Rapid Prototyping of Organ-on-a-Chip Devices Using Maskless Photolithography
Abstract
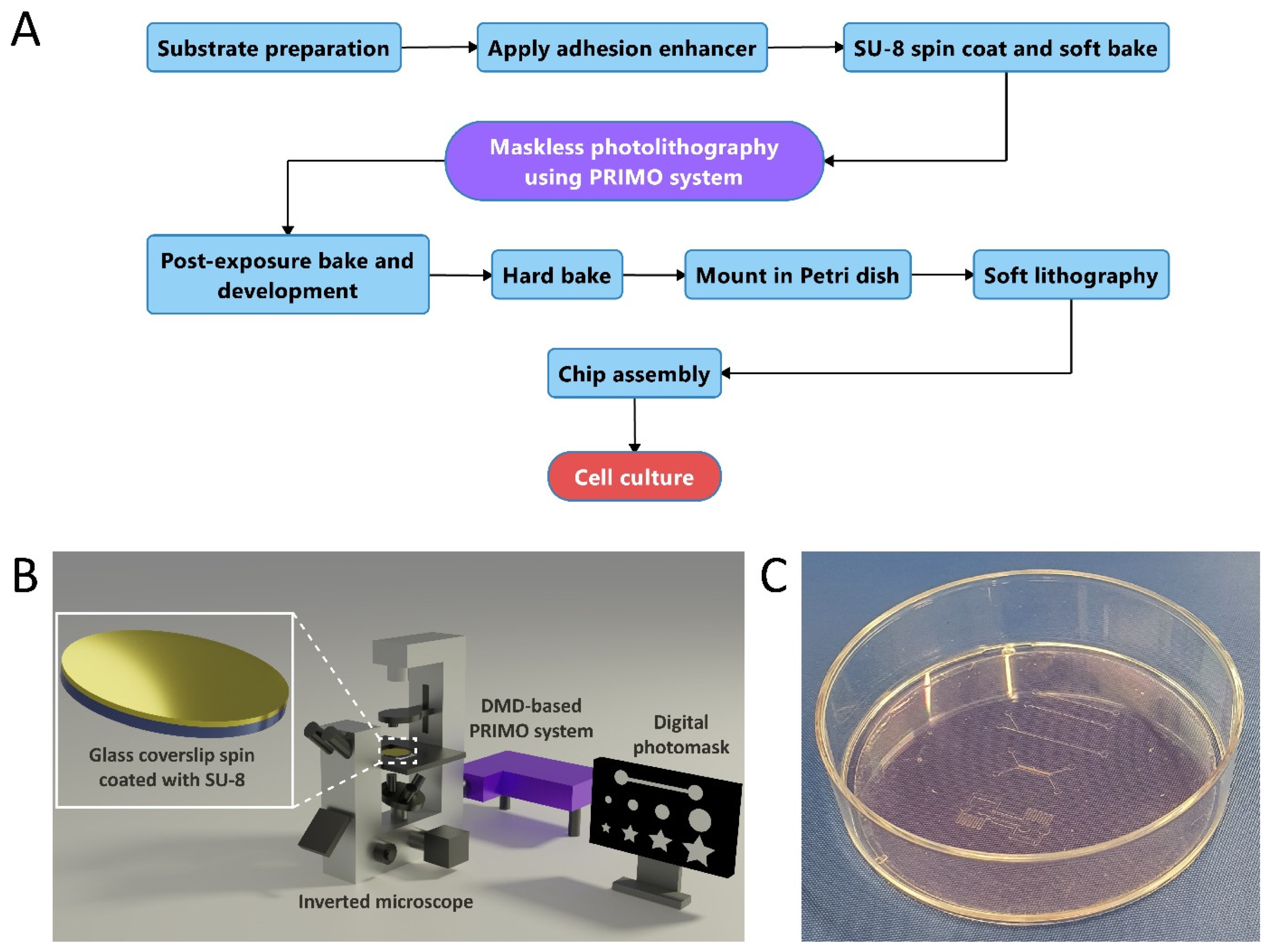
:1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. SU-8 Spin Coating and Soft Bake
- Spin coating
- (a)
- 500 rpm for a total of 10 s with 100 rpm/s acceleration
- (b)
- 1000, 2000, 3000, or 4000 rpm held for 30 s with 300 rpm/s acceleration
- (c)
- 300 rpm/s deceleration until stop
- Soft bake
2.3. Maskless Photolithography
2.4. Post-Exposure Bake, Development, and Hard-Bake
2.5. Realignment Procedure for Fabrication of the Multi-Level Neuron Chip
2.6. Scanning Electron Microscopy and Optical Profilometry
2.7. Soft Lithography and Chip Assembly
2.8. Cell Culture
2.9. On-Chip Cell Culture
2.9.1. Seeding and Culture of hiPSC-ECs in Microfluidic Chip
2.9.2. Seeding and Culture of hiPSC-vSMCs on Microgrooves
2.9.3. Seeding and Culture of hiPSC-Neurons in Multi-Level Chips
2.10. Immunofluorescence Staining
2.11. Fluorescence Imaging
3. Results and Discussion
3.1. Cleanroom-Free Microfabrication Process Flow for Organ-on-a-Chip Devices
3.2. Generation of SU-8 Microstructures Using Maskless Photolithography and Backside UV Exposure
3.3. Fabrication of Microfluidic Channels and Microgrooves
3.4. Two-Step Fabrication of Compartmentalized Multi-Level Organ-on-a-Chip Devices
3.5. One-Step Fabrication of Multi-Level Microstructures Using Grayscale Photolithography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Vunjak-Novakovic, G.; Ronaldson-Bouchard, K.; Radisic, M. Organs-on-a-chip models for biological research. Cell 2021, 184, 4597–4611. [Google Scholar] [CrossRef] [PubMed]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.; Loskill, P. Welcome to the special issue on organs-on-chip from the guest editors. Stem Cell Rep. 2021, 16, 2029–2032. [Google Scholar] [CrossRef]
- Passier, R.; Orlova, V.; Mummery, C. Complex Tissue and Disease Modeling using hiPSCs. Cell Stem Cell 2016, 18, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Voldman, J.; Gray, M.L.; Schmidt, M.A. Microfabrication in biology and medicine. Annu. Rev. Biomed. Eng. 1999, 401–425. [Google Scholar] [CrossRef] [Green Version]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Berthier, E.; Young, E.W.K.; Beebe, D. Engineers are from PDMS-land, biologists are from polystyrenia. Lab Chip 2012, 12, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Leester-Schädel, M.; Lorenz, T.; Jürgens, F.; Richter, C. Fabrication of Microfluidic Devices. In Microsystems for Pharmatechnology; Springer International Publishing: Cham, Switzerland, 2016; pp. 23–57. ISBN 9783319269207. [Google Scholar]
- Shakeri, A.; Khan, S.; Didar, T.F. Conventional and emerging strategies for the fabrication and functionalization of PDMS-based microfluidic devices. Lab Chip 2021, 21, 3053–3075. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Ng, S.H.; Li, K.H.H.; Yoon, Y.J. 3D printed microfluidics for biological applications. Lab Chip 2015, 15, 3627–3637. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [Green Version]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [Green Version]
- De Almeida Monteiro Melo Ferraz, M.; Nagashima, J.B.; Venzac, B.; Le Gac, S.; Songsasen, N. 3D printed mold leachates in PDMS microfluidic devices. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Venzac, B.; Deng, S.; Mahmoud, Z.; Lenferink, A.; Costa, A.; Bray, F.; Otto, C.; Rolando, C.; Le Gac, S. PDMS Curing Inhibition on 3D-Printed Molds: Why? Also, How to Avoid It? Anal. Chem. 2021, 93, 7180–7187. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Fu, J.Z.; Gao, Q.; Qiu, J.J. Developments of 3D Printing Microfluidics and Applications in Chemistry and Biology: A Review. Electroanalysis 2016, 28, 1658–1678. [Google Scholar] [CrossRef]
- Pinto, V.C.; Sousa, P.J.; Cardoso, V.F.; Minas, G. Optimized SU-8 processing for low-cost microstructures fabrication without cleanroom facilities. Micromachines 2014, 5, 738–755. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Byun, J.H.; Na, S.; Jeon, N.L. Fabrication of functional 3D multi-level microstructures on transparent substrates by one step back-side UV photolithography. RSC Adv. 2017, 7, 13353–13361. [Google Scholar] [CrossRef] [Green Version]
- Menon, R.; Patel, A.; Gil, D.; Smith, H.I. Maskless lithography. Mater. Today 2005, 8, 26–33. [Google Scholar] [CrossRef]
- Hasan, R.M.M.; Luo, X. Promising Lithography Techniques for Next-Generation Logic Devices. Nanomanufacturing Metrol. 2018, 1, 67–81. [Google Scholar] [CrossRef] [Green Version]
- Saghiri, A.A.; Kaden, M.; Rössler, K.; Wijnaendts, R.; Preuss, S.; Forozan, A. SU 8 multiple layer structuring by means of maskless photolithography (DWL66). Micromach. Technol. Micro-Optics Nano-Optics IV 2006, 6110, 611003. [Google Scholar] [CrossRef]
- Takahashi, K.; Setoyama, J. A UV-exposure system using DMD. Electron. Commun. Japan Part II Electron. 2000, 83, 56–58. [Google Scholar] [CrossRef]
- Dudley, D.; Duncan, W.M.; Slaughter, J. Emerging digital micromirror device (DMD) applications. MOEMS Disp. Imaging Syst. 2003, 4985, 14. [Google Scholar] [CrossRef]
- Hansotte, E.J.; Carignan, E.C.; Meisburger, W.D. High speed maskless lithography of printed circuit boards using digital micromirrors. Emerg. Digit. Micromirror Device Based Syst. Appl. III 2011, 7932, 793207. [Google Scholar] [CrossRef]
- Rammohan, A.; Dwivedi, P.K.; Martinez-Duarte, R.; Katepalli, H.; Madou, M.J.; Sharma, A. One-step maskless grayscale lithography for the fabrication of 3-dimensional structures in SU-8. Sens. Actuators B Chem. 2011, 153, 125–134. [Google Scholar] [CrossRef]
- Dinh, D.H.; Chien, H.L.; Lee, Y.C. Maskless lithography based on digital micromirror device (DMD) and double sided microlens and spatial filter array. Opt. Laser Technol. 2019, 113, 407–415. [Google Scholar] [CrossRef]
- Deng, Q.; Yang, Y.; Gao, H.; Zhou, Y.; He, Y.; Hu, S. Fabrication of micro-optics elements with arbitrary surface profiles based on one-step maskless grayscale lithography. Micromachines 2017, 8, 314. [Google Scholar] [CrossRef]
- Barbu, I.; Ivan, M.G.; Giesen, P.; Van de Moosdijk, M.; Meinders, E.R. Advances in maskless and mask-based optical lithography on plastic flexible substrates. Lithogr. Asia 2009 2009, 7520, 75200A. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Quaglio, M.; Pirri, F.; Cheng, Y.-C.; Busacker, D.; Cerrina, F. Patterning of SU-8 resist with digital micromirror device (DMD) maskless lithography. Opt. Microlithogr. XXII 2009, 7274, 72742O. [Google Scholar] [CrossRef]
- Zhong, K.; Gao, Y.; Li, F.; Zhang, Z.; Luo, N. Fabrication of PDMS microlens array by digital maskless grayscale lithography and replica molding technique. Optik (Stuttg) 2014, 125, 2413–2416. [Google Scholar] [CrossRef]
- Hamid, Q.; Wang, C.; Snyder, J.; Sun, W. Surface modification of SU-8 for enhanced cell attachment and proliferation within microfluidic chips. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 473–484. [Google Scholar] [CrossRef]
- Pilloni, O.; Madou, M.; Mendoza, D.; Muhl, S.; Oropeza-Ramos, L. Methodology and fabrication of adherent and crack-free SU-8 photoresist-derived carbon MEMS on fused silica transparent substrates. J. Micromechanics Microengineering 2019, 29, 027002. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, J.; Xiong, Z.; Liu, H.; Wang, L.; Gu, Y.; Lu, Z.; Li, J.; Huang, J. User-defined microstructures array fabricated by DMD based multistep lithography with dose modulation. Opt. Express 2019, 27, 31956. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Han, C.; Jeon, H. Submicrometer-scale pattern generation via maskless digital photolithography. Optica 2020, 7, 1788. [Google Scholar] [CrossRef]
- Basu, A.K.; Basak, A.; Bhattacharya, S. Geometry and thickness dependant anomalous mechanical behavior of fabricated SU-8 thin film micro-cantilevers. J. Micromanufacturing 2020, 3, 113–120. [Google Scholar] [CrossRef]
- Diez, S. The next generation of maskless lithography. Emerg. Digit. Micromirror Device Based Syst. Appl. VIII 2016, 9761, 976102. [Google Scholar] [CrossRef]
- Owens, B. The microscope makers. Nature 2017, 551, 659–662. [Google Scholar] [CrossRef] [Green Version]
- Souquet, B.; Opitz, M.; Vianay, B.; Brunet, S.; Théry, M. Manufacturing a Bone Marrow-On-A-Chip Using Maskless Photolithography. In Bone Marrow Environment: Methods and Protocols; Espéli, M., Balabanian, K., Eds.; Springer: New York, NY, USA, 2021; ISBN 978-1-0716-1425-9. [Google Scholar]
- Li, X.; Soler, M.; Özdemir, C.I.; Belushkin, A.; Yesilköy, F.; Altug, H. Plasmonic nanohole array biosensor for label-free and real-time analysis of live cell secretion. Lab Chip 2017, 17, 2208–2217. [Google Scholar] [CrossRef]
- Thurgood, P.; Suarez, S.A.; Chen, S.; Gilliam, C.; Pirogova, E.; Jex, A.R.; Baratchi, S.; Khoshmanesh, K. Self-sufficient, low-cost microfluidic pumps utilising reinforced balloons. Lab Chip 2019, 19, 2885–2896. [Google Scholar] [CrossRef]
- Thurgood, P.; Baratchi, S.; Arash, A.; Pirogova, E.; Jex, A.R.; Khoshmanesh, K. Asynchronous generation of oil droplets using a microfluidic flow focusing system. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Tovar-Lopez, F.J.; Scott, J.; Ghorbani, K. Differential microwave sensor for characterization of glycerol–water solutions. Sensors Actuators B Chem. 2020, 321, 128561. [Google Scholar] [CrossRef]
- Strale, P.O.; Azioune, A.; Bugnicourt, G.; Lecomte, Y.; Chahid, M.; Studer, V. Multiprotein Printing by Light-Induced Molecular Adsorption. Adv. Mater. 2016, 28, 2024–2029. [Google Scholar] [CrossRef] [PubMed]
- Pasturel, A.; Strale, P.O.; Studer, V. Tailoring Common Hydrogels into 3D Cell Culture Templates. Adv. Healthc. Mater. 2020, 2000519, 1–8. [Google Scholar] [CrossRef]
- Jimenez, A.J.; Schaeffer, A.; De Pascalis, C.; Letort, G.; Vianay, B.; Bornens, M.; Piel, M.; Blanchoin, L.; Théry, M. Acto-myosin network geometry defines centrosome position. Curr. Biol. 2021, 31, 1206–1220.e5. [Google Scholar] [CrossRef]
- PRIMO: Micropatterning, Microfabrication and Hydrogel Structuration. Available online: https://www.alveolelab.com/our-products/primo-micropatterning/ (accessed on 26 November 2021).
- Toro-Nahuelpan, M.; Zagoriy, I.; Senger, F.; Blanchoin, L.; Théry, M.; Mahamid, J. Tailoring cryo-electron microscopy grids by photo-micropatterning for in-cell structural studies. Nat. Methods 2020, 17, 50–54. [Google Scholar] [CrossRef]
- Fenech, M.; Girod, V.; Claveria, V.; Meance, S.; Abkarian, M.; Charlot, B. Microfluidic blood vasculature replicas using backside lithography. Lab Chip 2019, 19, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Halaidych, O.V.; Freund, C.; van den Hil, F.; Salvatori, D.C.F.; Riminucci, M.; Mummery, C.L.; Orlova, V.V. Inflammatory Responses and Barrier Function of Endothelial Cells Derived from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 1642–1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; D’Aniello, C.; Verkerk, A.O.; Wrobel, E.; Frank, S.; Ward-van Oostwaard, D.; Piccini, I.; Freund, C.; Rao, J.; Seebohm, G.; et al. Recessive cardiac phenotypes in induced pluripotent stem cell models of Jervell and Lange-Nielsen syndrome: Disease mechanisms and pharmacological rescue. Proc. Natl. Acad. Sci. USA 2014, 111, E5383–E5392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buijsen, R.A.M.; Gardiner, S.L.; Bouma, M.J.; van der Graaf, L.M.; Boogaard, M.W.; Pepers, B.A.; Eussen, B.; de Klein, A.; Freund, C.; van Roon-Mom, W.M.C. Generation of 3 spinocerebellar ataxia type 1 (SCA1) patient-derived induced pluripotent stem cell lines LUMCi002-A, B, and C and 2 unaffected sibling control induced pluripotent stem cell lines LUMCi003-A and B. Stem Cell Res. 2018, 29, 125–128. [Google Scholar] [CrossRef]
- Orlova, V.V.; Van Den Hil, F.E.; Petrus-Reurer, S.; Drabsch, Y.; Ten Dijke, P.; Mummery, C.L. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat. Protoc. 2014, 9, 1514–1531. [Google Scholar] [CrossRef] [Green Version]
- Halaidych, O.V.; Cochrane, A.; van den Hil, F.E.; Mummery, C.L.; Orlova, V.V. Quantitative Analysis of Intracellular Ca2+ Release and Contraction in hiPSC-Derived Vascular Smooth Muscle Cells. Stem Cell Rep. 2019, 12, 647–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Song, D.; Zong, G.; Yin, P.; Zhang, X.; Xu, Z.; Du, L.; Liu, C.; Wang, L. Fabrication of SU-8 moulds on glass substrates by using a common thin negative photoresist as an adhesive layer. J. Micromechanics Microengineering 2014, 24, 035009. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, J.; Zheng, Q.; Hu, Y. Multi-layer lithography using focal plane changing for SU-8 microstructures. Mater. Res. Express 2020, 7, 065306. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Fabrication of multi-layer SU-8 microstructures. J. Micromechanics Microengineering 2006, 16, 276–284. [Google Scholar] [CrossRef]
- Johari, S.; Tamilchelvan, N.; Nor, M.N.M.; Ramli, M.M.; Taib, B.N.; Mazalan, M.; Wahab, Y. The effect of softbaking temperature on SU-8 photoresist performance. In Proceedings of the 2014 IEEE International Conference on Semiconductor Electronics (ICSE2014), Kuala Lumpur, Malaysia, 27–29 August 2014; pp. 467–470. [Google Scholar] [CrossRef]
- Peterman, M.C.; Huie, P.; Bloom, D.M.; Fishman, H.A. Building thick photoresist structures from the bottom up. J. Micromechanics Microengineering 2003, 13, 380–382. [Google Scholar] [CrossRef]
- Lee, J.B.; Choi, K.H.; Yoo, K. Innovative SU-8 lithography techniques and their applications. Micromachines 2015, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Jackson-Holmes, E.L.; McDevitt, T.C.; Lu, H. A microfluidic trap array for longitudinal monitoring and multi-modal phenotypic analysis of individual stem cell aggregates. Lab Chip 2017, 17, 3634–3642. [Google Scholar] [CrossRef] [PubMed]
- Alford, P.W.; Nesmith, A.P.; Seywerd, J.N.; Grosberg, A.; Parker, K.K. Vascular smooth muscle contractility depends on cell shape. Integr. Biol. 2011, 3, 1063–1070. [Google Scholar] [CrossRef]
- Ye, G.J.C.; Nesmith, A.P.; Parker, K.K. The role of mechanotransduction on vascular smooth muscle myocytes cytoskeleton and contractile function. Anat. Rec. 2014, 297, 1758–1769. [Google Scholar] [CrossRef] [Green Version]
- Carrabba, M.; Madeddu, P. Current strategies for the manufacture of small size tissue engineering vascular grafts. Front. Bioeng. Biotechnol. 2018, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The tissue-engineered vascular graft—Past, present, and future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Kwong, G.; Marquez, H.A.; Yang, C.; Wong, J.Y.; Kotton, D.N. Generation of a Purified iPSC-Derived Smooth Muscle-like Population for Cell Sheet Engineering. Stem Cell Rep. 2019, 13, 499–514. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.M.; Rhee, S.W.; Tu, C.H.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. Microfluidic multicompartment device for neuroscience research. Langmuir 2003, 19, 1551–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef]
- Loomis, J.; Ratnayake, D.; McKenna, C.; Walsh, K.M. Grayscale lithography—automated mask generation for complex three-dimensional topography. J. Micro/Nanolithography MEMS MOEMS 2016, 15, 013511. [Google Scholar] [CrossRef]
- Lee, G.H.; Lee, J.S.; Oh, H.J.; Lee, S.H. Reproducible construction of surface tension-mediated honeycomb concave microwell arrays for engineering of 3D microtissues with minimal cell loss. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| 50 °C | 65 °C | 95 °C | |
|---|---|---|---|
| 1000 rpm | 6 min | 15 min | 45 min |
| 2000 rpm | 4 min | 10 min | 25 min |
| 3000 rpm | 2 min | 5 min | 12 min |
| 4000 rpm | 2 min | 5 min | 10 min |
| 50 °C | 65 °C | 95 °C | |
|---|---|---|---|
| 2000 rpm | Not needed | 2 min | 4 min |
| 4000 rpm | Not needed | 2 min | 4 min |
| 50 °C | 65 °C | 95 °C | |
|---|---|---|---|
| 1000 rpm | 6 min | 12 min | 25 min |
| 2000 rpm | 4 min | 10 min | 20 min |
| 3000 rpm | 2 min | 4 min | 10 min |
| 4000 rpm | 2 min | 4 min | 10 min |
| 50 °C | 65 °C | 95 °C | |
|---|---|---|---|
| 2000 rpm | Not needed | 2 min | 4 min |
| 4000 rpm | Not needed | 2 min | 4 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasi, D.G.; de Graaf, M.N.S.; Motreuil-Ragot, P.A.; Frimat, J.-P.M.S.; Ferrari, M.D.; Sarro, P.M.; Mastrangeli, M.; van den Maagdenberg, A.M.J.M.; Mummery, C.L.; Orlova, V.V. Rapid Prototyping of Organ-on-a-Chip Devices Using Maskless Photolithography. Micromachines 2022, 13, 49. https://doi.org/10.3390/mi13010049
Kasi DG, de Graaf MNS, Motreuil-Ragot PA, Frimat J-PMS, Ferrari MD, Sarro PM, Mastrangeli M, van den Maagdenberg AMJM, Mummery CL, Orlova VV. Rapid Prototyping of Organ-on-a-Chip Devices Using Maskless Photolithography. Micromachines. 2022; 13(1):49. https://doi.org/10.3390/mi13010049
Chicago/Turabian StyleKasi, Dhanesh G., Mees N. S. de Graaf, Paul A. Motreuil-Ragot, Jean-Phillipe M. S. Frimat, Michel D. Ferrari, Pasqualina M. Sarro, Massimo Mastrangeli, Arn M. J. M. van den Maagdenberg, Christine L. Mummery, and Valeria V. Orlova. 2022. "Rapid Prototyping of Organ-on-a-Chip Devices Using Maskless Photolithography" Micromachines 13, no. 1: 49. https://doi.org/10.3390/mi13010049
APA StyleKasi, D. G., de Graaf, M. N. S., Motreuil-Ragot, P. A., Frimat, J.-P. M. S., Ferrari, M. D., Sarro, P. M., Mastrangeli, M., van den Maagdenberg, A. M. J. M., Mummery, C. L., & Orlova, V. V. (2022). Rapid Prototyping of Organ-on-a-Chip Devices Using Maskless Photolithography. Micromachines, 13(1), 49. https://doi.org/10.3390/mi13010049








