Utilising Commercially Fabricated Printed Circuit Boards as an Electrochemical Biosensing Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Roughness Characterisation
2.2. PCB Cleaning and Electrochemical Analytical Techniques
2.3. Electrochemical Sensor for E. coli DNA Detection
2.4. Electrochemical Detection of PCT Protein
3. Results and Discussion
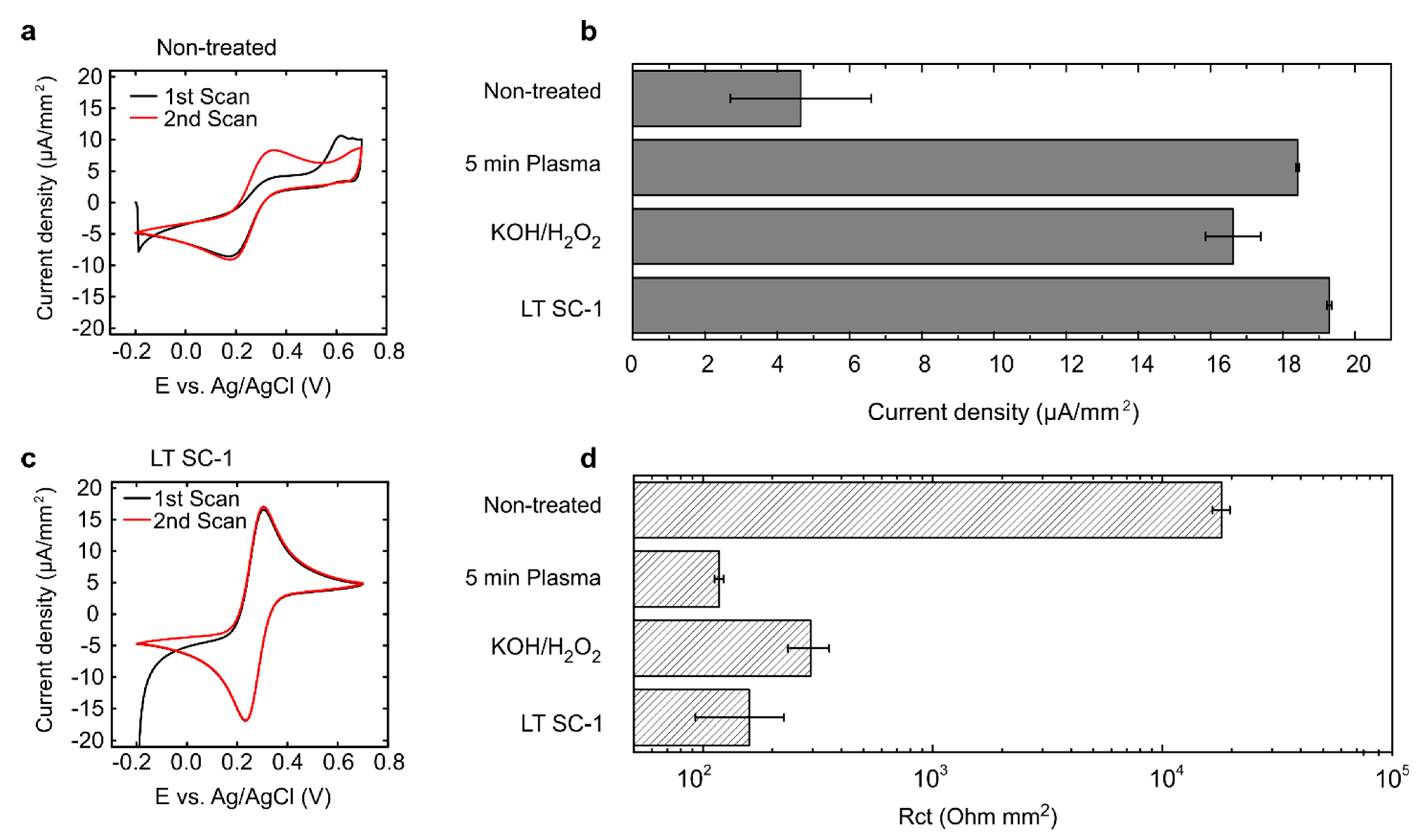
3.1. Removal of Surface Impurities in Commercially Manufactured PCB Electrodes
3.2. Evaluation of PCB Surface Roughness
3.3. Electrochemical Protein Quantification Using Commercial PCB Electrodes
3.4. Detection of DNA Using Commercial PCB Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Güth, F.; Arki, P.; Löher, T.; Ostmann, A.; Joseph, Y. Electrochemical Sensors Based on Printed Circuit Board Technologies. Procedia Eng. 2016, 168, 452–455. [Google Scholar] [CrossRef]
- Shamkhalichenar, H.; Bueche, C.; Choi, J.-W. Printed Circuit Board (PCB) Technology for Electrochemical Sensors and Sensing Platforms. Biosensors 2020, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Lammerink, T.; Spiering, V.; Elwenspoek, M.; Fluitman, J.; Berg, A.V.D. Modular concept for fluid handling systems. A demonstrator micro analysis system. In Proceedings of the Ninth International Workshop on Micro Electromechanical Systems, San Diego, CA, USA, 11–15 February 1996; Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA, 1996. [Google Scholar]
- Moschou, D.; Tserepi, A. The lab-on-PCB approach: Tackling the μTAS commercial upscaling bottleneck. Lab Chip 2017, 17, 1388–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolly, P.; Rainbow, J.; Regoutz, A.; Estrela, P.; Moschou, D. A PNA-based Lab-on-PCB diagnostic platform for rapid and high sensitivity DNA quantification. Biosens. Bioelectron. 2019, 123, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Land, K.J.; Boeras, D.I.; Chen, X.-S.; Ramsay, A.R.; Peeling, R.W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 2019, 4, 46–54. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Ali, K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.D.; Buckley, D.T.; Morrissey, J.A. Novel view of the electrochemistry of gold. Analyst 1994, 119, 841–845. [Google Scholar] [CrossRef]
- Dutta, G.; Jallow, A.A.; Paul, D.; Moschou, D. Label-Free Electrochemical Detection of S. mutans Exploiting Commercially Fabricated Printed Circuit Board Sensing Electrodes. Micromachines 2019, 10, 575. [Google Scholar] [CrossRef] [Green Version]
- Moschou, D. Amperometric IFN-gamma immunosensors with commercially fabricated PCB sensing elec-trodes. Biosens. Bioelectron. 2016, 86, 805–810. [Google Scholar] [CrossRef]
- Raiber, K.; Terfort, A.; Benndorf, C.; Krings, N.; Strehblow, H.-H. Removal of self-assembled monolayers of alkanethiolates on gold by plasma cleaning. Surf. Sci. 2005, 595, 56–63. [Google Scholar] [CrossRef]
- Fierro, L.; Getty, J. Plasma Processes for Printed Circuit Board Manufacturing. 1 January 2003. Available online: https://www.yumpu.com/en/document/read/626798/plasma-processes-for-printed-circuit-board-manufacturing (accessed on 10 April 2020).
- Heiskanen, A.; Spégel, C.F.; Kostesha, N.; Ruzgas, T.; Emnéus, J. Monitoring of Saccharomyces cerevisiae Cell Proliferation on Thiol-Modified Planar Gold Microelectrodes Using Impedance Spectroscopy. Langmuir 2008, 24, 9066–9073. [Google Scholar] [CrossRef] [PubMed]
- Kern, W. The Evolution of Silicon Wafer Cleaning Technology. J. Electrochem. Soc. 1990, 137, 1887–1892. [Google Scholar] [CrossRef]
- Goosey, M. Printed Circuits Handbook. Circuit World 2010, 36. [Google Scholar] [CrossRef]
- Tkac, J.; Davis, J.J. An optimised electrode pre-treatment for SAM formation on polycrystalline gold. J. Electroanal. Chem. 2008, 621, 117–120. [Google Scholar] [CrossRef]
- Labuda, A.; Paul, W.; Pietrobon, B.; Lennox, R.B.; Grutter, P.H.; Bennewitz, R. High-resolution friction force microscopy under electrochemical control. Rev. Sci. Instrum. 2010, 81, 83701. [Google Scholar] [CrossRef]
- Wahl, A.; Dawson, K.; Sassiat, N.; Quinn, A.J.; O’Riordan, A. Nanomolar Trace Metal Analysis of Copper at Gold Microband Arrays. J. Phys. Conf. Ser. 2011, 307, 012061. [Google Scholar] [CrossRef]
- Cherevko, S.; Topalov, A.A.; Zeradjanin, A.R.; Katsounaros, I.; Mayrhofer, K. Gold dissolution: Towards understanding of noble metal corrosion. RSC Adv. 2013, 3, 16516–16527. [Google Scholar] [CrossRef]
- Ho, L.S.J.; Limson, J.L.; Fogel, R. Certain Methods of Electrode Pretreatment Create Misleading Responses in Impedimetric Aptamer Biosensors. ACS Omega 2019, 4, 5839–5847. [Google Scholar] [CrossRef]
- Butterworth, A.; Blues, E.; Williamson, P.; Cardona, M.; Gray, L.; Corrigan, D.K. SAM Composition and Electrode Roughness Affect Performance of a DNA Biosensor for Antibiotic Resistance. Biosensors 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Hoogvliet, J. Electrochemical pretreatment of polycrystalline gold electrodes to produce a reproducible sur-face roughness for self-assembly: A study in phosphate buffer pH 7.4. Anal. Chem. 2000, 72, 2016–2021. [Google Scholar] [CrossRef] [PubMed]
- Obaje, E.A. Carbon screen-printed electrodes on ceramic substrates for label-free molecular detection of anti-biotic resistance. J. Interdiscip. Nanomed. 2016, 1, 93–109. [Google Scholar] [CrossRef]
- Collinson, M. Nanoporous Gold Electrodes and Their Applications in Analytical Chemistry. ISRN Anal. Chem. 2013, 2013, 1–21. [Google Scholar] [CrossRef]
- Benites, T.A. Efeitos da rugosidade superficial nas propriedades de passivação de monocamadas orgânicas automontadas. Quim. Nova 2014, 37, 1533–1537. [Google Scholar]
- Santos, A.; Piccoli, J.P.; Santos-Filho, N.A.; Cilli, E.M.; Bueno, P.R. Redox-tagged peptide for capacitive diagnostic assays. Biosens. Bioelectron. 2015, 68, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.; Rushdi, A.I.; Bowen, J.; Sabouri, A.; Anthony, C.J.; Mendes, P.M.; Preece, J.A. Room temperature thermally evaporated thin Au film on Si suitable for application of thiol self-assembled monolayers in micro/nano-electro-mechanical-systems sensors. J. Vac. Sci. Technol. A 2017, 35, 041514. [Google Scholar] [CrossRef] [Green Version]
- Chan, T.; Gu, F. Early diagnosis of sepsis using serum biomarkers. Expert Rev. Mol. Diagn. 2011, 11, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Faix, J.D. Biomarkers of sepsis. Crit. Rev. Clin. Lab. Sci. 2013, 50, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Yazdanparast, M.; Behzadi, E.; Salmanian, A.H.; Mousavi, S.L.; Nazarian, S.; Amani, J. A review on strategies for decreasing E. coli O157:H7 risk in animals. Microb. Pathog. 2017, 103, 186–195. [Google Scholar] [CrossRef]





| Cleaning Method | Procedure |
|---|---|
| Oxygen plasma treatment | 3, 5, or 10 min at 100 W and 0.2 mbar (Diener Zepto System, Diener electronic, Ebhausen, Germany). |
| KOH/H2O2 treatment | Immersion in a solution of 30% H2O2 and 50 mM KOH for 10 min. |
| LT SC-1 clean | Step 1: Immersion in a solution of 30% NH4OH, 30% H2O2, and MQ water in a ratio of 1:1:5 for 15 min. Step 2: Immersion in >99% acetone solution for 5 min. Step 3: Immersion in >99% IPA solution for 5 min. Step 4: Immersion in MQ water for 5 min. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zupančič, U.; Rainbow, J.; Estrela, P.; Moschou, D. Utilising Commercially Fabricated Printed Circuit Boards as an Electrochemical Biosensing Platform. Micromachines 2021, 12, 793. https://doi.org/10.3390/mi12070793
Zupančič U, Rainbow J, Estrela P, Moschou D. Utilising Commercially Fabricated Printed Circuit Boards as an Electrochemical Biosensing Platform. Micromachines. 2021; 12(7):793. https://doi.org/10.3390/mi12070793
Chicago/Turabian StyleZupančič, Uroš, Joshua Rainbow, Pedro Estrela, and Despina Moschou. 2021. "Utilising Commercially Fabricated Printed Circuit Boards as an Electrochemical Biosensing Platform" Micromachines 12, no. 7: 793. https://doi.org/10.3390/mi12070793
APA StyleZupančič, U., Rainbow, J., Estrela, P., & Moschou, D. (2021). Utilising Commercially Fabricated Printed Circuit Boards as an Electrochemical Biosensing Platform. Micromachines, 12(7), 793. https://doi.org/10.3390/mi12070793







