Loading and Release of Charged and Neutral Fluorescent Dyes into and from Mesoporous Materials: A Key Role for Sensing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Buffer Solutions
2.3. Determination of Molar Absorption Coefficient of the Dyes
2.4. Synthesis of Mesoporous MCM-41-Type Silica Nanoparticles (MSN)
2.5. Materials Characterisation
2.6. Loading of Dyes into MCM-41
2.7. Quantification of Dye Loaded
2.8. Dye Release Experiments
3. Results
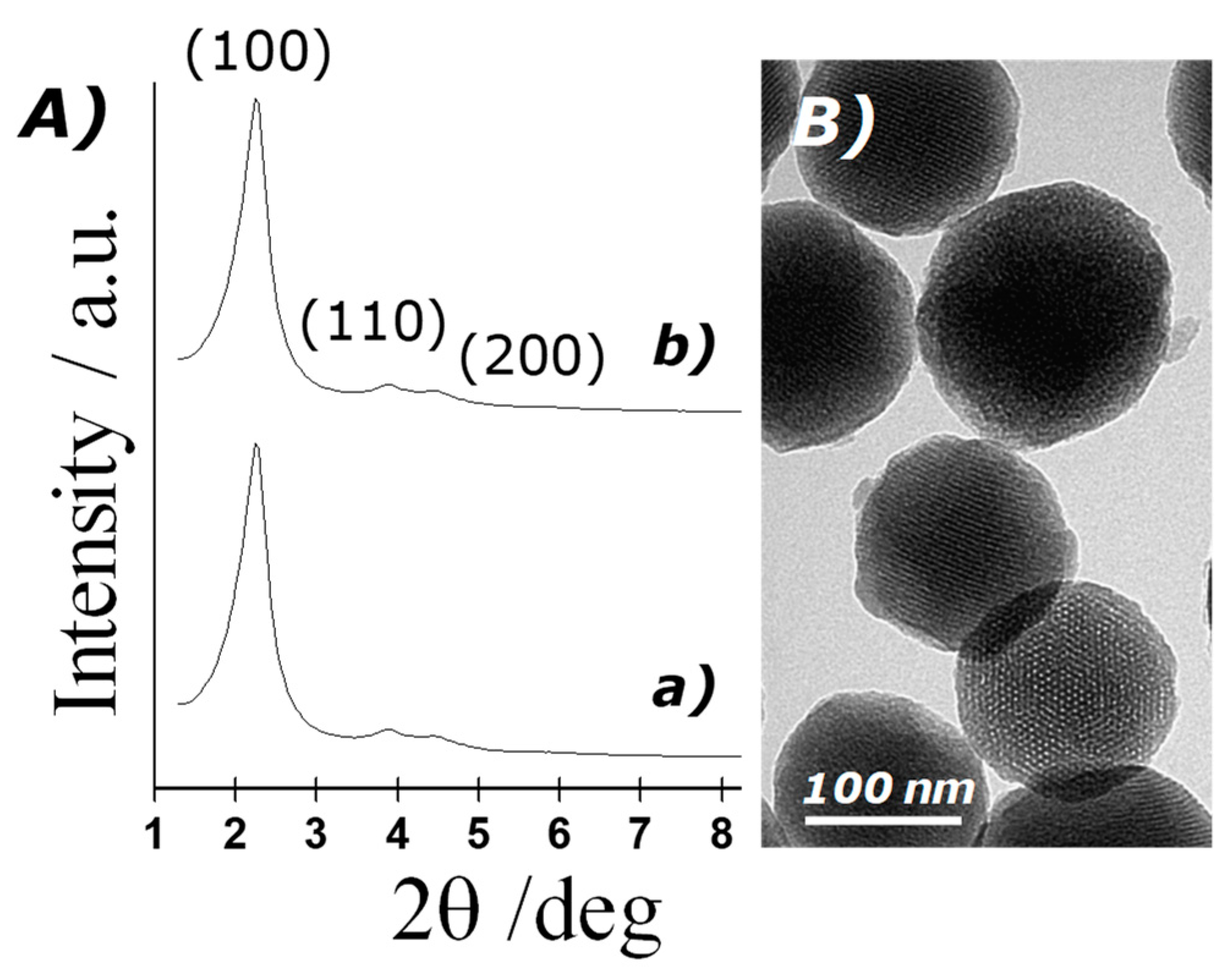
3.1. Characterisation of Silica Mesoporous Nanoparticles
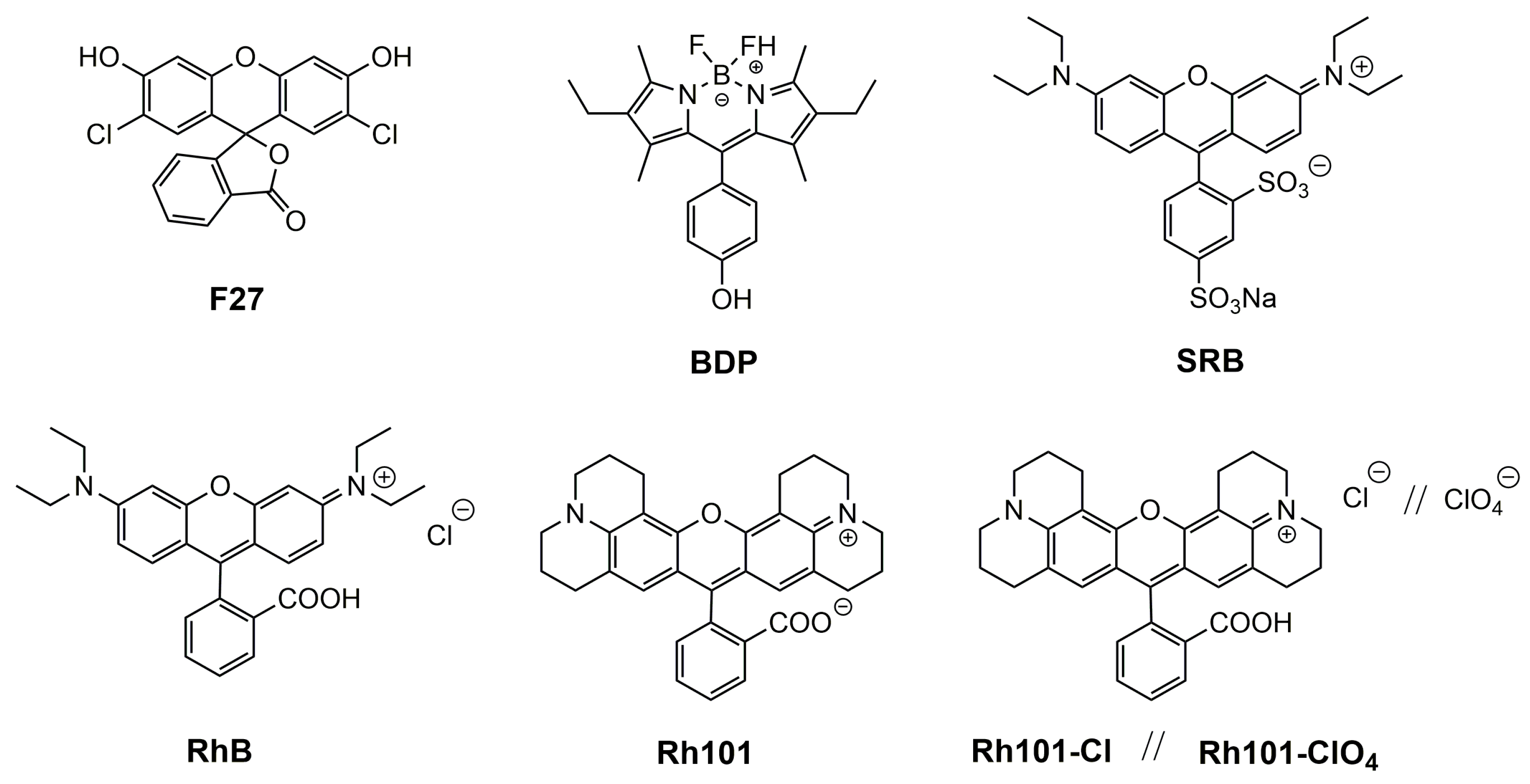
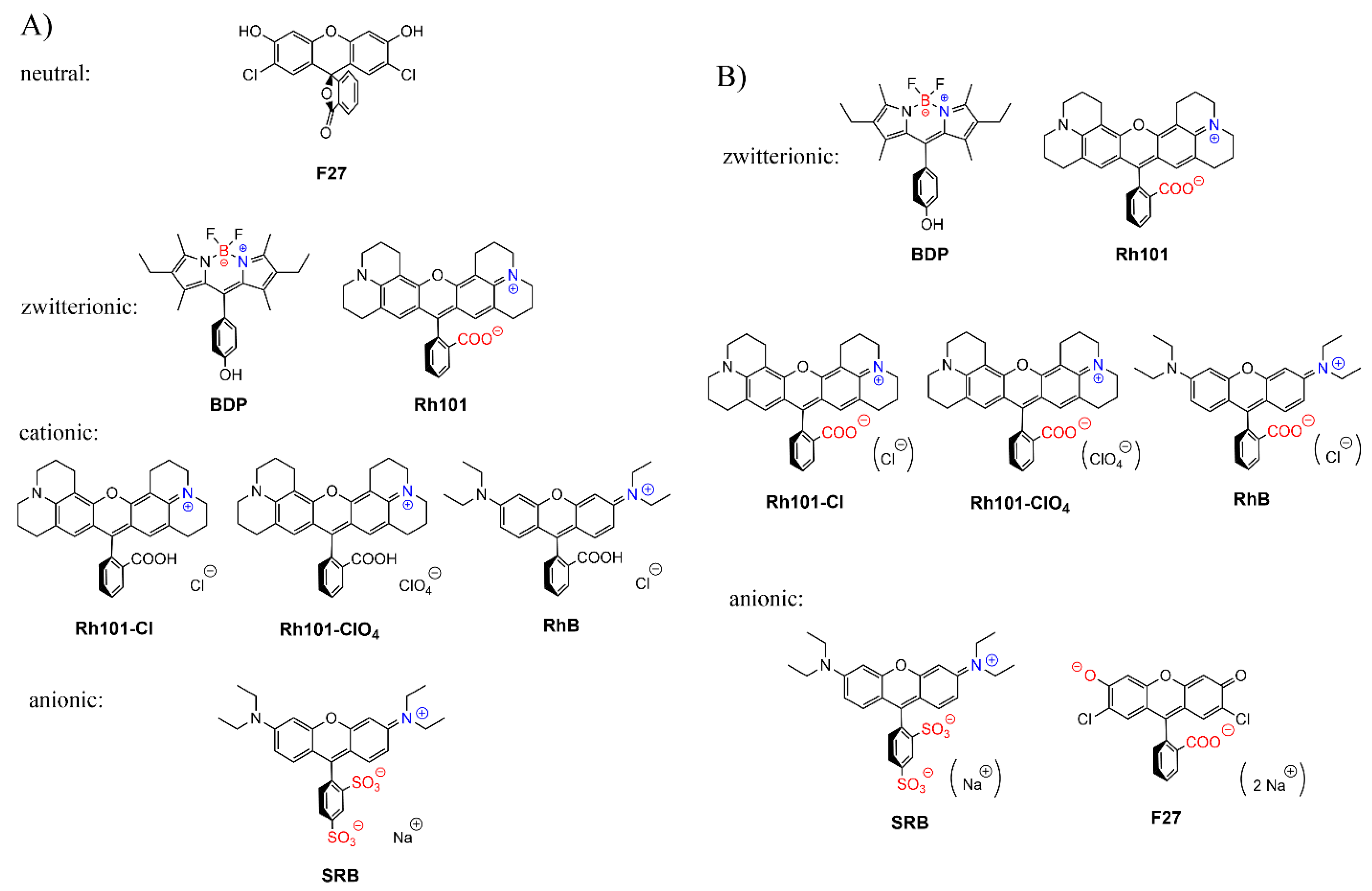
3.2. Selection of Dyes and Loading Procedure
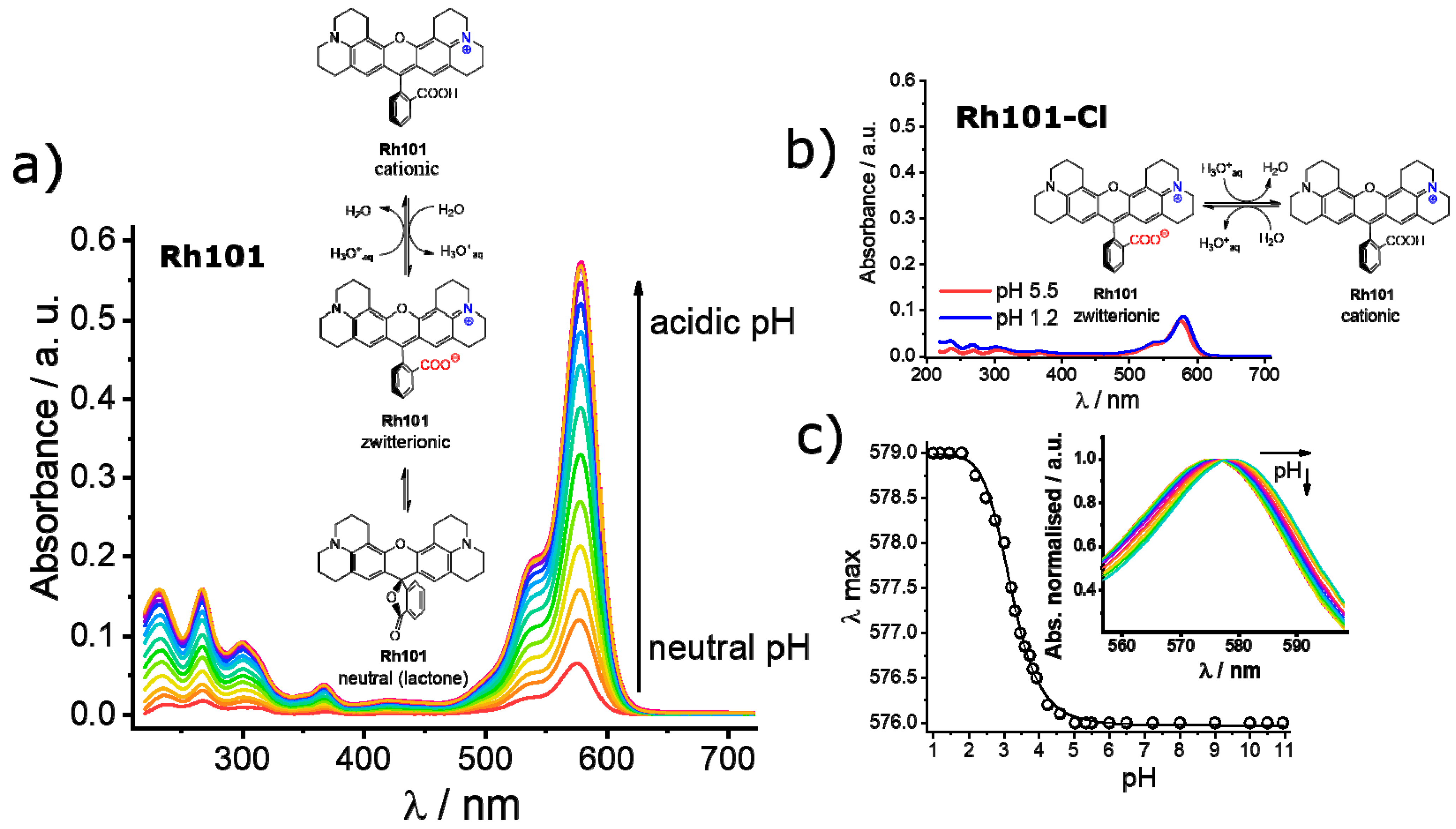
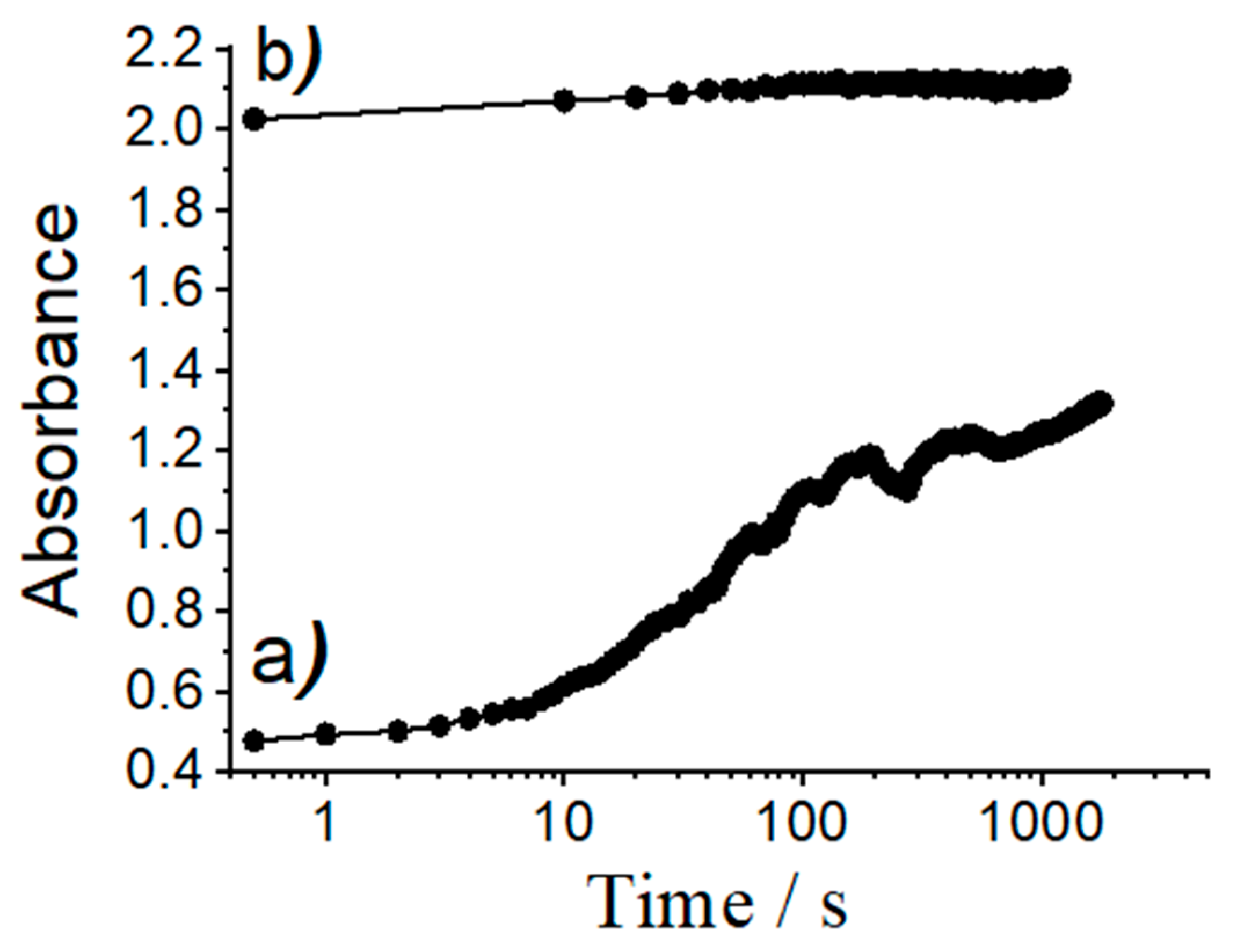
3.3. Loading and Release Studies
4. Discussion
- (i)
- Dye and solvent: the solvation process of the dye is mainly affected by dipole interactions and polarizability properties, depending on dipole moments and charge delocalisation. In addition, if a counter-ion is present, the equilibrium between well-solvated dye ion (D)S and well-solvated counter-ion (I)S, a solvated ternary complex of a dye, a solvent molecule and a counter-ion (D•S•I)S as well as a solvated yet tightly bound ion pair (D•I)S has to be considered as a consequence of the electrostatic forces at play. These effects can stabilise the dissolution of the compound and reduce the adsorption ability of the dye within the material. On the other hand, a low solubility in the bulk solvent can enhance the adsorption into the pores of a material because of the favoured affinity between the surface functional groups and dye molecule.
- (ii)
- Dye and dye: hydrophobic interactions such as π stacking between two dye molecules can influence the loading and release process.
- (iii)
- Dye and silica surface: a satisfactory degree of loading can only be achieved if the interactions of the dye and the material are sufficiently strong. However, too strong interactions are not favoured because they would hinder a fast desorption process.
- (iv)
- Silica surface: the surface pH of the porous material can affect the dissolution of the dye as a function of its own charge state, which is connected to the solvent’s bulk pH. Furthermore, the pore size and shape may have an influence on the mass transfer as well [65].
- A-materials: BDP < F27 < SRB < Rh101-ClO4 < Rh101-Cl ~ Rh101 < RhB
- B-materials: BDP < Rh101-ClO4 < Rh101-Cl < F27 < RhB < Rh101 < SRB
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Koryakina, I.; Kuznetsova, D.S.; Zuev, D.A.; Milichko, V.A.; Timin, A.S.; Zyuzin, M.V. Optically responsive delivery platforms: From the design considerations to biomedical applications. Nanophotonics 2020, 9, 39–74. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mazumder, P.; Joshi, M.; Joshi, C.; Dalvi, S.V.; Kumar, M. Biomedical application, drug delivery and metabolic pathway of antiviral nanotherapeutics for combating viral pandemic: A review. Environ. Res. 2020, 191, 110119. [Google Scholar] [CrossRef] [PubMed]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camara, M.C.; Campos, E.V.R.; Monteiro, R.A.; do Espirito Santo Pereira, A.; de Freitas Proença, P.L.; Fraceto, L.F. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol. 2019, 17, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Sung, W.; Kim, T.Y.; Yang, S.J.; Kim, S.; Lee, G.; Cho, K.; Hahn, S.K. Upconversion nanoparticles coated organic photovoltaics for near infrared light controlled drug delivery systems. Nano Energy 2021, 81, 105650. [Google Scholar] [CrossRef]
- Andersson Trojer, M.; Nordstierna, L.; Bergek, J.; Blanck, H.; Holmberg, K.; Nydén, M. Use of microcapsules as controlled release devices for coatings. Adv. Colloid Interface Sci. 2015, 222, 18–43. [Google Scholar] [CrossRef]
- Costa, R.; Santos, L. Delivery systems for cosmetics—From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Xu, Y.; He, K.; Wang, H.; Li, M.; Shen, T.; Liu, X.; Yuan, C.; Dai, L. Self-Assembly Behavior and pH-Stimuli-Responsive Property of POSS-Based Amphiphilic Block Copolymers in Solution. Micromachines 2018, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.C.M.; Liao, P.; Wei, T.; Zhang, L.; Sun, D. Magnetically Powered Biodegradable Microswimmers. Micromachines 2020, 11, 404. [Google Scholar] [CrossRef]
- Campbell, J.; Kastania, G.; Volodkin, D. Encapsulation of Low-Molecular-Weight Drugs into Polymer Multilayer Capsules Templated on Vaterite CaCO3 Crystals. Micromachines 2020, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Iturrioz-Rodríguez, N.; Correa-Duarte, M.A.; Fanarraga, M.L. Controlled drug delivery systems for cancer based on mesoporous silica nanoparticles. Int. J. Nanomed. 2019, 14, 3389–3401. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous Silica Nanoparticles for Intracellular Controlled Drug Delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Lozano, D.; Gonzalez, B.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regi, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: An update. Expert Opin. Drug Deliv. 2019, 16, 415–439. [Google Scholar] [CrossRef] [PubMed]
- De Vos, D.E.; Dams, M.; Sels, B.F.; Jacobs, P.A. Ordered Mesoporous and Microporous Molecular Sieves Functionalized with Transition Metal Complexes as Catalysts for Selective Organic Transformations. Chem. Rev. 2002, 102, 3615–3640. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.B.; Zou, R.Q.; Zhao, Y.L. Heterogeneous Catalysis in Zeolites, Mesoporous Silica, and Metal-Organic Frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Na, J.; Konarova, M.; Wakihara, T.; Yamauchi, Y.; Salomon, C.; Gawande, M.B. Functional Mesoporous Silica Nanomaterials for Catalysis and Environmental Applications. Bull. Chem. Soc. Jpn. 2020, 93, 1459–1496. [Google Scholar] [CrossRef]
- Kumar, P.; Guliants, V.V. Periodic mesoporous organic-inorganic hybrid materials: Applications in membrane separations and adsorption. Microporous Mesoporous Mater. 2010, 132, 1–14. [Google Scholar] [CrossRef]
- Crandall, B.S.; Zhang, J.Y.; Stavila, V.; Allendorf, M.D.; Li, Z.L. Desulfurization of Liquid Hydrocarbon Fuels with Microporous and Mesoporous Materials: Metal-Organic Frameworks, Zeolites, and Mesoporous Silicas. Ind. Eng. Chem. Res. 2019, 58, 19322–19352. [Google Scholar] [CrossRef]
- Mathieu, Y.; Tzanis, L.; Soulard, M.; Patarin, J.; Vierling, M.; Moliere, M. Adsorption of SOx by oxide materials: A review. Fuel Process. Technol. 2013, 114, 81–100. [Google Scholar] [CrossRef]
- Peluso, A.; Gargiulo, N.; Aprea, P.; Pepe, F.; Caputo, D. Nanoporous Materials as H2S Adsorbents for Biogas Purification: A Review. Sep. Purif. Rev. 2019, 48, 78–89. [Google Scholar] [CrossRef]
- Li, P.Z.; Zhao, Y.L. Nitrogen-Rich Porous Adsorbents for CO2 Capture and Storage. Chem. Asian J. 2013, 8, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Oschatz, M.; Antonietti, M. A search for selectivity to enable CO2 capture with porous adsorbents. Energy Environ. Sci. 2018, 11, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Ispas, C.; Sokolov, I.; Andreescu, S. Enzyme-functionalized mesoporous silica for bioanalytical applications. Anal. Bioanal. Chem. 2009, 393, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Izquierdo-barba, I.; Ruiz-González, M.; Doadrio, J.; Gonzalez-Calbet, J.; Vallet-Regí, M. Tissue regeneration: A new property of mesoporous materials. Solid State Sci. 2005, 7, 983–989. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Chenab, K.K.; Taheri-Ledari, R.; Mosafer, J.; Hashemi, S.M.; Mokhtarzadeh, A.; Maleki, A.; Hamblin, M.R. Recent advances in the application of mesoporous silica-based nanomaterials for bone tissue engineering. Mater. Sci. Eng. C 2020, 107, 110267. [Google Scholar] [CrossRef]
- Gibson, L.T. Mesosilica materials and organic pollutant adsorption: Part B removal from aqueous solution. Chem. Soc. Rev. 2014, 43, 5173–5182. [Google Scholar] [CrossRef]
- Li, C.M.; Wang, X.P.; Jiao, Z.H.; Zhang, Y.S.; Yin, X.B.; Cui, X.M.; Wei, Y.Z. Functionalized Porous Silica-Based Nano/Micro Particles for Environmental Remediation of Hazard Ions. Nanomaterials 2019, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Thirunavukkarasu, A.; Nithya, R.; Sivashankar, R. A review on the role of nanomaterials in the removal of organic pollutants from wastewater. Rev. Environ. Sci. Bio/Technol. 2020, 19, 751–778. [Google Scholar] [CrossRef]
- Hecht, M.; Climent, E.; Biyikal, M.; Sancenón, F.; Martínez-Máñez, R.; Rurack, K. Gated hybrid delivery systems: En route to sensory materials with inherent signal amplification. Coord. Chem. Rev. 2013, 257, 2589–2606. [Google Scholar] [CrossRef]
- Pla, L.; Lozano-Torres, B.; Martinez-Manez, R.; Sancenon, F.; Ros-Lis, J.V. Overview of the Evolution of Silica-Based Chromo-Fluorogenic Nanosensors. Sensors 2019, 19, 5138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.Y.; Qiu, P.P.; Yang, J.P.; Fan, Y.C.; Wang, L.J.; Jiang, W.; Cheng, X.W.; Deng, Y.H.; Luo, W. Mesoporous Materials-Based Electrochemical Biosensors from Enzymatic to Nonenzymatic. Small 2019, 1904022, e1904022. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.G.; Ma, X.Q.; Gao, Y.E.; Hou, M.L.; Xue, P.; Li, C.M.; Kang, Y.J. Multifunctional silica nanoparticles as a promising theranostic platform for biomedical applications. Mater. Chem. Front. 2017, 1, 1257–1272. [Google Scholar] [CrossRef]
- Kumar, P.; Tambe, P.; Paknikar, K.M.; Gajbhiye, V. Mesoporous silica nanoparticles as cutting-edge theranostics: Advancement from merely a carrier to tailor-made smart delivery platform. J. Control. Release 2018, 287, 35–57. [Google Scholar] [CrossRef]
- Zuo, B.; Li, W.F.; Wu, X.Q.; Wang, S.G.; Deng, Q.Y.; Huang, M.X. Recent Advances in the Synthesis, Surface Modifications and Applications of Core-Shell Magnetic Mesoporous Silica Nanospheres. Chem. Asian J. 2020, 15, 1248–1265. [Google Scholar] [CrossRef]
- Aznar, E.; Oroval, M.; Pascual, L.; Murguía, J.R.; Martínez-Máñez, R.; Sancenón, F. Gated Materials for On-Command Release of Guest Molecules. Chem. Rev. 2016, 116, 561–718. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Saldarriaga, C.; Montes, C.; Garces, J.; Crowdert, C. A molecular sieve with eighteen-membered rings. Nature 1988, 331, 698–699. [Google Scholar] [CrossRef]
- Moore, P.B.; Shen, J. An X-ray structural study of cacoxenite, a mineral phosphate. Nature 1983, 306, 356–358. [Google Scholar] [CrossRef]
- Estermann, M.; McCusker, L.B.; Baerlocher, C.; Merrouche, A.; Kessler, H. A synthetic gallophosphate molecular sieve with a 20-tetrahedral-atom pore opening. Nature 1991, 352, 320–323. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-Based Mesoporous Organic–Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Brunel, D.; Cauvel, A.; Di Renzo, F.; Fajula, F.; Fubini, B.; Onida, B.; Garrone, E. Preferential grafting of alkoxysilane coupling agents on the hydrophobic portion of the surface of micelle-templated silica. New J. Chem. 2000, 24, 807–813. [Google Scholar] [CrossRef]
- Wu, X.; Wu, M.; Zhao, J.X. Recent development of silica nanoparticles as delivery vectors for cancer imaging and therapy. Nanomedicine 2014, 10, 297–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Descalzo, A.B.; Martinez-Manez, R.; Sancenon, R.; Hoffmann, K.; Rurack, K. The supramolecular chemistry of organic-inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 5924–5948. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Climent, E.; Ast, S.; Weller, M.G.; Canning, J.; Rurack, K. Development of a lateral flow test for rapid pyrethroid detection using antibody-gated indicator-releasing hybrid materials. Analyst 2020, 145, 3490–3494. [Google Scholar] [CrossRef]
- Climent, E.; Biyikal, M.; Gröninger, D.; Weller, M.G.; Martínez-Máñez, R.; Rurack, K. Multiplexed Detection of Analytes on Single Test Strips with Antibody-Gated Indicator-Releasing Mesoporous Nanoparticles. Angew. Chem. Int. Ed. 2020, 59, 23862–23869. [Google Scholar] [CrossRef] [PubMed]
- Ibragimova, A.R.; Gabdrakhmanov, D.R.; Khamatgalimov, A.R.; Saifina, A.F.; Gubaidullin, A.T.; Egorova, S.R.; Lamberov, A.A.; Danilaev, M.P.; Zakharova, L.Y. Nanosized carriers for hydrophobic compounds based on mesoporous silica: Synthesis and adsorption properties. Russ. Chem. Bull. 2019, 68, 1358–1365. [Google Scholar] [CrossRef]
- Colilla, M.; Manzano, M.; Vallet-Regí, M. Recent advances in ceramic implants as drug delivery systems for biomedical applications. Int. J. Nanomed. 2008, 3, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Latifi, L.; Sohrabnezhad, S. Influence of pore size and surface area of mesoporous silica materilas (MCM-41 and KIT-6) on the drug loading and release. J. Sol-Gel Sci. Technol. 2018, 87, 626–638. [Google Scholar] [CrossRef]
- Fernández-Núñez, M.; Zorrilla, D.; Montes, A.; Mosquera, M.J. Ibuprofen Loading in Surfactant-Templated Silica: Role of the Solvent According to the Polarizable Continuum Model. J. Phys. Chem. A 2009, 113, 11367–11375. [Google Scholar] [CrossRef]
- Šoltys, M.; Kovačík, P.; Dammer, O.; Beránek, J.; Štěpánek, F. Effect of solvent selection on drug loading and amorphisation in mesoporous silica particles. Int. J. Pharm. 2019, 555, 19–27. [Google Scholar] [CrossRef]
- Rimola, A.; Costa, D.; Sodupe, M.; Lambert, J.-F.; Ugliengo, P. Silica Surface Features and Their Role in the Adsorption of Biomolecules: Computational Modeling and Experiments. Chem. Rev. 2013, 113, 4216–4313. [Google Scholar] [CrossRef] [Green Version]
- Lian, H.-Y.; Liang, Y.-H.; Yamauchi, Y.; Wu, K.C.W. A Hierarchical Study on Load/Release Kinetics of Guest Molecules into/from Mesoporous Silica Thin Films. J. Phys. Chem. C 2011, 115, 6581–6590. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhi, Z.; Jiang, T.; Zhang, J.; Wang, Z.; Wang, S. Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J. Control. Release 2010, 145, 257–263. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin. Drug Delivery 2019, 16, 219–237. [Google Scholar] [CrossRef]
- Li, Z.; Nyalosaso, J.L.; Hwang, A.A.; Ferris, D.P.; Yang, S.; Derrien, G.; Charnay, C.; Durand, J.-O.; Zink, J.I. Measurement of Uptake and Release Capacities of Mesoporous Silica Nanoparticles Enabled by Nanovalve Gates. J. Phys. Chem. C 2011, 115, 19496–19506. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Jian, D.; Wang, S. Investigation of loading and release of guest molecules from hollow mesoporous silica spheres. Micro Nano Lett. 2011, 6, 802–805. [Google Scholar] [CrossRef]
- Kärger, J.; Valiullin, R. Mass transfer in mesoporous materials: The benefit of microscopic diffusion measurement. Chem. Soc. Rev. 2013, 42. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Brackmann, U. Lambdachrome® Laser Dyes, 3rd ed.; Lambda Physik AG: Gottingen, Germany, 2000. [Google Scholar]
- Karpiuk, J.; Grabowski, Z.R.; De Schryver, F.C. Photophysics of the Lactone Form of Rhodamine 101. J. Phys. Chem. 1994, 98, 3247–3256. [Google Scholar] [CrossRef]
- Ferreira, L.F.V.; Lemos, M.J.; Reis, M.J.; do Rego, A.M.B. UV-Vis absorption, luminescence, and X-ray photoelectron spectroscopic studies of rhodamine dyes adsorbed onto different pore size silicas. Langmuir 2000, 16, 5673–5680. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, A.; Yang, W.; Araya, T.; Huang, Y.; Zhao, P.; Johnson, D.; Wang, J.; Ren, Z.J. Complex Formation via Hydrogen bonding between Rhodamine B and Montmorillonite in Aqueous Solution. Sci. Rep. 2018, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- Gotor, R.; Ashokkumar, P.; Hecht, M.; Keil, K.; Rurack, K. Optical pH Sensor Covering the Range from pH 0–14 Compatible with Mobile-Device Readout and Based on a Set of Rationally Designed Indicator Dyes. Anal. Chem. 2017, 89, 8437–8444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadzadeh, S.; Olya, M.E.; Arabi, A.M.; Shariati, A.; Khosravi Nikou, M.R. Synthesis, characterization and application of ZnO-Ag as a nanophotocatalyst for organic compounds degradation, mechanism and economic study. J. Environ. Sci. 2015, 35, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, H.; Gordon, L.; Livingston, R. Acid-base equilibriums of fluorescein and 2’,7’-dichlorofluorescein in their ground and fluorescent states. J. Phys. Chem. 1971, 75, 245–249. [Google Scholar] [CrossRef]
- Balas, F.; Manzano, M.; Horcajada, P.; Vallet-Regí, M. Confinement and Controlled Release of Bisphosphonates on Ordered Mesoporous Silica-Based Materials. J. Am. Chem. Soc. 2006, 128, 8116–8117. [Google Scholar] [CrossRef]
- Ukmar, T.; Maver, U.; Planinšek, O.; Pintar, A.; Kaučič, V.; Godec, A.; Gaberšček, M. Guest–host van der Waals interactions decisively affect the molecular transport in mesoporous media. J. Mater. Chem. 2012, 22, 1112–1120. [Google Scholar] [CrossRef] [Green Version]
- Piazza, F.; Traytak, S.D. Diffusion-influenced reactions in a hollow nano-reactor with a circular hole. Phys. Chem. Chem. Phys. 2015, 17, 10417–10425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, J.B.S.; Kamali-Zare, P.; Brismar, H.; Bergström, L. Release and Molecular Transport of Cationic and Anionic Fluorescent Molecules in Mesoporous Silica Spheres. Langmuir 2008, 24, 11096–11102. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.; Climent, E.; Hecht, M.; Buurman, M.; Rurack, K. Combining a Droplet-Based Microfluidic Tubing System with Gated Indicator Releasing Nanoparticles for Mercury Trace Detection. ACS Sens. 2016, 1, 334–338. [Google Scholar] [CrossRef]
- Costa, E.; Climent, E.; Gawlitza, K.; Wan, W.; Weller, M.G.; Rurack, K. Optimization of analytical assay performance of antibody-gated indicator-releasing mesoporous silica particles. J. Mater. Chem. B 2020, 8, 4950–4961. [Google Scholar] [CrossRef]



| Solvent | Dyes | ||||||
|---|---|---|---|---|---|---|---|
| Rh101 | Rh101-Cl | Rh101-ClO4 | RhB | SRB | F27 | BDP | |
| MeCN | ARh101 | ARh101-Cl | ARh101-ClO4 | ARhB | ASRB | AF27 | ABDP |
| PBS | BRh101 | BRh101-Cl | BRh101-ClO4 | BRhB | BSRB | BF27 | BBDP |
| Dye | Solvent | λmax (nm) | εmax a (M−1 cm−1) |
|---|---|---|---|
| Rh101 | PBS | 576 | 44580 |
| Rh101 | MeCN | 576 | 12690 |
| Rh101-Cl | PBS | 576 | 86630 |
| Rh101-Cl | MeCN | 576 | 86330 |
| Rh101-ClO4 | PBS | 576 | 96890 |
| Rh101-ClO4 | MeCN | 572 | 96160 |
| SRB | PBS | 564 | 95950 |
| SRB | MeCN | 550 | 73320 |
| RhB | PBS | 555 | 97520 |
| RhB | MeCN | 554 | 87120 |
| F27 | PBS | 503 | 92510 |
| F27 | MeCN | 503 | 300 |
| BDP | PBS | - b | - b |
| BDP | MeCN | 507 | 69950 |
| Material | mmol g−1 SiO2 Loaded; via EA | mmol g− SiO2 Loaded; via UV | mmol g− SiO2 Delivered | % Loaded (Average) a | % Delivered |
|---|---|---|---|---|---|
| BRh101 | 0.229 ± 0.015 | 0.354 ± 0.035 | 0.103 ± 0.001 | 36.5 | 35.2 |
| ARh101 | 0.577 ± 0.040 | 0.658 ± 0.045 | 0.156 ± 0.015 | 77.2 | 25.3 |
| BRh101-Cl | 0.559 ± 0.089 | 0.603 ± 0.053 | 0.103 ± 0.013 | 72.6 | 17.8 |
| ARh101-Cl | 0.229 ± 0.008 | 0.091 ± 0.038 | 0.005 ± 0.001 | 20.0 | 3.0 |
| BRh101-ClO4 | 0.501 ± 0.067 | 0.299 ± 0.005 | 0.096 ± 0.007 | 50.0 | 23.9 |
| ARh101-ClO4 | 0.177 ± 0.007 | 0.111 ± 0.011 | 0.004 ± 0.001 | 18.0 | 2.7 |
| BSRB | 0.097 ± 0.104 | 0.082 ± 0.001 | 0.066 ± 0.003 | 11.2 | 73.1 |
| ASRB | 0.756 ± 0.029 | 0.682 ± 0.002 | 0.436 ± 0.011 | 89.9 | 60.7 |
| BRhB | 0.467 ± 0.034 | 0.631 ± 0.002 | 0.156 ± 0.002 | 68.6 | 28.3 |
| ARhB | 0.300 ± 0.017 | 0.533 ± 0.028 | 0.037 ± 0.005 | 52.1 | 8.9 |
| BF27 | 0.091 ± 0.012 | 0.0980 ± 0.020 | 0.042 ± 0.005 | 11.8 | 44.6 |
| AF27 | 0.115 ± 0.035 | 0.1230 ± 0.030 | 0.0200 ± 0.002 | 14.9 | 16.8 |
| BBDP | 0.130 ± 0.002 | - | - | 13.8 | - |
| ABDP | 0.071 ± 0.010 | 0.0621 ± 0.045 | 0.006 ± 0.010 | 8.3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Climent, E.; Hecht, M.; Rurack, K. Loading and Release of Charged and Neutral Fluorescent Dyes into and from Mesoporous Materials: A Key Role for Sensing Applications. Micromachines 2021, 12, 249. https://doi.org/10.3390/mi12030249
Climent E, Hecht M, Rurack K. Loading and Release of Charged and Neutral Fluorescent Dyes into and from Mesoporous Materials: A Key Role for Sensing Applications. Micromachines. 2021; 12(3):249. https://doi.org/10.3390/mi12030249
Chicago/Turabian StyleCliment, Estela, Mandy Hecht, and Knut Rurack. 2021. "Loading and Release of Charged and Neutral Fluorescent Dyes into and from Mesoporous Materials: A Key Role for Sensing Applications" Micromachines 12, no. 3: 249. https://doi.org/10.3390/mi12030249






