Effect of Ultrasonic Excitation on Discharge Performance of a Button Zinc–Air Battery
Abstract
:1. Introduction
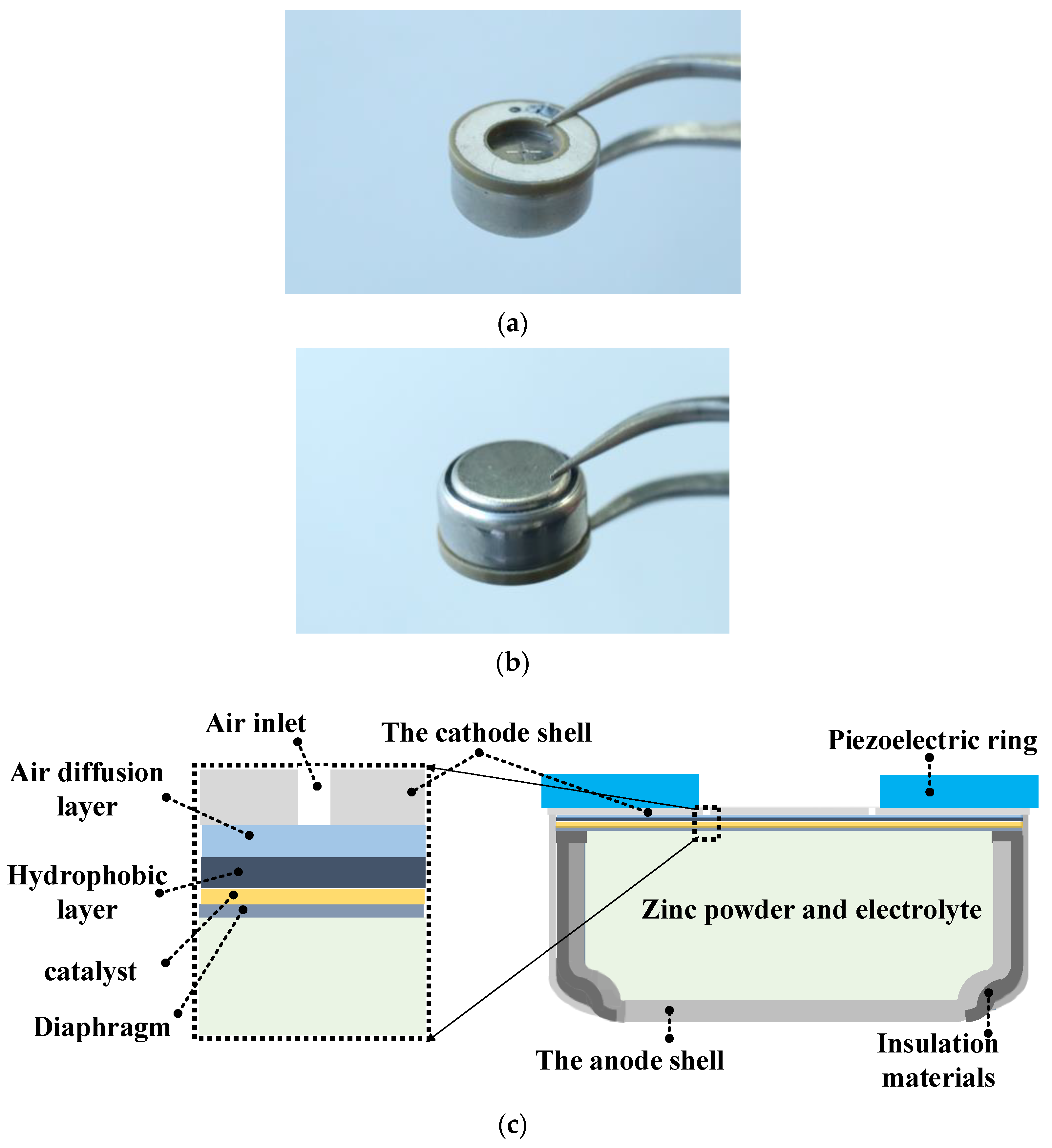
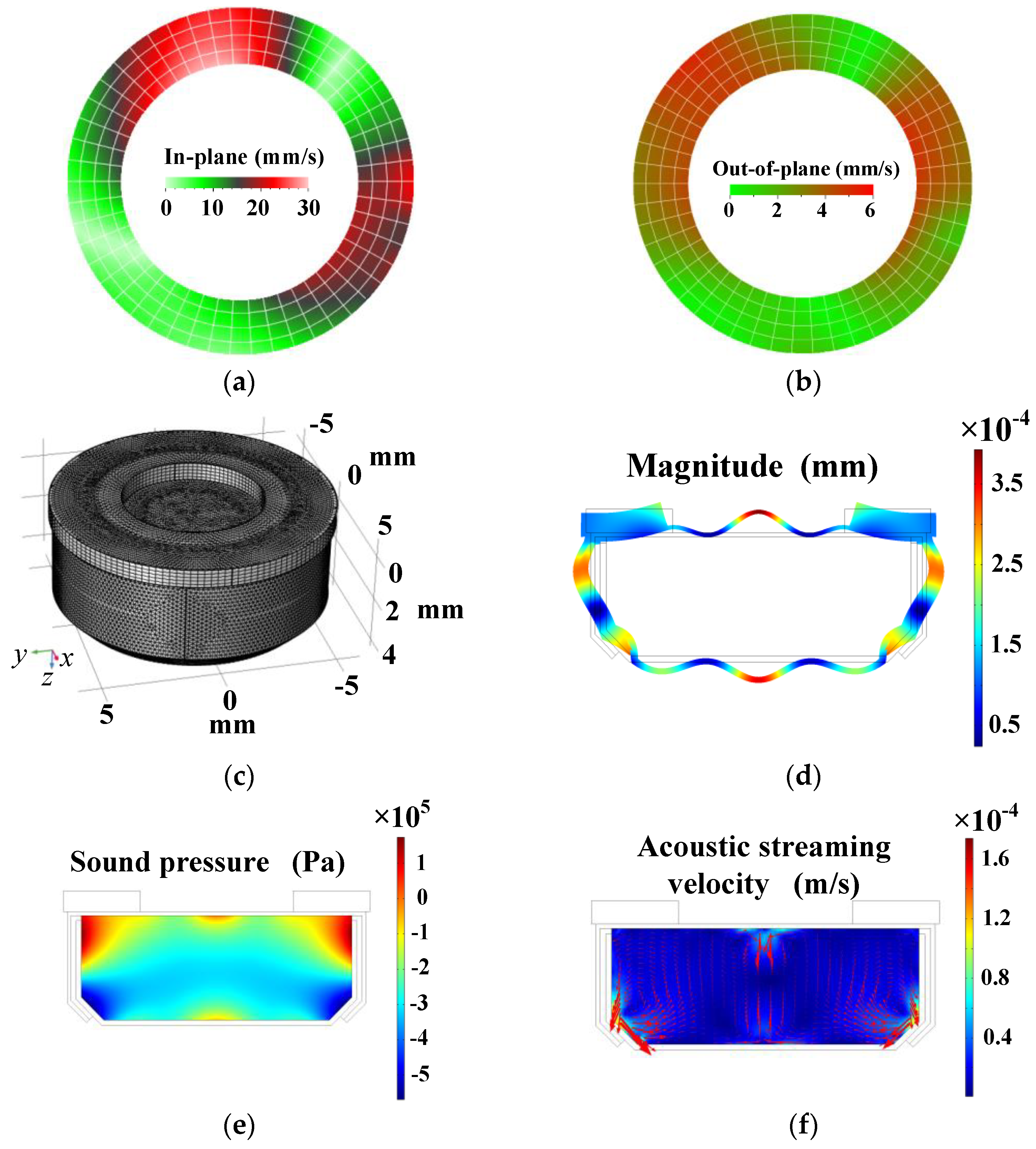
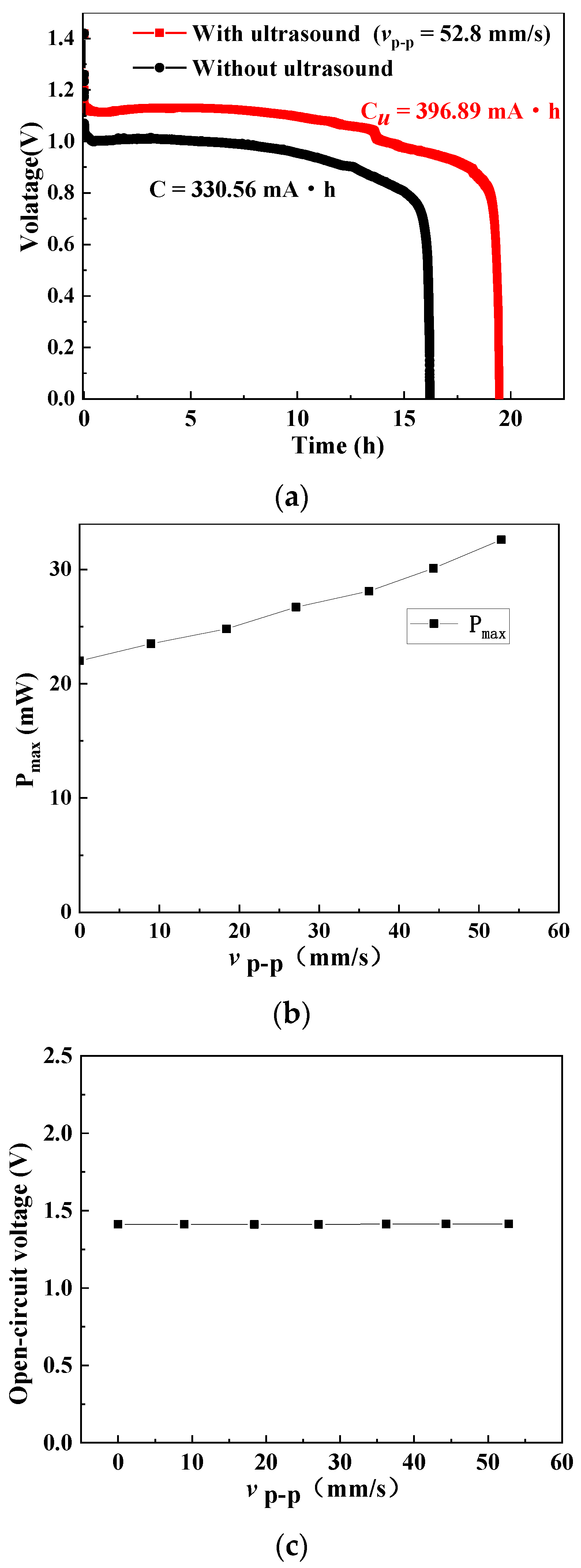
2. Results, Analyses, and Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4, 1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, J.; Liang, R.; Liu, G.; Yu, A.; Bai, Z.; Yang, L.; Chen, Z. Recent progress in electrically rechargeable zinc-air batteries. Adv. Mater. 2018, 31, 1805230. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, H. Recent advances in zinc-air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef] [Green Version]
- Lim, B.; Jiang, M.; Camargo, P.H.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, T.; Song, H.; Cho, J.; Kim, B. Ionic liquid modified graphene nanosheets anchoring manganese oxide nanoparticles as efficient electrocatalysts for Zn-air batteries. Energy Environ. Sci. 2011, 4, 4148. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, Y.; Zhang, L.; Chen, J. Recent advances in isolated single-atom catalysts for zinc air batteries: A focus review. Nanomaterials 2019, 9, 1402. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.C.; Xu, Z.; Gasteiger, H.A.; Chen, S.; Hamad-Schifferli, K.; Shao-Horn, Y. Platinum-gold nanoparticles: A highly active bifunctional electrocatalyst for rechargeable lithium-air batteries. J. Am. Chem. Soc. 2010, 132, 12170–12171. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kaempgen, M.; Nopphawan, P.; Wee, G.; Mhaisalkar, S.; Srinivasan, M. Silver nanoparticle-decorated carbon nanotubes as bifunctional gas-diffusion electrodes for zinc-air batteries. J. Power Sources 2010, 195, 4350–4355. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, F. Facile preparation of Ag-Cu bifunctional electrocatalysts for zinc-air batteries. Electrochim. Acta 2015, 158, 437–445. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, M.; Shang, N.; Gao, S.; Wang, J.; Wang, C.; Wang, Z.; Wang, H.; Gao, Y. Preparation of N, S co-decorated carbon supported iron species for oxygen reduction and zinc air batteries. J. Alloys Compd. 2020, 848, 156367. [Google Scholar] [CrossRef]
- Peng, Z.; Zhao, J.; Gu, L.; Sun, X.; Jia, H.; Guan, M.; Ma, S. S-doped hierarchical graphene decorated with Co-porphyrins as an efficient electrocatalyst for zinc-air batteries. New J. Chem. 2020, 44, 14343–14349. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, H.; Zhu, J.; Li, W.; Zhang, C.; Zhao, J.; Luo, F.; Sun, Z.; Mu, S. 3D flower-like ZnFe-ZIF derived hierarchical Fe, N-codoped carbon architecture for enhanced oxygen reduction in both alkaline and acidic media, and zinc-air battery performance. Carbon 2020, 161, 502–509. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wu, M.; Wei, Z.; Cui, C.; Mao, M.; Zhang, J.; Han, X.; Liu, Q.; Ma, J. Nitrogen, fluorine, and boron ternary doped carbon fibers as cathode electrocatalysts for zinc-air batteries. Small 2018, 14, 1800737. [Google Scholar] [CrossRef]
- Wang, X.; Sunarso, J.; Lu, Q.; Zhou, Z.; Dai, J.; Guan, D.; Zhou, W.; Shao, Z. High-performance platinum-perovskite composite bifunctional oxygen electrocatalyst for rechargeable Zn-air battery. Adv. Energy Mater. 2019, 10, 1903271. [Google Scholar] [CrossRef]
- Li, P.; Wei, B.; Lü, Z.; Wu, Y.; Zhang, Y.; Huang, X. La1.7Sr0.3Co0.5Ni0.5O4+δ layered perovskite as an efficient bifunctional electrocatalyst for rechargeable zinc-air batteries. Appl. Surf. Sci. 2019, 464, 494–501. [Google Scholar] [CrossRef]
- Majee, R.; Mondal, S.; Bhattacharyya, S. Charge transfer from perovskite oxide nanosheets to N-doped carbon nanotubes to promote enhanced performance of a zinc-air battery. Chem. Commun. 2020, 56, 8277–8280. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Yu, P.; Tian, C.; Sun, F.; Ma, J.; Li, W.; Fu, H. A stable bifunctional catalyst for rechargeable zinc-air batteries: Iron-cobalt nanoparticles embedded in a nitrogen-doped 3D carbon matrix. Angew. Chem. Int. Ed. Engl. 2018, 130, 16166–16170. [Google Scholar] [CrossRef]
- Liu, X.; Park, M.; Kim, M.G.; Gupta, S.; Wu, G.; Cho, J. Integrating NiCo alloys with their oxides as efficient bifunctional cathode catalysts for rechargeable zinc-air batteries. Angew. Chem. Int. Ed. Engl. 2015, 54, 9654–9658. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, M.; Hertzberg, B.J.; Gupta, T.; Davies, D.; Bhadra, S.; Van Tassell, B.; Erdonmez, C.; Steingart, D.A. Hyper-dendritic nanoporous zinc foam anodes. NPG Asia Mater. 2015, 7, e178. [Google Scholar] [CrossRef]
- Parker, J.F.; Chervin, C.N.; Nelson, E.S.; Rolison, D.R.; Long, J.W. Wiring zinc in three dimensions re-writes battery performance-dendrite-free cycling. Energy Environ. Sci. 2014, 7, 1117–1124. [Google Scholar] [CrossRef]
- Li, M.; Meng, J.; Li, Q.; Huang, M.; Liu, X.; Owusu, K.A.; Liu, Z.; Mai, L. Finely crafted 3D electrodes for dendrite-free and high-performance flexible fiber-shaped Zn-Co batteries. Adv. Funct. Mater. 2018, 28, 1802016. [Google Scholar] [CrossRef]
- Li, P.; Jin, Z.; Xiao, D. Three-dimensional nanotube-array anode enables a flexible Ni/Zn fibrous battery to ultrafast charge and discharge in seconds. Energy Storage Mater. 2018, 12, 232–240. [Google Scholar] [CrossRef]
- Liu, J.; Guan, C.; Zhou, C.; Fan, Z.; Ke, Q.; Zhang, G.; Liu, C.; Wang, J. A flexible quasi-solid-state nickel-zinc battery with high energy and power densities based on 3D electrode design. Adv. Mater. 2016, 28, 8732–8739. [Google Scholar] [CrossRef]
- Othman, R.; Basirun, W.J.; Yahaya, A.H.; Arof, A.K. Hydroponics gel as a new electrolyte gelling agent for alkaline zinc-air cells. J. Power Sources 2001, 103, 34–41. [Google Scholar] [CrossRef]
- Mohamad, A.A. Zn/gelled 6M KOH/O2 zinc-air battery. J. Power Sources 2006, 159, 752–757. [Google Scholar] [CrossRef]
- Peter, K.; Cook, J.A. Nonwovens as separators for alkaline batteries an overview. J. Electrochem. Soc. 2007, 154, A481–A494. [Google Scholar]
- Dewi, E.L.; Oyaizu, K.; Nishide, H.; Tsuchida, E. Cationic polysulfonium membrane as separator in zinc-air cell. J. Power Sources 2003, 115, 149–152. [Google Scholar] [CrossRef]
- Chao, W.; Lee, C.; Shieu, S.; Chou, C.; Shieu, F. Clay as a dispersant in the catalyst layer for zinc-air fuel cells. J. Power Sources 2008, 177, 637–642. [Google Scholar] [CrossRef]
- Wu, G.M.; Lin, S.J.; You, J.H.; Yang, C.C. Study of high-anionic conducting sulfonated microporous membranes for zinc-air electrochemical cells. Mater. Chem. Phy. 2008, 112, 798–804. [Google Scholar] [CrossRef]
- Saputra, H.; Othman, R.; Sutjipto, A.G.E.; Muhida, R. MCM-41 as a new separator material for electrochemical cell: Application in zinc-air system. J. Membrane Sci. 2011, 367, 152–157. [Google Scholar] [CrossRef]
- Sapkota, P.; Kim, H. Zinc-air fuel cell, a potential candidate for alternative energy. J. Ind. Eng. Chem. 2009, 15, 445–450. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Z.; Karahan, H.E.; Shao, Q.; Wei, L.; Chen, Y. Recent advances in materials and design of electrochemically rechargeable zinc–air batteries. Small 2018, 14, e1703843. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Tai Kim, S.; Cao, R.; Choi, N.; Liu, M.; Lee, K.T.; Cho, J. Metal-air batteries with high energy density: Li-air versus Zn-air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Gu, P.; Zheng, M.; Zhao, Q.; Xiao, X.; Xue, H.; Pang, H. Rechargeable zinc–air batteries: A promising way to green energy. J. Mater. Chem. A 2017, 5, 7651–7666. [Google Scholar] [CrossRef]
- Pourfarzad, H.; Shabani-Nooshabadi, M.; Ganjali, M.R. Novel bi-functional electrocatalysts based on the electrochemical synthesized bimetallicmetal organic frameworks: Towards high energy advanced reversible zinc-air batteries. J. Power Sources 2020, 451, 227768. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, C.; Du, Y.; Wang, X.; Ding, L.; Omar, A.; Mikhailova, D. Uniform Zn deposition achieved by Ag coating for improved aqueous zinc-ion batteries. ACS Appl. Mater. Inter. 2021, 13, 16869–16875. [Google Scholar] [CrossRef]
- Song, Z.; Ding, J.; Liu, B.; Liu, X.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. A rechargeable Zn-air battery with high energy efficiency and long life enabled by a highly water-retentive gel electrolyte with reaction modifier. Adv. Mater. 2020, 32, e1908127. [Google Scholar] [CrossRef]
- Cheng, H.; Tan, C. Reduction of CO2 concentration in a zinc/air battery by absorption in a rotating packed bed. J. Power Sources 2006, 162, 1431–1436. [Google Scholar] [CrossRef]
- Jia, Z.; Dai, C.; Chen, L. Electrochemical Measurement Method; Chemical Industry Press: Beijing, China, 2006; pp. 49–52. [Google Scholar]
- Hamann, C.H.; Hamnett, A.; Vielstich, W. Electrochemistry; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Gao, P.; Zhu, Y.; Yu, C. Basic Course of Electrochemistry; Chemical Industry Press: Beijing, China, 2019; pp. 138–140. [Google Scholar]
- Hu, J. Ultrasonic Micro/Nano Manipulations: Principles and Examples; World Scientific Publishing: Singapore, 2014; pp. 217–220. [Google Scholar]



| Material Parameters | Value |
|---|---|
| KOH (at temperature T = 26 °C) | |
| Dynamic viscosity (Pa s) | 2.4 |
| Density (kg/m3) | 1305 |
| Diffusion coefficient (m2/s) | 3.79 × 10−9 |
| Velocity of sound (m/s) | 1988 |
| Lead Zirconate Titanate (PZT-4) | |
| Density (kg/m3) | 7500 |
| Piezoelectric coefficient e33 (C/m2) | 15.1 |
| Relative dielectric constant εr33 | 663.2 |
| Structural steel | |
| Density (kg/m3) | 7850 |
| Young’s modulus (Pa) | 200 × 109 |
| Poisson’s ratio | 0.3 |
| Nylon | |
| Density (kg/m3) | 1150 |
| Young’s modulus (Pa) | 2 × 109 |
| Poisson’s ratio | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Tang, Q.; Hu, J. Effect of Ultrasonic Excitation on Discharge Performance of a Button Zinc–Air Battery. Micromachines 2021, 12, 792. https://doi.org/10.3390/mi12070792
Luo Z, Tang Q, Hu J. Effect of Ultrasonic Excitation on Discharge Performance of a Button Zinc–Air Battery. Micromachines. 2021; 12(7):792. https://doi.org/10.3390/mi12070792
Chicago/Turabian StyleLuo, Zhao, Qiang Tang, and Junhui Hu. 2021. "Effect of Ultrasonic Excitation on Discharge Performance of a Button Zinc–Air Battery" Micromachines 12, no. 7: 792. https://doi.org/10.3390/mi12070792







