Passivated Porous Silicon Membranes and Their Application to Optical Biosensing
Abstract
:1. Introduction
2. Materials and Methods
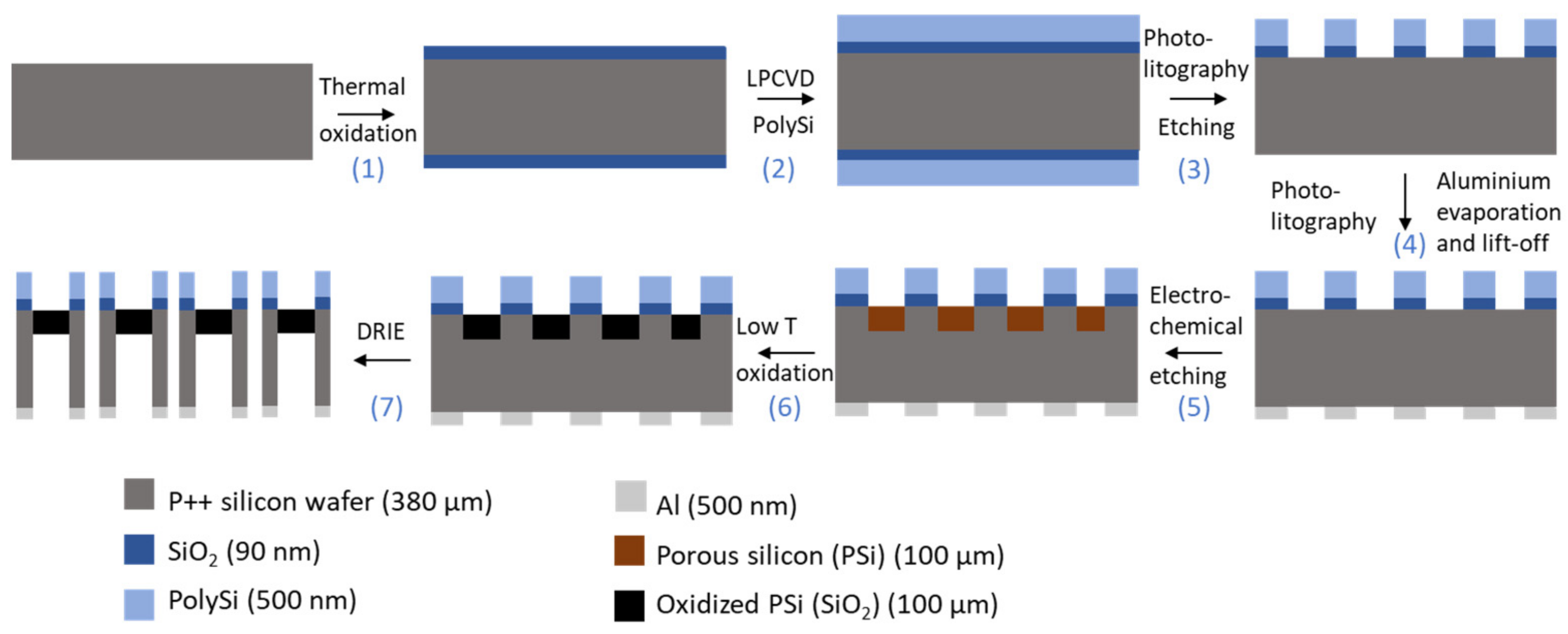
2.1. Fabrication and Characterization of Porous Silicon Membranes
2.1.1. Materials
2.1.2. Fabrication and ALD Passivation
2.1.3. Optical Determination of Thickness and Porosity
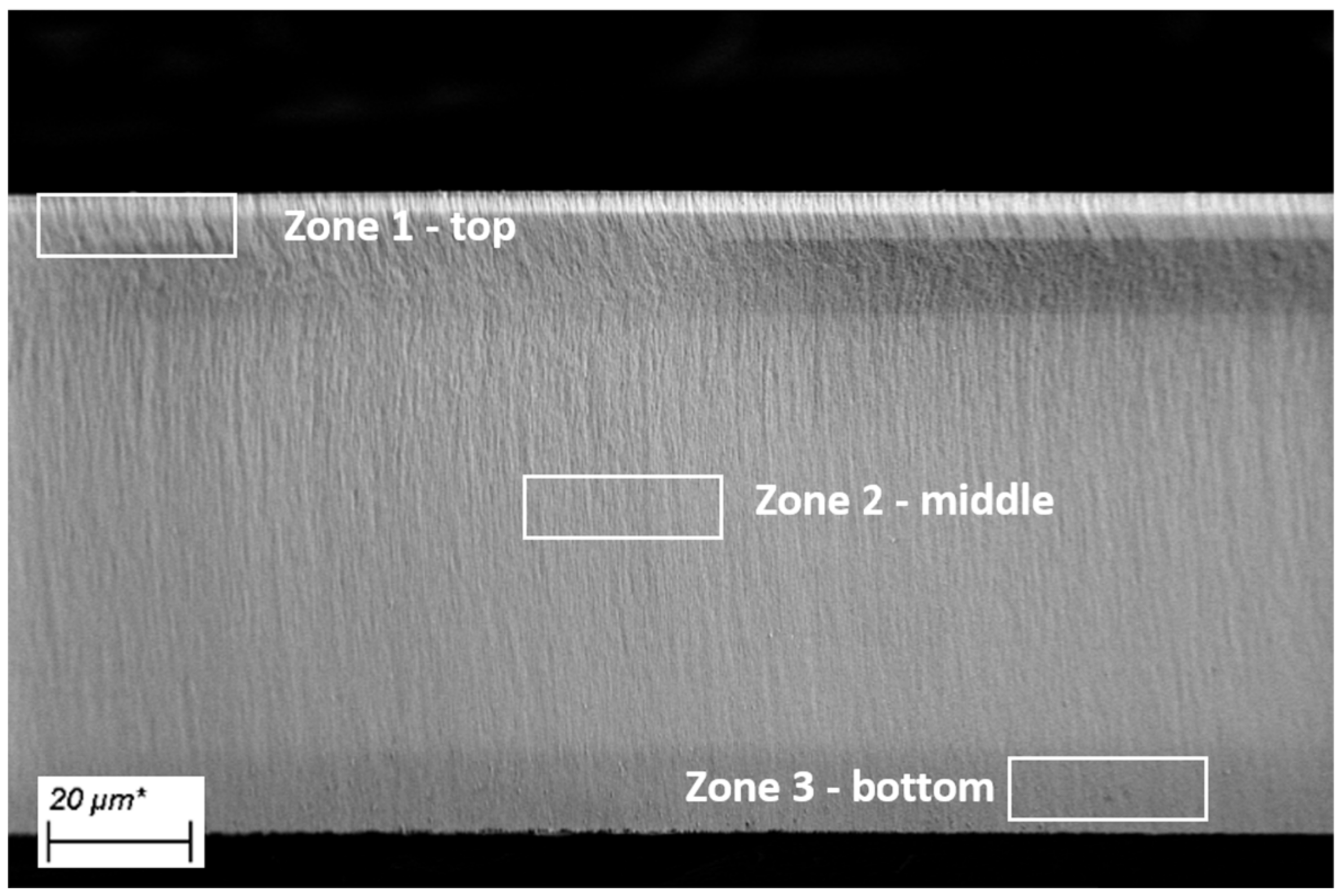
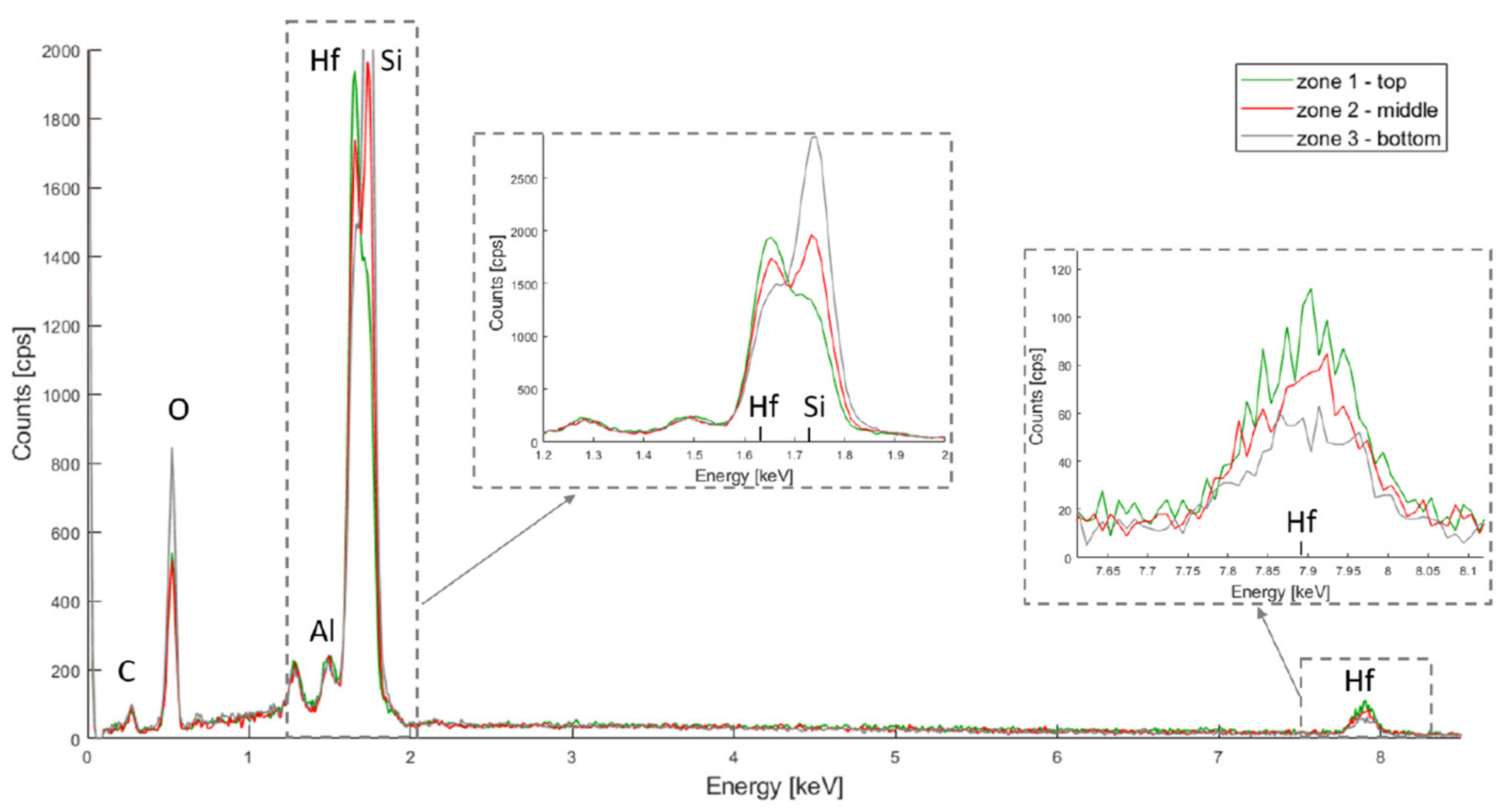
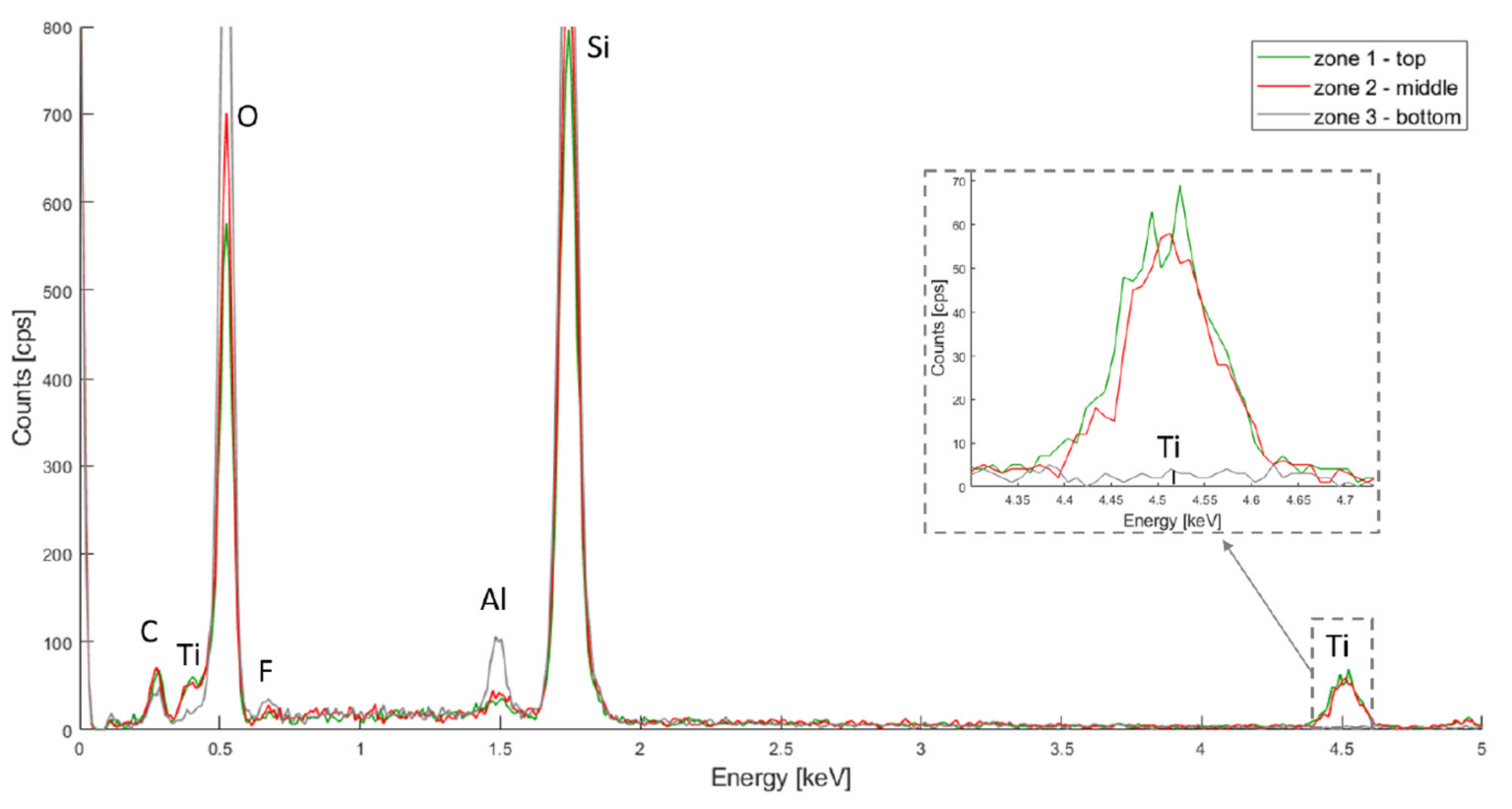
2.1.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
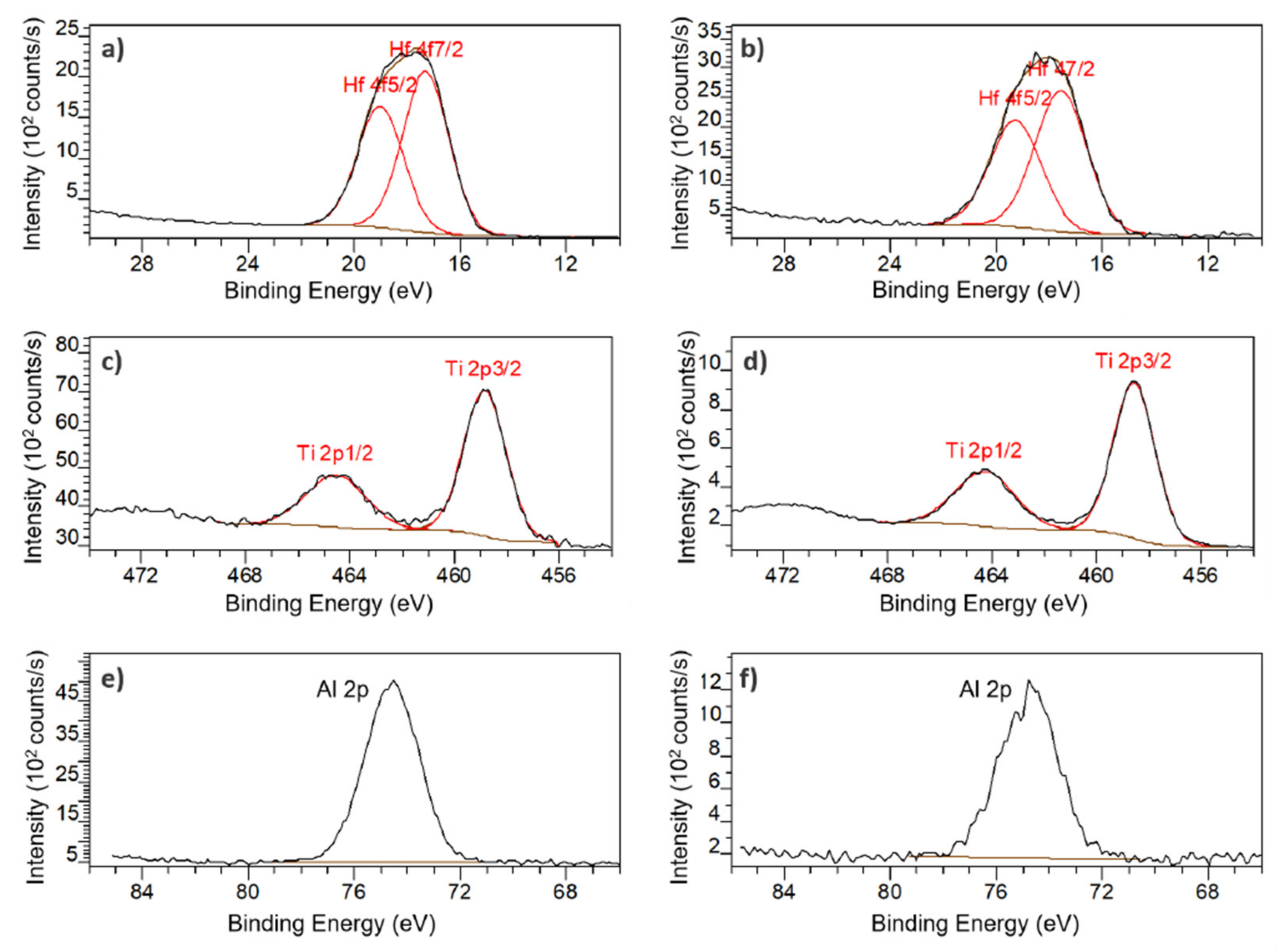
2.1.5. X-ray Photoelectron Spectroscopy (XPS)
2.2. Optical Stability Measurements
2.2.1. Noise, Sensitivity and Limit of Detection Determination
2.2.2. Optical Detection of Bacterial Lysate
3. Results and Discussion
3.1. PSi Characterization
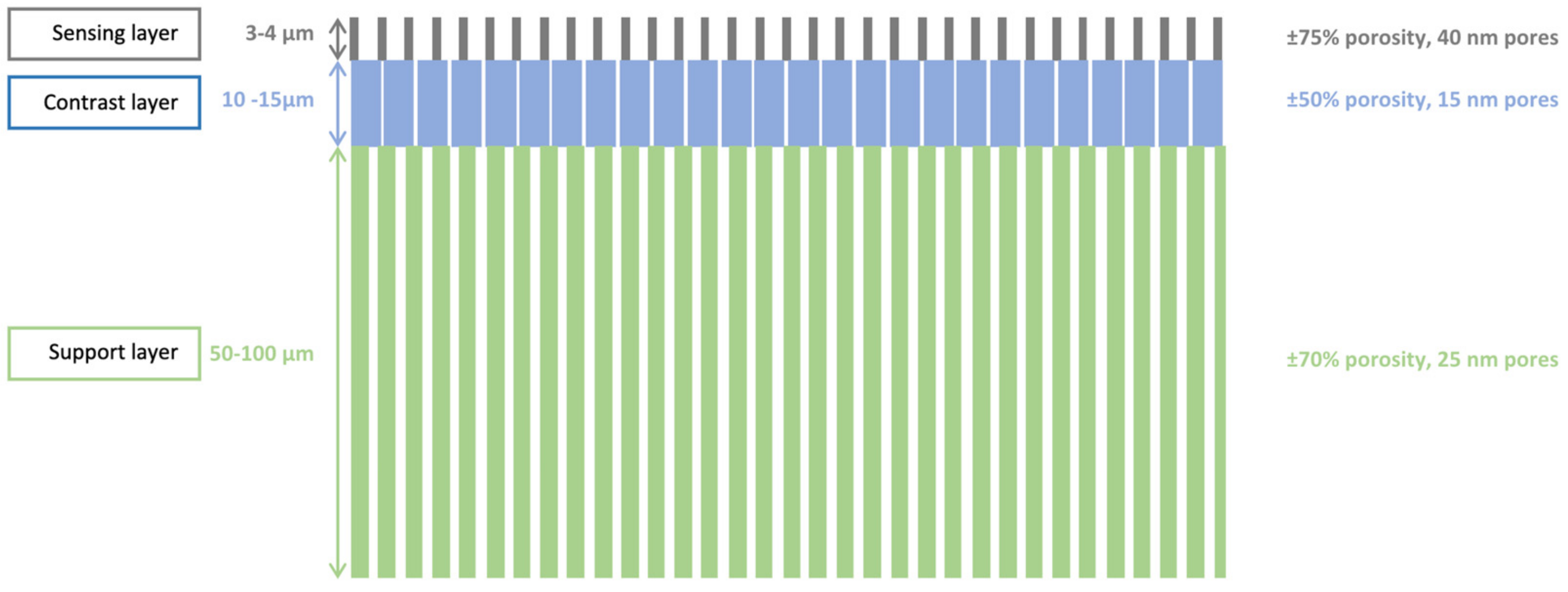
3.1.1. Porosity, Thickness and Pore Morphology
3.1.2. Porous Structure Passivation
3.2. Stability in Aqueous Media
3.3. Preliminary Results for the Optical Detection of Bacterial Lysate
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tieu, T.; Alba, M.; Elnathan, R.; Cifuentes-Rius, A.; Voelcker, N.H. Advances in Porous Silicon–Based Nanomaterials for Diagnostic and Therapeutic Applications. Adv. Ther. 2019, 2, 1800095. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Huh, Y.; Kim, D. Recent Advances in Surface Engineering of Porous Silicon Nanomaterials for Biomedical Applications. Microporous Mesoporous Mater. 2021, 310, 110673. [Google Scholar] [CrossRef]
- Abu-Thabit, N.; Ratemi, E. Hybrid Porous Silicon Biosensors Using Plasmonic and Fluorescent Nanomaterials: A Mini Review. Front. Chem. 2020, 8, 454. [Google Scholar] [CrossRef] [PubMed]
- Moretta, R.; De Stefano, L.; Terracciano, M.; Rea, I. Porous Silicon Optical Devices: Recent Advances in Biosensing Applications. Sensors 2021, 21, 1336. [Google Scholar] [CrossRef] [PubMed]
- Canham, L. Handbook of Porous Silicon; Springer: New York, NY, USA, 2014; ISBN 978-3-319-05743-9. [Google Scholar]
- Herino, R.; Bomchil, G.; Barla, K.; Bertrand, C.; Ginoux, J.L. Porosity and Pore Size Distributions of Porous Silicon Layers. J. Electrochem. Soc. 1987, 134, 1994. [Google Scholar] [CrossRef]
- Harraz, F.A. Porous Silicon Chemical Sensors and Biosensors: A Review. Sens. Actuators B Chem. 2014, 202, 897–912. [Google Scholar] [CrossRef]
- Vercauteren, R.; Scheen, G.; Raskin, J.-P.; Francis, L.A. Porous Silicon Membranes and Their Applications: Recent Advances. Sens. Actuators A Phys. 2020, 318, 112486. [Google Scholar] [CrossRef]
- Anderson, S.H.C.; Elliott, H.; Wallis, D.J.; Canham, L.T.; Powell, J.J. Dissolution of Different Forms of Partially Porous Silicon Wafers under Simulated Physiological Conditions. Phys. Status Solidi A Appl. Res. 2003, 197, 331–335. [Google Scholar] [CrossRef]
- De Stefano, L. Porous Silicon Optical Biosensors: Still a Promise or a Failure? Sensors 2019, 19, 4776. [Google Scholar] [CrossRef] [Green Version]
- Sailor, M.J. Porous Silicon in Practice: Preparation, Characterization and Applications; Wiley: Weinheim, Germany, 2012; ISBN 978-3-527-64191-8. [Google Scholar]
- Pap, A.E.; Kordás, K.; Tóth, G.; Levoska, J.; Uusimäki, A.; Vähäkangas, J.; Leppävuori, S.; George, T.F. Thermal Oxidation of Porous Silicon: Study on Structure. Appl. Phys. Lett. 2005, 86, 041501. [Google Scholar] [CrossRef]
- Stewart, M.P.; Robins, E.G.; Geders, T.W.; Allen, M.J.; Choi, H.C.; Buriak, J.M. Three Methods for Stabilization and Functionalization of Porous Silicon Surfaces via Hydrosilylation and Electrografting Reactions. Phys. Status Solidi A Appl. Res. 2000, 182, 109–115. [Google Scholar] [CrossRef]
- Terracciano, M.; De Stefano, L.; Rendina, I.; Oliviero, G.; Piccialli, G.; Borbone, N.; Rea, I. Aminosilane-Modified Mesoporous Oxidized Silicon for in Situ Oligonucleotides Synthesis and Detection. In Proceedings of the 2014 Fotonica AEIT Italian Conference on Photonics Technologies, Naples, Italy, 12–14 May 2014; pp. 1–4. [Google Scholar]
- Terracciano, M.; Rea, I.; Politi, J.; De Stefano, L. Optical Characterization of Aminosilane-Modified Silicon Dioxide Surface for Biosensing. J. Eur. Opt. Soc. Rapid Publ. 2013, 8, 13075. [Google Scholar] [CrossRef] [Green Version]
- Shabir, Q.; Webb, K.; Nadarassan, D.K.; Loni, A.; Canham, L.T.; Terracciano, M.; Stefano, L.D.; Rea, I. Quantification and Reduction of the Residual Chemical Reactivity of Passivated Biodegradable Porous Silicon for Drug Delivery Applications. Silicon 2018, 10, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Salonen, J.; Björkqvist, M.; Laine, E.; Niinistö, L. Stabilization of Porous Silicon Surface by Thermal Decomposition of Acetylene. Appl. Surf. Sci. 2004, 225, 389–394. [Google Scholar] [CrossRef]
- Franssila, S. Introduction to Microfabrication; Wiley: Hoboken, NJ, USA, 2004; ISBN 978-0-470-85106-7. [Google Scholar]
- Iatsunskyi, I.; Kempiński, M.; Jancelewicz, M.; Załęski, K.; Jurga, S.; Smyntyna, V. Structural and XPS Characterization of ALD Al2O3 Coated Porous Silicon. Vacuum 2015, 113, 52–58. [Google Scholar] [CrossRef]
- Rasson, J.; Francis, L.A. Improved Stability of Porous Silicon in Aqueous Media via Atomic Layer Deposition of Oxides. J. Phys. Chem. C 2018, 122, 331–338. [Google Scholar] [CrossRef]
- Vercauteren, R.; Leprince, A.; Mahillon, J.; Francis, L.A. Porous Silicon Biosensor for the Detection of Bacteria through Their Lysate. Biosensors 2021, 11, 27. [Google Scholar] [CrossRef]
- He, Y.; Leïchlé, T. Fabrication of Lateral Porous Silicon Membranes for Planar Microfluidics by Means of Ion Implantation. Sens. Actuators B Chem. 2017, 239, 628–634. [Google Scholar] [CrossRef]
- He, Y.; Bourrier, D.; Imbernon, E.; Leïchlé, T. Lateral Porous Silicon Membranes with Size and Charge Selectivity. In Proceedings of the 2017 IEEE 12th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Los Angeles, CA, USA, 9–12 April 2017; pp. 770–773. [Google Scholar]
- Gautier, G.; Kouassi, S. Integration of Porous Silicon in Microfuel Cells: A Review. Int. J. Energy Res. 2015, 39, 1–25. [Google Scholar] [CrossRef]
- Rasson, J. Stable Porous Silicon-Based Optical Sensing Platform towards Detection of Bacterial Lysis. Ph.D. Thesis, Université Catholique de Louvain, Louvain-la-Neuve, Belgium, 2018. [Google Scholar]
- Leprince, A.; Nuytten, M.; Gillis, A.; Mahillon, J. Characterization of PlyB221 and PlyP32, Two Novel Endolysins Encoded by Phages Preying on the Bacillus Cereus Group. Viruses 2020, 12, 1052. [Google Scholar] [CrossRef]
- Robertson, J. High Dielectric Constant Oxides. Eur. Phys. J. Appl. Phys. 2004, 28, 265–291. [Google Scholar] [CrossRef] [Green Version]
- Iatsunskyi, I.; Jancelewicz, M.; Nowaczyk, G.; Kempiński, M.; Peplińska, B.; Jarek, M.; Załęski, K.; Jurga, S.; Smyntyna, V. Atomic Layer Deposition TiO2 Coated Porous Silicon Surface: Structural Characterization and Morphological Features. Thin Solid Films 2015, 589, 303–308. [Google Scholar] [CrossRef]
- Tenenbaum, E.; Segal, E. Optical Biosensors for Bacteria Detection by a Peptidomimetic Antimicrobial Compound. Analyst 2015, 140, 7726–7733. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Rahimi, F.; Negahdari, B.; Rezayan, A.H.; Shafiekhani, A. A Lectin-Coupled Porous Silicon-Based Biosensor: Label-Free Optical Detection of Bacteria in a Real-Time Mode. Sci. Rep. 2020, 10, 16017. [Google Scholar] [CrossRef]
- Urmann, K.; Walter, J.-G.; Scheper, T.; Segal, E. Label-Free Optical Biosensors Based on Aptamer-Functionalized Porous Silicon Scaffolds. Anal. Chem. 2015, 87, 1999–2006. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Z.; Luo, Q.; Liu, J.; Wu, J. Bacteria Detection Based on Its Blockage Effect on Silicon Nanopore Array. Biosens. Bioelectron. 2016, 79, 715–720. [Google Scholar] [CrossRef]
- Groner, M.D.; Fabreguette, F.H.; Elam, J.W.; George, S.M. Low-Temperature Al2O3 Atomic Layer Deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Porro, S.; Jasmin, A.; Bejtka, K.; Conti, D.; Perrone, D.; Guastella, S.; Pirri, C.F.; Chiolerio, A.; Ricciardi, C. Low-Temperature Atomic Layer Deposition of TiO2 Thin Layers for the Processing of Memristive Devices. J. Vac. Sci. Technol. A Vac. Surf. Films 2016, 34, 01A147. [Google Scholar] [CrossRef]




| HfO2 | Al2O3 | TiO2 | |
|---|---|---|---|
| Precursor Pulse 1 | 0.3 s | 0.5 s | 0.2 s |
| Wait | 120 s | 120 s | 120 s |
| Purge | 360 s | 360 s | 360 s |
| Precursor Pulse 2 | 0.1 s | 0.1 s | 0.2 s |
| Wait | 120 s | 120 s | 120 s |
| Purge | 360 s | 360 s | 360 s |
| Total number of cycles | 20 | 40 | 50 |
| Deposition temperature | 100 °C | 100 °C | 150 °C |
| SLIM | SEM | |||
|---|---|---|---|---|
| Thickness [µm] | Open Porosity [%] | nskeleton [RIU] | Pore Size [nm] | |
| PSiO2 | 3.66 ± 0.35 | 74.7 ± 5.8 | 2.52 ± 0.17 | 30.3 ± 14.8 |
| PSiO2/HfO2 | 3.67 ± 0.21 | 56.9 ± 4.5 | 2.38 ± 0.20 | 26.4 ± 14.0 |
| PSiO2/TiO2 | 3.60 ± 0.13 | 62.1 ± 6.1 | 2.57 ± 0.25 | 25.5 ± 11.4 |
| PSiO2/Al2O3 | 3.56 ± 0.21 | 62.4 ± 4.7 | 2.09 ± 0.17 | 23.2 ± 12.0 |
| σN [%] | Sensitivity [%/RIU] | LoD ×10−3 [RIU] | |
|---|---|---|---|
| PSiO2 | 0.080 | 59.6 | 4.0 |
| PSiO2/HfO2 | 0.019 | 38.7 | 1.5 |
| PSiO2/TiO2 | 0.089 | 35.6 | 7.5 |
| PSiO2/Al2O3 | 0.030 | 48.6 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whyte Ferreira, C.; Vercauteren, R.; Francis, L.A. Passivated Porous Silicon Membranes and Their Application to Optical Biosensing. Micromachines 2022, 13, 10. https://doi.org/10.3390/mi13010010
Whyte Ferreira C, Vercauteren R, Francis LA. Passivated Porous Silicon Membranes and Their Application to Optical Biosensing. Micromachines. 2022; 13(1):10. https://doi.org/10.3390/mi13010010
Chicago/Turabian StyleWhyte Ferreira, Clara, Roselien Vercauteren, and Laurent A. Francis. 2022. "Passivated Porous Silicon Membranes and Their Application to Optical Biosensing" Micromachines 13, no. 1: 10. https://doi.org/10.3390/mi13010010
APA StyleWhyte Ferreira, C., Vercauteren, R., & Francis, L. A. (2022). Passivated Porous Silicon Membranes and Their Application to Optical Biosensing. Micromachines, 13(1), 10. https://doi.org/10.3390/mi13010010








