; Perret, G.; Jalabert, L.; Collard, D.; Kim, B.J.; Tarhan, M.C. Direct measurement of the mechanism by which magnesium specifically modifies the mechanical properties of DNA. Biomicrofluidics 2017, 11, 051102. [Google Scholar] [CrossRef]
- Tauran, Y.; Kumemura, M.; Tarhan, M.C.; Perret, G.; Perret, F.; Jalabert, L.; Collard, D.; Fujita, H.; Coleman, A.W. Direct measurement of the mechanical properties of a chromatin analog and the epigenetic effects of para -sulphonato-calix[4]arene. Sci. Rep. 2019, 9, 5816. [Google Scholar] [CrossRef] [Green Version]
- Pekin, D.; Perret, G.; Rezard, Q.; Gerbedoen, J.C.; Meignan, S.; Collard, D.; Lagadec, C.; Tarhan, M.C. Subcellular Imaging during Single Cell Mechanical Characterization. In Proceedings of the 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), Vancouver, BC, Canada, 18–22 January 2020; pp. 62–65. [Google Scholar]
- Somà, A.; Iamoni, S.; Voicu, R.; Müller, R.; Al-Zandi, M.H.M.; Wang, C. Design and experimental testing of an electro-thermal microgripper for cell manipulation. Microsyst. Technol. 2018, 24, 1053–1060. [Google Scholar] [CrossRef]
- Hashiguchi, G.; Goda, T.; Hosogi, M.; Hirano, K.; Kaji, N.; Baba, Y.; Kakushima, K.; Fujita, H. DNA Manipulation and Retrieval from an Aqueous Solution with Micromachined Nanotweezers. Anal. Chem. 2003, 75, 4347–4350. [Google Scholar] [CrossRef]
- Nakahara, K.; Sakuma, S.; Hayakawa, T.; Arai, F. On-Chip Transportation and Measurement of Mechanical Characteristics of Oocytes in an Open Environment. Micromachines 2015, 6, 648–659. [Google Scholar] [CrossRef] [Green Version]
- Barazani, B.; Warnat, S.; Fine, A.; Hubbard, T. MEMS squeezer for the measurement of single cell rupture force, stiffness change, and hysteresis. J. Micromech. Microeng. 2017, 27, 025002. [Google Scholar] [CrossRef]
- Eppell, S.J.; Smith, B.N.; Kahn, H.; Ballarini, R. Nano measurements with micro-devices: Mechanical properties of hydrated collagen fibrils. J. R. Soc. Interface 2006, 3, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Rezard, Q.; Perret, G.; Gerbedoen, J.C.; Pekin, D.; Cleri, F.; Collard, D.; Lagadec, C.; Tarhan, M.C. Developing A Mems Device for High-Throughput Multi-Parameter Single Cell Biophysical Analysis. In Proceedings of the 2021 IEEE 34th International Conference on Micro Electro Mechanical Systems (MEMS), Gainesville, FL, USA, 25–29 January 2021; pp. 494–497. [Google Scholar]
- Warnat, S.; King, H.; Forbrigger, C.; Hubbard, T. PolyMUMPs MEMS device to measure mechanical stiffness of single cells in aqueous media. J. Micromech. Microeng. 2015, 25, 025011. [Google Scholar] [CrossRef]
- Barazani, B.; Piercey, M.; Paulson, A.; Warnat, S.; Hubbard, T.; MacIntosh, A.J. Rehydration of active dried yeast: Impact on strength and stiffness of yeast cells measured using microelectromechanical systems. J. Inst. Brew. 2019, 125, 53–59. [Google Scholar] [CrossRef]
- Shen, Z.L.; Dodge, M.R.; Kahn, H.; Ballarini, R.; Eppell, S.J. Stress-strain experiments on individual collagen fibrils. Biophys. J. 2008, 95, 3956–3963. [Google Scholar] [CrossRef] [Green Version]
- Ilic, B.; Yang, Y.; Aubin, K.; Reichenbach, R.; Krylov, S.; Craighead, H.G. Enumeration of DNA Molecules Bound to a Nanomechanical Oscillator. Nano Lett. 2005, 5, 925–929. [Google Scholar] [CrossRef]
- Chang, D.; Sakuma, S.; Kera, K.; Uozumi, N.; Arai, F. Measurement of the mechanical properties of single Synechocystis sp. strain PCC6803 cells in different osmotic concentrations using a robot-integrated microfluidic chip. Lab Chip 2018, 18, 1241–1249. [Google Scholar] [CrossRef]
- Ko, J.; Jeong, J.; Son, S.; Lee, J. Cellular and biomolecular detection based on suspended microchannel resonators. Biomed. Eng. Lett. 2021, 11, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Hirate, T.; Uehara, C.; Maruyama, H.; Uozumi, N.; Arai, F. Evaluating Young’s Modulus of Single Yeast Cells Based on Compression Using an Atomic Force Microscope with a Flat Tip. Microsc. Microanal. 2021, 27, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, H.; Gan, X.; Xia, X.; Xu, P.; Li, J.; Liu, M.; Li, Y. Integrated MEMS/NEMS Resonant Cantilevers for Ultrasensitive Biological Detection. J. Sens. 2009, 2009, 637874. [Google Scholar] [CrossRef]
- Johnson, B.N.; Mutharasan, R. Biosensing using dynamic-mode cantilever sensors: A review. Biosens. Bioelectron. 2012, 32, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Nair, P.R.; Akin, D.; Ladisch, M.R.; Broyles, S.; Alam, M.A.; Bashir, R. Anomalous resonance in a nanomechanical biosensor. Proc. Natl. Acad. Sci. USA 2006, 103, 13362–13367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, L.; Gupta, A.K.; Ghafoor, A.; Akin, D.; Bashir, R. Characterization of vaccinia virus particles using microscale silicon cantilever resonators and atomic force microscopy. Sens. Actuator B-Chem 2006, 115, 189–197. [Google Scholar] [CrossRef]
- Ricciardi, C.; Castagna, R.; Ferrante, I.; Frascella, F.; Marasso, S.L.; Ricci, A.; Canavese, G.; Lorè, A.; Prelle, A.; Gullino, M.L.; et al. Development of a microcantilever-based immunosensing method for mycotoxin detection. Biosens. Bioelectron. 2013, 40, 233–239. [Google Scholar] [CrossRef]
- Ferrante, I.; Ciprianetti, N.; Stassi, S.; Santoro, K.; Ferrero, S.; Scaltrito, L.; Ricciardi, C. High-Throughput Characterization of Microcantilever Resonator Arrays for Low-Concentration Detection of Small Molecules. J. Microelectromech. Syst. 2017, 26, 246–254. [Google Scholar] [CrossRef]
- Puiggalí-Jou, A.; del Valle, L.J.; Alemán, C.; Pérez-Madrigal, M.M. Weighing biointeractions between fibrin(ogen) and clot-binding peptides using microcantilever sensors. J. Pept. Sci. 2017, 23, 162–171. [Google Scholar] [CrossRef]
- Park, J.; Nishida, S.; Lambert, P.; Kawakatsu, H.; Fujita, H. High-resolution cantilever biosensor resonating at air-liquid in a microchannel. Lab Chip 2011, 11, 4187–4193. [Google Scholar] [CrossRef]
- Park, J.; Karsten, S.L.; Nishida, S.; Kawakatsu, H.; Fujita, H. Application of a new microcantilever biosensor resonating at the air-liquid interface for direct insulin detection and continuous monitoring of enzymatic reactions. Lab Chip 2012, 12, 4115–4119. [Google Scholar] [CrossRef] [Green Version]
- Rust, P.; Cereghetti, D.; Dual, J. A micro-liter viscosity and density sensor for the rheological characterization of DNA solutions in the kilo-hertz range. Lab Chip 2013, 13, 4794–4799. [Google Scholar] [CrossRef]
- von Muhlen, M.G.; Brault, N.D.; Knudsen, S.M.; Jiang, S.; Manalis, S.R. Label-Free Biomarker Sensing in Undiluted Serum with Suspended Microchannel Resonators. Anal. Chem. 2010, 82, 1905–1910. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Liu, X.; Zhang, Y.; Cheng, J.; Yu Wu, X.; Sun, Y. Elastic and viscoelastic characterization of microcapsules for drug delivery using a force-feedback MEMS microgripper. Biomed. Microdevices 2009, 11, 421–427. [Google Scholar] [CrossRef]
- Brunetti, G.; Padovani, F.; De Pastina, A.; Rotella, C.; Monahan, A.; Hoffman, S.L.; Jongo, S.A.; Abdulla, S.; Corradin, G.; Pluschke, G.; et al. Nanotechnological immunoassay for rapid label-free analysis of candidate malaria vaccines. Nanoscale 2021, 13, 2338–2349. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, H.; Lee, G.; Kim, Y.R.; Ahn, M.-J.; Park, H.J.; Eom, K.; Kwon, T. Blood Droplet-Based Cancer Diagnosis via Proteolytic Activity Measurement in Cancer Progression. Theranostics 2017, 7, 2878–2887. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, M.; Fariña, D.; Escuela, A.M.; Sendra, J.R.; Lechuga, L.M. Development of a surface plasmon resonance and nanomechanical biosensing hybrid platform for multiparametric reading. Rev. Sci. Instrum. 2013, 84, 015008. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Yu, H.; Xu, P.; Xu, W.; Chen, W.; Chen, C.; Li, X. Real-time enzyme-digesting identification of double-strand DNA in a resonance-cantilever embedded micro-chamber. Lab Chip 2014, 14, 1206–1214. [Google Scholar] [CrossRef]
- Duffy, J.; Padovani, F.; Brunetti, G.; Noy, P.; Certa, U.; Hegner, M. Hegner Towards personalised rapid label free miRNA detection for cancer and liver injury diagnostics in cell lysates and blood based samples. Nanoscale 2018, 10, 12797–12804. [Google Scholar] [CrossRef]
- Braun, T.; Ghatkesar, M.K.; Backmann, N.; Grange, W.; Boulanger, P.; Letellier, L.; Lang, H.-P.; Bietsch, A.; Gerber, C.; Hegner, M. Quantitative time-resolved measurement of membrane protein–ligand interactions using microcantilever array sensors. Nat. Nanotechnol. 2009, 4, 179–185. [Google Scholar] [CrossRef]
- Kwon, T.; Park, J.; Yang, J.; Yoon, D.S.; Na, S.; Kim, C.-W.; Suh, J.-S.; Huh, Y.-M.; Haam, S.; Eom, K. Nanomechanical In Situ Monitoring of Proteolysis of Peptide by Cathepsin B. PLoS ONE 2009, 4, e6248. [Google Scholar] [CrossRef]
- Campbell, G.A.; Mutharasan, R. Detection and quantification of proteins using self-excited PZT-glass millimeter-sized cantilever. Biosens. Bioelectron. 2005, 21, 597–607. [Google Scholar] [CrossRef]
- Dhayal, B.; Henne, W.A.; Doorneweerd, D.D.; Reifenberger, R.G.; Low, P.S. Detection of Bacillus subtilis spores using peptide-functionalized cantilever arrays. J. Am. Chem. Soc. 2006, 128, 3716–3721. [Google Scholar] [CrossRef]
- Bansal, M.; Farrugia, A.; Balboni, S.; Martin, G. Relative survival benefit and morbidity with fluids in severe sepsis—A network meta-analysis of alternative therapies. Curr. Drug Saf. 2013, 8, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Maloney, N.; Lukacs, G.; Ball, S.L.; Hegner, M. Device for filamentous fungi growth monitoring using the multimodal frequency response of cantilevers. Rev. Sci. Instrum. 2014, 85, 015003. [Google Scholar] [CrossRef] [Green Version]
- Nugaeva, N.; Gfeller, K.Y.; Backmann, N.; Lang, H.-P.; Düggelin, M.; Hegner, M. Micromechanical cantilever array sensors for selective fungal immobilization and fast growth detection. Biosens. Bioelectron. 2005, 21, 849–856. [Google Scholar] [CrossRef]
- Maloney, N.; Lukacs, G.; Nugaeva, N.; Grange, W.; Ramseyer, J.P.; Jensen, J.; Hegner, M. Fibre Optic Readout of Microcantilever Arrays for Fast Microorganism Growth Detection. J. Sens. 2012, 2012, 405281. [Google Scholar] [CrossRef]
- Baëtens, T.; Perret, G.; Takayama, Y.; Kumemura, M.; Jalabert, L.; Meignan, S.; Lagadec, C.; Fujita, H.; Collard, D.; Tarhan, M.C. A Practical Single Cell Analysis Method for Mechanical Characterization of Cancer Cells. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 608–611. [Google Scholar]
- Desmaële, D.; Boukallel, M.; Régnier, S. Actuation means for the mechanical stimulation of living cells via microelectromechanical systems A critical review. J. Biomech. 2011, 44, 1433–1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, R.G. The role of matrix stiffness in regulating cell behavior. Hepatology 2008, 47, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Cho, C.H.; Park, E.K.; Jung, M.-H.; Yoon, K.-S.; Park, H.-K. AFM-Detected Apoptotic Changes in Morphology and Biophysical Property Caused by Paclitaxel in Ishikawa and HeLa Cells. PLoS ONE 2012, 7, e30066. [Google Scholar] [CrossRef] [Green Version]
- Baker, E.L.; Lu, J.; Yu, D.; Bonnecaze, R.T.; Zaman, M.H. Cancer Cell Stiffness: Integrated Roles of Three-Dimensional Matrix Stiffness and Transforming Potential. Biophys. J. 2010, 99, 2048–2057. [Google Scholar] [CrossRef] [Green Version]
- Torzilli, P.A.; Bourne, J.W.; Cigler, T.; Vincent, C.T. A new paradigm for mechanobiological mechanisms in tumor metastasis. Semin. Cancer Biol. 2012, 22, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Carey, S.P.; D’Alfonso, T.M.; Shin, S.J.; Reinhart-King, C.A. Mechanobiology of tumor invasion: Engineering meets oncology. Crit. Rev. Oncol. Hematol. 2012, 83, 170–183. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Mezencev, R.; Kim, B.; Wang, L.; McDonald, J.; Sulchek, T. Cell Stiffness Is a Biomarker of the Metastatic Potential of Ovarian Cancer Cells. PLoS ONE 2012, 7, e46609. [Google Scholar] [CrossRef] [Green Version]
- Gfeller, K.Y.; Nugaeva, N.; Hegner, M. Rapid Biosensor for Detection of Antibiotic-Selective Growth of Escherichia coli. Appl. Environ. Microbiol. 2005, 71, 2626–2631. [Google Scholar] [CrossRef] [Green Version]
- Gagino, M.; Katsikis, G.; Olcum, S.; Virot, L.; Cochet, M.; Thuaire, A.; Manalis, S.R.; Agache, V. Suspended Nanochannel Resonator Arrays with Piezoresistive Sensors for High-Throughput Weighing of Nanoparticles in Solution. ACS Sens. 2020, 5, 1230–1238. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Cukierman, E. Taking Cell-Matrix Adhesions to the Third Dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Eom, K.; Park, H.S.; Yoon, D.S.; Kwon, T. Nanomechanical resonators and their applications in biological/chemical detection: Nanomechanics principles. Phys. Rep. 2011, 503, 115–163. [Google Scholar] [CrossRef] [Green Version]
- Stockslager, M.A.; Olcum, S.; Knudsen, S.M.; Kimmerling, R.J.; Cermak, N.; Payer, K.R.; Agache, V.; Manalis, S.R. Rapid and high-precision sizing of single particles using parallel suspended microchannel resonator arrays and deconvolution. Rev. Sci. Instrum. 2019, 90, 085004. [Google Scholar] [CrossRef]

| Device Type | Sample | Parameters | Stimulation/ Sensing | Key Fabrication Steps | Ref. |
|---|---|---|---|---|---|
| Suspended structures | |||||
 | Molecules, Proteins, Nucleic acids, Viruses | Mass, Viscosity, Density | Thermal/Optical |
| [8,107] [108] |
| Piezoelectric (ext)/ Optical |
| [109,110] [111] | |||
| Piezoelectric/ Optical |
| [9] | |||
| Optical/ Optical |
| [112,113] | |||
| [101] | ||||
| Electromagnetic/ Electromagnetic |
| [114] | |||
 | Proteins | Mass | Piezoelectric (ext)/ Optical |
| [62,71,72] |
 | Proteins | Mass | Piezoelectric (ext)/ Optical |
| [73] |
| Cells | Cell mass, Cell growth, Stiffness, Viscoelasticity | Magnetic/ Optical |
| [12,42,43,74,75] | |
| Suspended channel devices | |||||
 | Proteins, Nucleic acids, Exosomes, Cells | Mass, Cell density, Cell volume, Cell growth, Deformability, Mass accum. rate | Electrostatic/ Optical |
| [55] |
| Piezoceramic (ext)/ Optical |
| [11,13,35,38,56,80,81,82,115] | |||
| [79] | |||||
| Electrostatic/ Piezoresist |
| [70] | |||
| Piezoceramic (ext)/ Piezoresist | [39,40] | ||||
 | Cells | Mass | Optical/ Optical |
| [63,83,84] |
 | Buffers, Solutions | Mass | Electrostatic/ Electrostatic |
| [64,85] |
| MEMS squeezers | |||||
 | DNA, Cells, Animals | Force, Stiffness, Young’s modulus, Viscosity, Elastic modulus, | Electrostatic/ Capacitive |
| [15,48,57,58,88,89,90,91] |
| Drug capsules | Electrothermal/ Capacitive | [116] | |||
 | Proteins, Cells, Cell spheroids, Microorganism | Force, Stiffness, Young’s modulus, Viscosity, Elastic modulus | Piezoactuator (ext)/Optical |
| [14,34,94,102,104] |
| Electrostatic or Electrothermal/ Optical |
| [95,98,99] | |||
| Electrostatic/ Capacitive |
| [65,97] | |||
| Electrostatic/ Optical |
| [96,100] | |||
| Electromagnetic/ Optical |
| [47] | |||
| Target Sample | Parameter | Purpose | Device Type | Condition: Sample/Measure | Ref. |
|---|---|---|---|---|---|
| Molecules and proteins | |||||
| Aflatoxins | Mass | Detection | 1 | Vacuum/Vacuum | [109,110] |
| Ochratoxin A | [109] | ||||
| ALCAM | Mass | Cancer biomarker detection | 4 | Liquid/Vacuum | [115] |
| Tetrapeptide | Mass | Detection of proteolysis | 1 | Liquid/Liquid | [123] |
| Fibrinogen | Mass | Cancer biomarker detection | 1 | Air/Air | [111] |
| Collagen fibres | Stress, strain | Tensile mechanical resistance | 8 | Humid/Humid | [96,100] |
| Antigen, antibodies, (IgG, biotin, avidin, EP9, SP3-E6, etc.) | Mass | Surface coating | 1 | Air/Air | [107] |
| Detection | 4 | Liquid/Vacuum | [55] | ||
| Detection | 1 | Liquid/(partially) air | [112] | ||
| Detecting binding rate | Liquid/Liquid | [124] | |||
| Detection | 2 | Vacuum/Vacuum | [62] | ||
| Testing malaria vaccine | 1 | Liquid/Liquid | [117] | ||
| PSA | Mass | Cancer biomarker detection | 3 | Vacuum/Vacuum | [73] |
| Insulin | Mass | Detection | 1 | Liquid/(partially) air | [113] |
| SOD1 | Proteinase K enzyme reaction | ||||
| Matrix metallo-proteinase | Mass | Cancer diagnosis | 1 | Liquid/Liquid | [118] |
| Nucleic acids | |||||
| miRNA | Mass | Detection for cancer and liver injury diagnostics | 1 | Liquid/Liquid | [121] |
| ssDNA | Mass | Detection | 1 | Air/Air | [17] |
| Enumeration | Vacuum/Vacuum | [101] | |||
| Hybridisation kinetics | Liquid/Liquid | [61] | |||
| Detecting hybridisation | [119] | ||||
| DNA 110 bp,10 kbp | Viscosity, Density | Rheological characterisation | 1 | Liquid/Liquid | [114] |
| DNA λ-phage | Stiffness | Effects of irradiation Effect of ions Effect of compounds | 7 | Liquid/Air | [88] [57,58,89] [58,90] |
| DNA 3776 bp | Mass | Enzymatic reaction monitoring | 1 | Liquid/Liquid | [120] |
| Viruses and exosomes | |||||
| Baculovirus | Mass | Single virus detection | 1 | Vacuum/Vacuum | [9] |
| Vaccinia virus | Mass | Single virus detection | 1 | Air/Air | [8,108] |
| T5 virus | Mass | Detection | 1 | Humid/Humid | [122] |
| Bovine Herpesvirus1 | Mass | Detection | 3 | Vacuum/Vacuum | [76] |
| Exosomes | Mass | Mass distribution | 4 | Liquid/Vacuum | [79] |
| Target Sample | Parameter | Purpose | Device Type | Condition: Sample/Measure | Ref. |
|---|---|---|---|---|---|
| Bacterial and parasite cells | |||||
| E. coli | Mass | Detection | 1 | Air/Air | [10,138] |
| Mass | Detection | 4 | Liquid/Vacuum | [11] | |
| Cell growth | Instantaneous growth | 4 | Liquid/Vacuum | [80] | |
| B. subtilis | Mass | Detection | 1 | Liquid/Liquid | [125] |
| Mass | Detection | 4 | Liquid/Vacuum | [11] | |
| Cell growth | Instantaneous growth | 4 | Liquid/Vacuum | [80] | |
| Synechocystis sp. strain PCC6803 | Young’s modulus | Osmoadaptation mechanism of cell membrane | 8 | Liquid/Liquid | [102] |
| P. falciparum | Density | Drug treatment | 4 | Liquid/Vacuum | [81] |
| Fungal cells | |||||
| S. cerevisiae | Cell growth | Fast growth detection | 1 | Humid/Humid | [128] |
| Mass, density, vol. | Growth during cell cycle | 4 | Liquid/Vacuum | [13] | |
| Mass | Budding yeast cells | 4 | Liquid/Vacuum | [70] | |
| Cell growth | Detecting growth rate | 4 | Liquid/Vacuum | [80] | |
| Mass | Combined optical observation | 5 | Liquid/Air | [84] | |
| Stiffness | Discriminating viable cells | 8 | Liquid/Liquid | [98] | |
| Force | Cell rupture analysis | 8 | Liquid/Liquid | [95] | |
| Young’s modulus | Force-deformation curve | 8 | Liquid/Liquid | [104] | |
| A. niger | Cell growth | Fast growth detection | 1 | Humid/Humid | [127,128,129] |
| S. pastorianus | Stiffness | Rehydration effect on mechanical properties | 8 | Liquid/Liquid | [99] |
| Mammalian cells | |||||
| Colon cancer cell lines (human) HT-29 | Mass, growth | Adherent cell growth | 3 | Liquid/Liquid | [12] |
| Viscoelasticity | Cell discrimination by mechanical properties | 3 | Liquid/Liquid | [75] | |
| Breast cancer cell lines (human) MCF7, MCF10A, MDA-MB-231, SUM159-PT | Mass | Long-term growth meas. | 3 | Liquid/Liquid | [42] |
| Mass, growth | Discriminating pathological cells | 3 | Liquid/Liquid | [43] | |
| Mass + reflectivity | Discriminating pathological cells | 5 | Liquid/Air | [63] | |
| Stiffness | Discriminating cells | 1 | Liquid/Liquid | [74] | |
| 7 | Liquid/Air | [130] | |||
| Lung cancer cell lines (human, mouse) H1650, H1975, HCC827, Tmet … | Mass, density | Comparing physical properties | 4 | Liquid/Vacuum | [56] |
| Deformability | Comparing metastatic potential | 4 | Liquid/Vacuum | [35] | |
| Multiple myeloma cell lines | Mass accumulation Rate (MAR) | Detecting drug sensitivity and predicting therapeutic response | 4 | Liquid/Vacuum | [40] |
| Glioblastoma cell lines U87, BT145, BT159… | Mass accumulation Rate (MAR) | Defining drug sensitivity or resistance | 4 | Liquid/Vacuum | [38] |
| Lymphoblastic leukaemia cell lines (mouse) L1210 | Deformability | Comparing metastatic potential | 4 | Liquid/Vacuum | [35] |
| Mass, density | Comparing physical properties | 4 | Liquid/Vacuum | [56] | |
| Mass accumulation Rate (MAR) | Defining drug sensitivity or resistance | 4 | Liquid/Vacuum | [38] | |
| Mass + SNACS | Single cell mechanics | 4 | Liquid/Vacuum | [41] | |
| Growth rate | Drug response | 4 | Liquid/Vacuum | [39] | |
| Mass | Growth efficiency monitoring | 4 | Liquid/Vacuum | [36] | |
| B cell acute lymphoblastic leukaemia primary cells | Mass accumulation Rate (MAR) | Defining drug sensitivity or resistance | 4 | Liquid/Vacuum | [38] |
| HeLa | Mass, growth | Fast mass fluctuations | 1 | Liquid/Liquid | [44] |
| Fibroblast (mouse) | Mass, growth | Fast mass fluctuations | 1 | Liquid/Liquid | [44] |
| Deformability | Mechanical characteristics | 4 | Liquid/Vacuum | [35] | |
| MDCK cells | Force | Mechanical characteristics | 8 | Liquid/Liquid | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumemura, M.; Pekin, D.; Menon, V.A.; Van Seuningen, I.; Collard, D.; Tarhan, M.C. Fabricating Silicon Resonators for Analysing Biological Samples. Micromachines 2021, 12, 1546. https://doi.org/10.3390/mi12121546
Kumemura M, Pekin D, Menon VA, Van Seuningen I, Collard D, Tarhan MC. Fabricating Silicon Resonators for Analysing Biological Samples. Micromachines. 2021; 12(12):1546. https://doi.org/10.3390/mi12121546
Chicago/Turabian StyleKumemura, Momoko, Deniz Pekin, Vivek Anand Menon, Isabelle Van Seuningen, Dominique Collard, and Mehmet Cagatay Tarhan. 2021. "Fabricating Silicon Resonators for Analysing Biological Samples" Micromachines 12, no. 12: 1546. https://doi.org/10.3390/mi12121546
APA StyleKumemura, M., Pekin, D., Menon, V. A., Van Seuningen, I., Collard, D., & Tarhan, M. C. (2021). Fabricating Silicon Resonators for Analysing Biological Samples. Micromachines, 12(12), 1546. https://doi.org/10.3390/mi12121546








