Microfluidics Integration into Low-Noise Multi-Electrode Arrays
Abstract
:1. Introduction
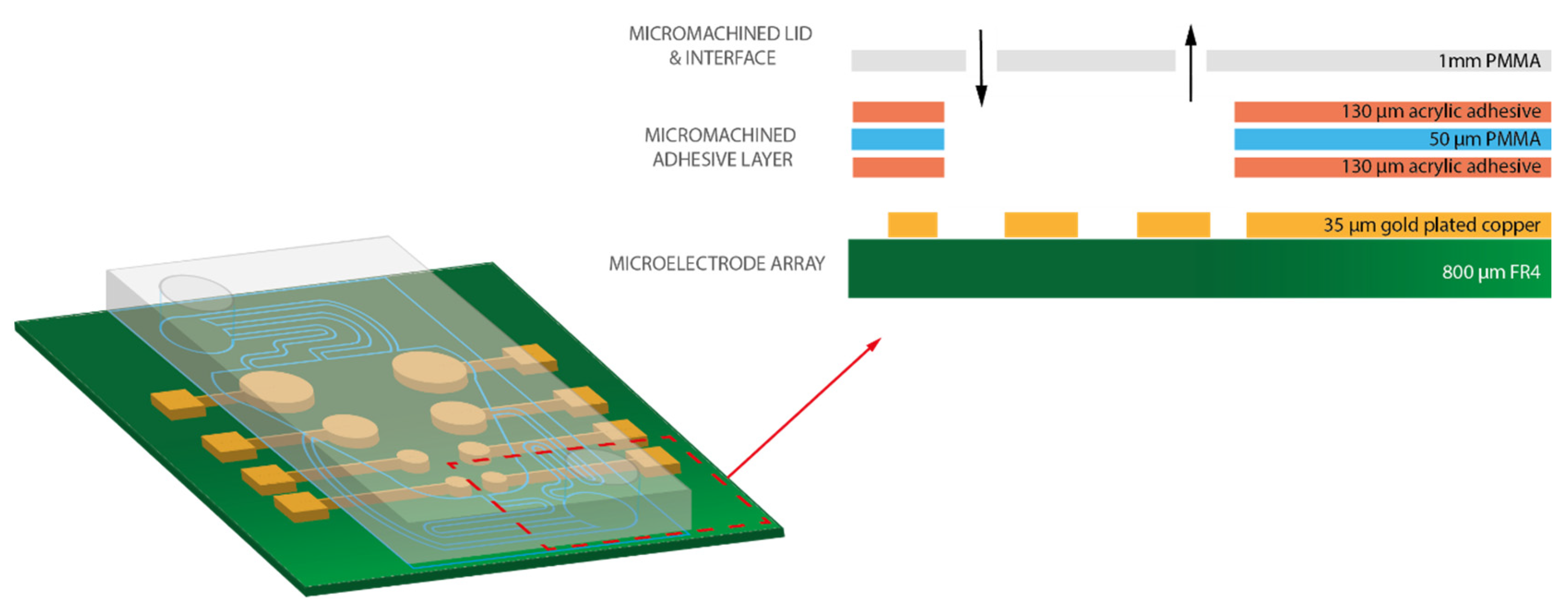
2. Materials and Methods
3. Results
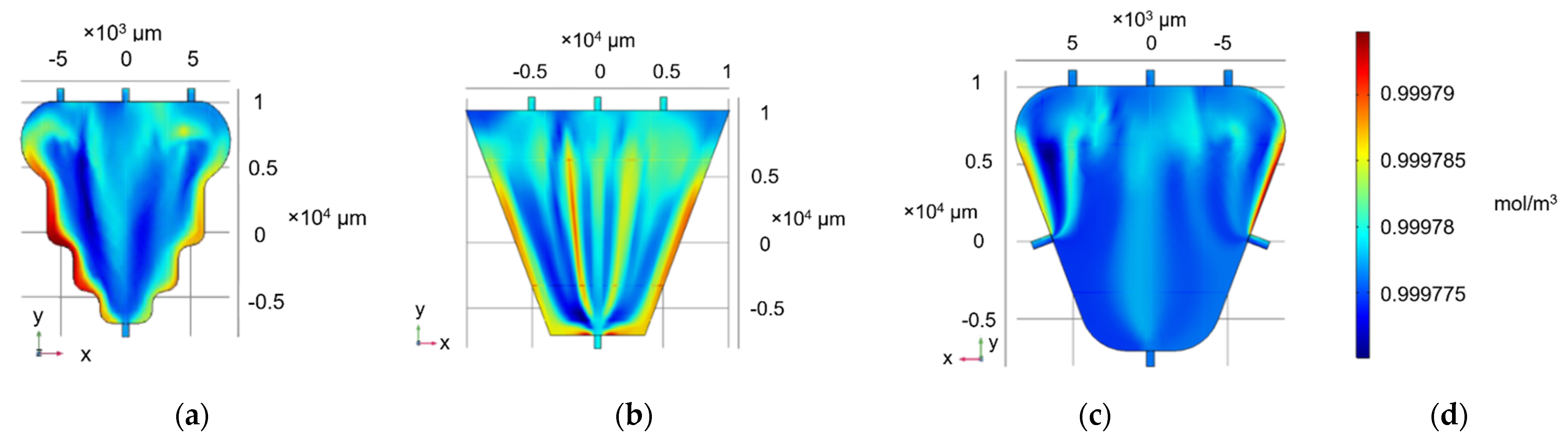
3.1. Concentration Profile
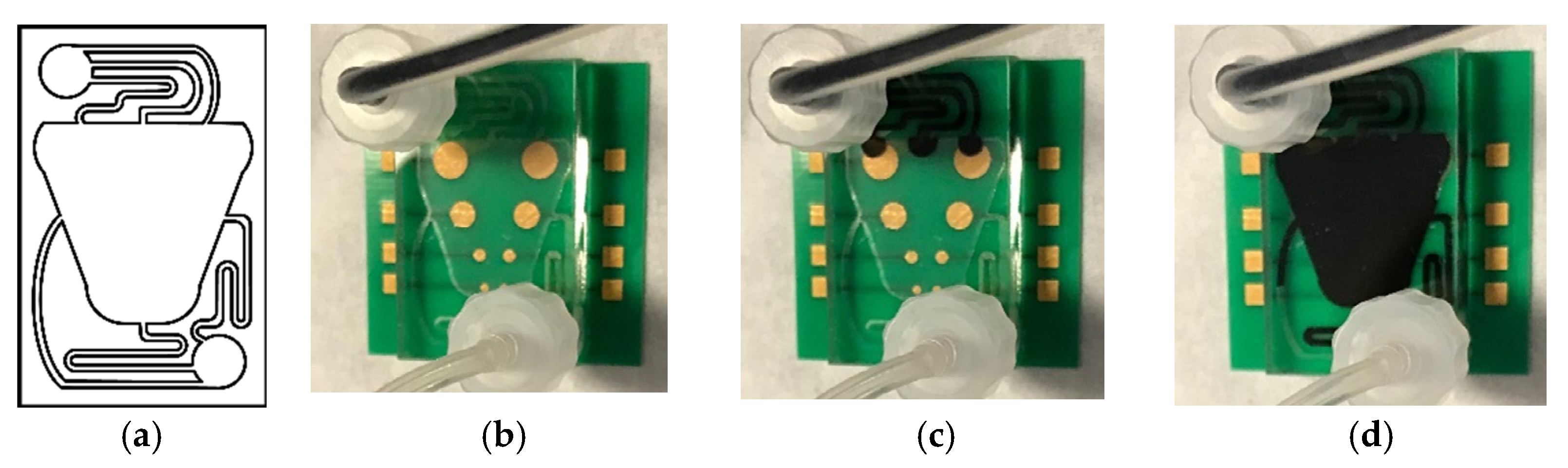
3.2. Microfluidic Prototype Implementation
3.3. Electrical Characterisation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [PubMed] [Green Version]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve RD productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Radisic, M. Organ-on-A-chip devices advance to market. Lab Chip 2017, 17, 2395–2420. [Google Scholar] [CrossRef]
- Eastwood, D.; Findlay, L.; Poole, S.; Bird, C.; Wadhwa, M.; Moore, M.; Burns, C.; Thorpe, R.; Stebbings, R. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4 + effector memory T-cells. Br. J. Pharmacol. 2010, 161, 512–526. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.I.; Oleaga, C.; Long, C.J.; Esch, M.B.; McAleer, C.W.; Miller, P.G.; Hickman, J.J.; Shuler, M.L. Self-contained, low-cost Body-on-a-Chip systems for drug development. Exp. Biol. Med. 2017, 242, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef] [Green Version]
- Esch, M.B.; King, T.L.; Shuler, M.L. The role of body-on-a-chip devices in drug and toxicity studies. Annu. Rev. Biomed. Eng. 2011, 13, 55–72. [Google Scholar] [CrossRef] [Green Version]
- Habibey, R.; Latifi, S.; Mousavi, H.; Pesce, M.; Arab-Tehrany, E.; Blau, A. A multielectrode array microchannel platform reveals both transient and slow changes in axonal conduction velocity. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Rocha, P.R.F.; Schlett, P.; Schneider, L.; Dröge, M.; Mailänder, V.; Gomes, H.L.; Blom, P.W.M.; De Leeuw, D.M. Low frequency electric current noise in glioma cell populations. J. Mater. Chem. B 2015, 3, 5035–5039. [Google Scholar] [CrossRef] [Green Version]
- Rocha, P.R.F.; Schlett, P.; Kintzel, U.; Mailänder, V.; Vandamme, L.K.J.; Zeck, G.; Gomes, H.L.; Biscarini, F.; De Leeuw, D.M. Electrochemical noise and impedance of Au electrode/electrolyte interfaces enabling extracellular detection of glioma cell populations. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, M.C.R.; Mestre, A.; Inácio, P.; Asgarif, S.; Araújo, I.M.; Hubbard, P.C.; Velez, Z.; Cancela, M.L.; Rocha, P.R.F.; de Leeuw, D.M.; et al. An electrical method to measure low-frequency collective and synchronized cell activity using extracellular electrodes. Sens. Bio-Sens. Res. 2016, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.; Elghajiji, A.; Fraser, S.P.; Burke, Z.D.; Tosh, D.; Djamgoz, M.B.A.; Rocha, P.R.F. Human Breast Cancer Cells Demonstrate Electrical Excitability. Front. Neurosci. 2020, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cabello, M.; Ge, H.; Aracil, C.; Moschou, D.; Estrela, P.; Quero, J.M.; Pascu, S.I.; Rocha, P.R.F. Extracellular electrophysiology in the prostate cancer cell model PC-3. Sensors 2019, 19, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschou, D.; Tserepi, A. The lab-on-PCB approach: Tackling the μTAS commercial upscaling bottleneck. Lab Chip 2017, 17, 1388–1405. [Google Scholar] [CrossRef] [Green Version]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Searson, P.C.; Dawson, J.L. Analysis of Electrochemical Noise Generated by Corroding Electrodes under Open-Circuit Conditions. J. Electrochem. Soc. 1988, 135, 1908–1915. [Google Scholar] [CrossRef]
- Yi, C.; Du, X.; Yang, Y.; Zhu, B.; Zhang, Z. Correlation between the corrosion rate and electrochemical noise energy of copper in chloride electrolyte. RSC Adv. 2018, 8, 19208–19212. [Google Scholar] [CrossRef] [Green Version]
- Elveflow. Microfluidic Flow Controller (OB1 MK3+). 2019. Available online: https://www.elveflow.com/microfluidic-products/microfluidics-flow-control-systems/ob1-pressure-controller/ (accessed on 18 June 2021).
- Elveflow. Microfluidic Flow Sensor 3 (MFS3). 2019. Available online: https://www.elveflow.com/microfluidic-products/microfluidics-flow-measurement-sensors/microfluidic-liquid-mass-flow-sensors/ (accessed on 18 June 2021).
- Arjmandi, N.; Liu, C.; Van Roy, W.; Lagae, L.; Borghs, G. Method for flow measurement in microfluidic channels based on electrical impedance spectroscopy. Microfluid. Nanofluid. 2012, 12, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Perrier, R.; Pirog, A.; Jaffredo, M.; Gaitan, J.; Catargi, B.; Renaud, S.; Raoux, M.; Lang, J. Bioelectronic organ-based sensor for microfluidic real-time analysis of the demand in insulin. Biosens. Bioelectron. 2018, 117, 253–259. [Google Scholar] [CrossRef]
- Pancrazio, J.J.; Gray, S.A.; Shubin, Y.S.; Kulagina, N.; Cuttino, D.S.; Shaffer, K.M.; Eisemann, K.; Curran, A.; Zim, B.; Gross, G.W.; et al. A portable microelectrode array recording system incorporating cultured neuronal networks for neurotoxin detection. Biosens. Bioelectron. 2003, 18, 1339–1347. [Google Scholar] [CrossRef]
- Chung, B.G.; Choo, J. Microfluidic gradient platforms for controlling cellular behavior. Electrophoresis 2010, 31, 3014–3027. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; Zeringue, H.C.; Beebe, D.J. Microenvironment design considerations for cellular scale studies. Lab Chip 2004, 4, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Young, E.W.K.; Beebe, D.J. Fundamentals of microfluidic cell culture in controlled microenvironments. Chem. Soc. Rev. 2010, 39, 1036–1048. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, M.; Ali, P.; Metcalfe, B.; Moschou, D.; Rocha, P.R.F. Microfluidics Integration into Low-Noise Multi-Electrode Arrays. Micromachines 2021, 12, 727. https://doi.org/10.3390/mi12060727
Ribeiro M, Ali P, Metcalfe B, Moschou D, Rocha PRF. Microfluidics Integration into Low-Noise Multi-Electrode Arrays. Micromachines. 2021; 12(6):727. https://doi.org/10.3390/mi12060727
Chicago/Turabian StyleRibeiro, Mafalda, Pamela Ali, Benjamin Metcalfe, Despina Moschou, and Paulo R. F. Rocha. 2021. "Microfluidics Integration into Low-Noise Multi-Electrode Arrays" Micromachines 12, no. 6: 727. https://doi.org/10.3390/mi12060727
APA StyleRibeiro, M., Ali, P., Metcalfe, B., Moschou, D., & Rocha, P. R. F. (2021). Microfluidics Integration into Low-Noise Multi-Electrode Arrays. Micromachines, 12(6), 727. https://doi.org/10.3390/mi12060727






