Study on Flexible sEMG Acquisition System and Its Application in Muscle Strength Evaluation and Hand Rehabilitation
Abstract
1. Introduction
2. Materials and Methods
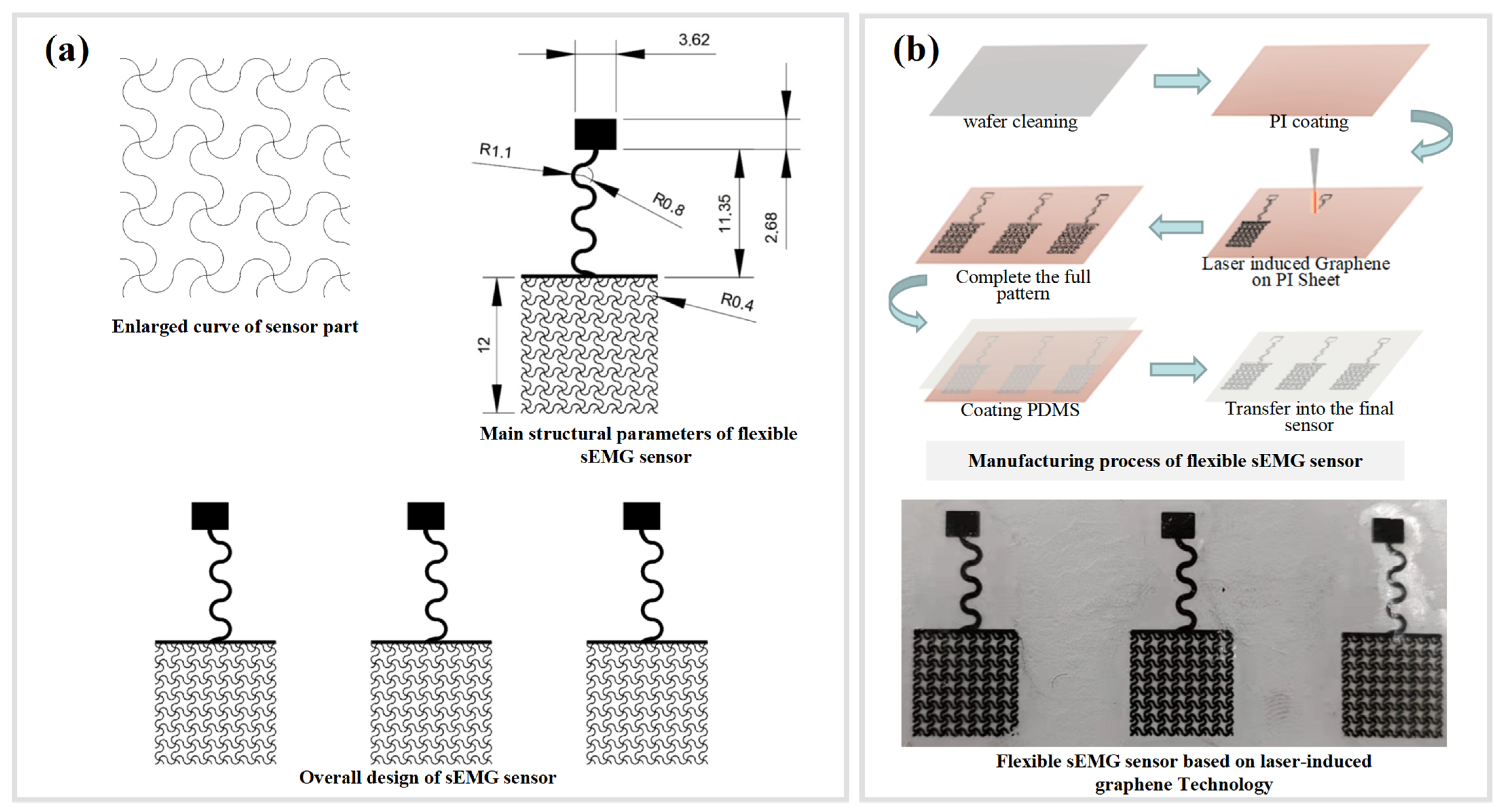
2.1. Preparation of Flexible sEMG Sensors
2.2. Flexible sEMG Acquisition Hardware Preparation and Mechanical Property Testing
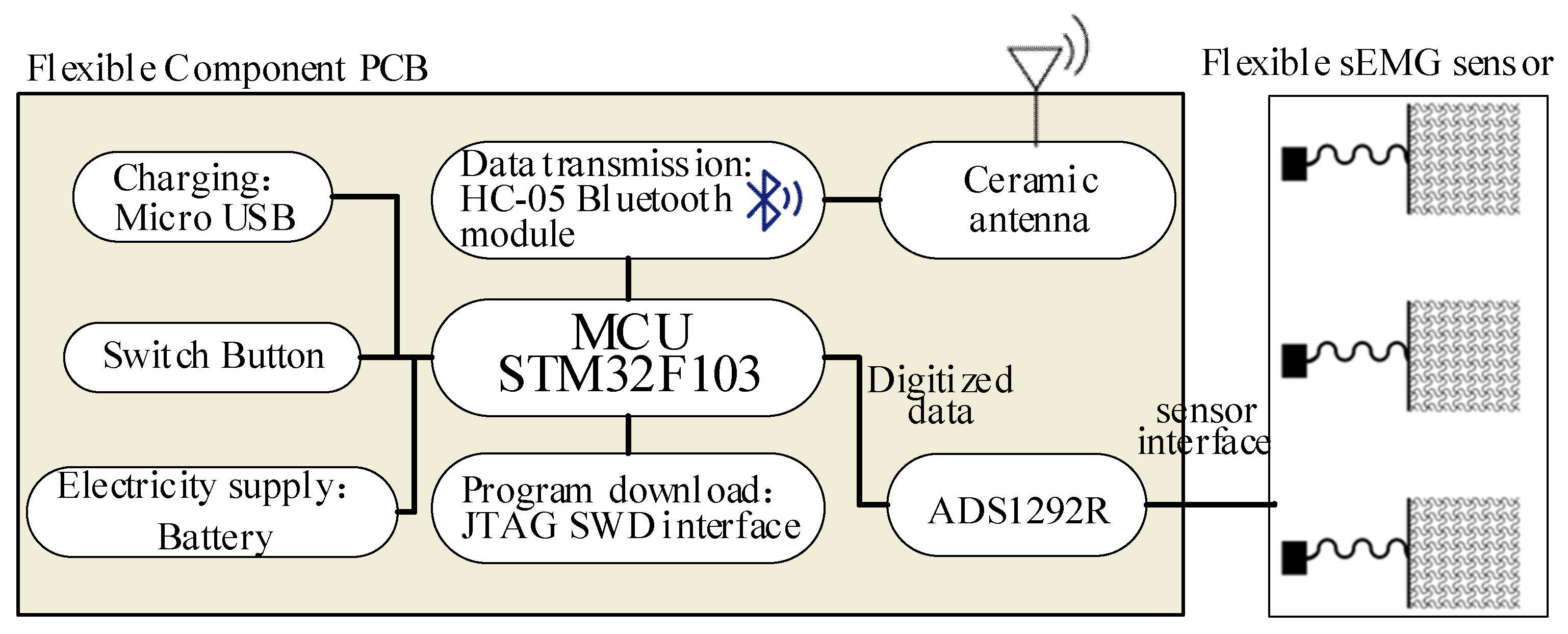
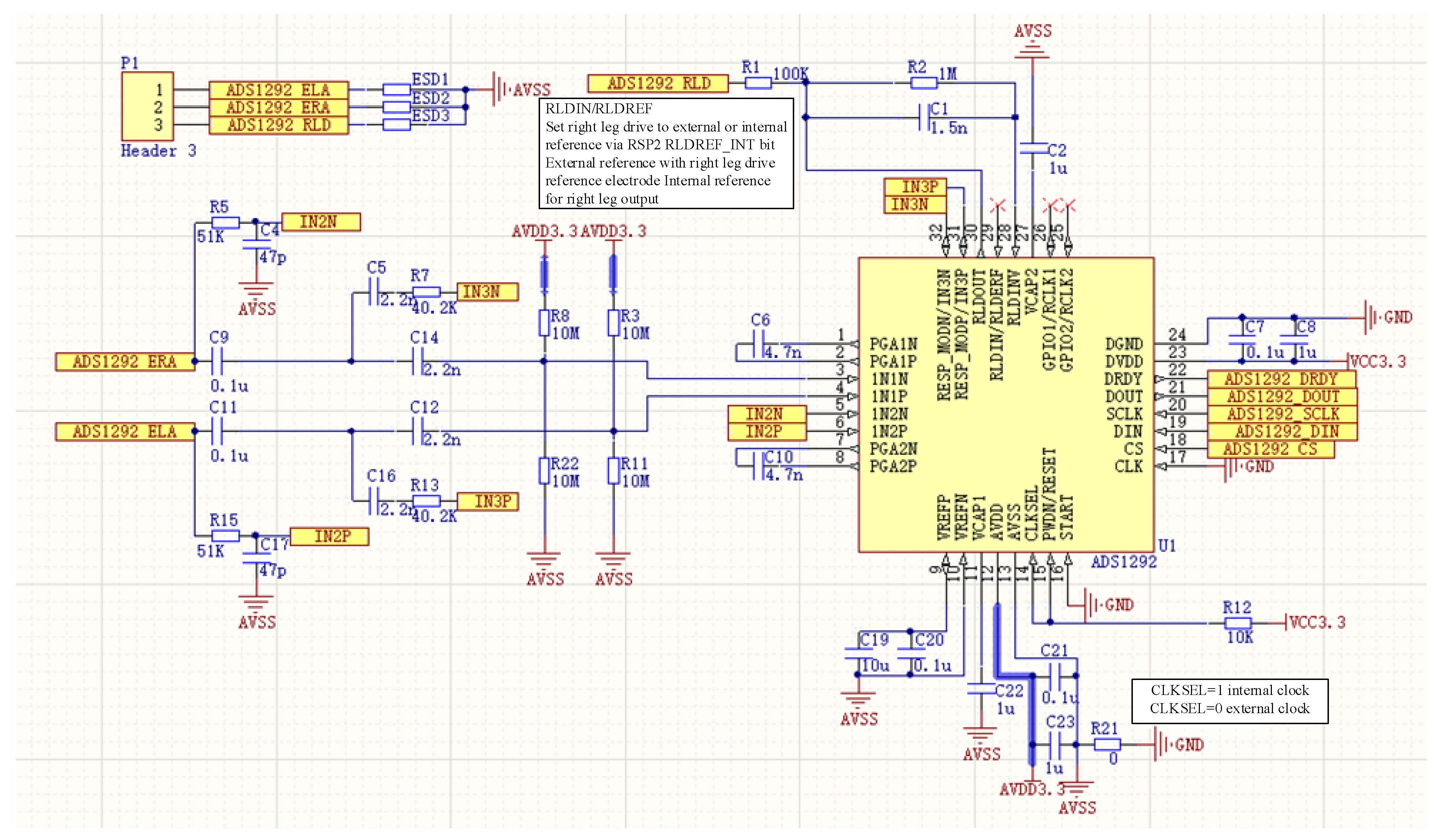
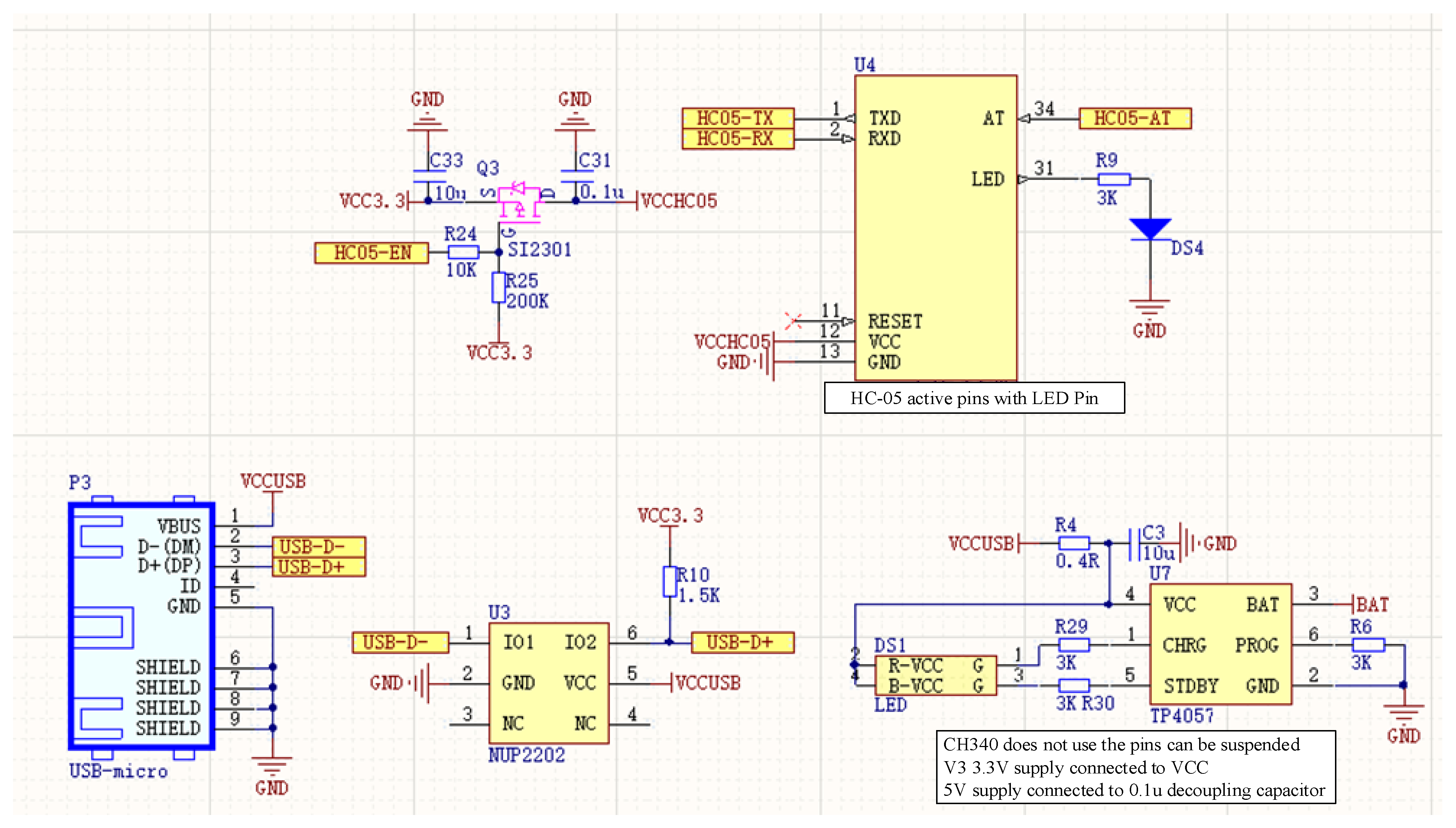
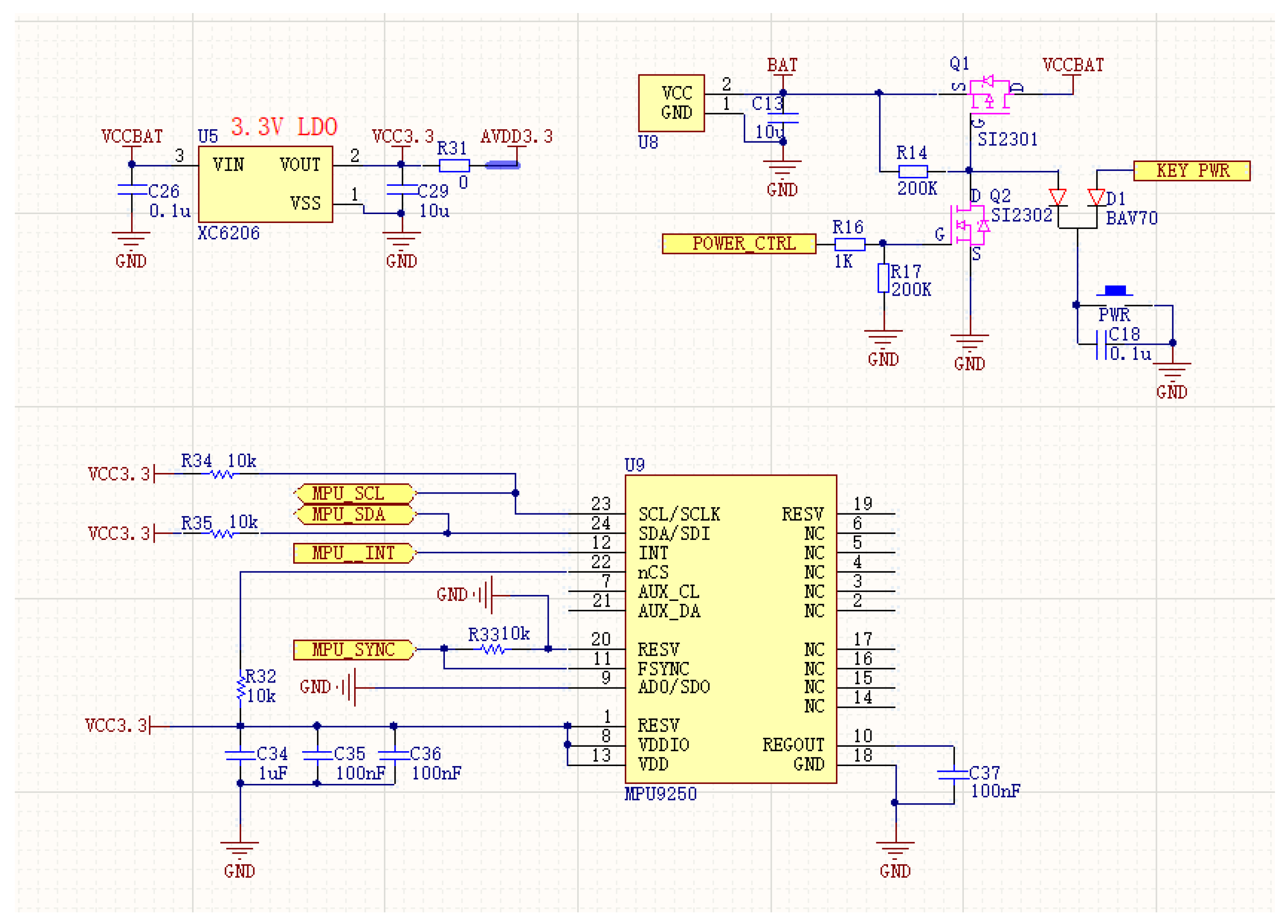
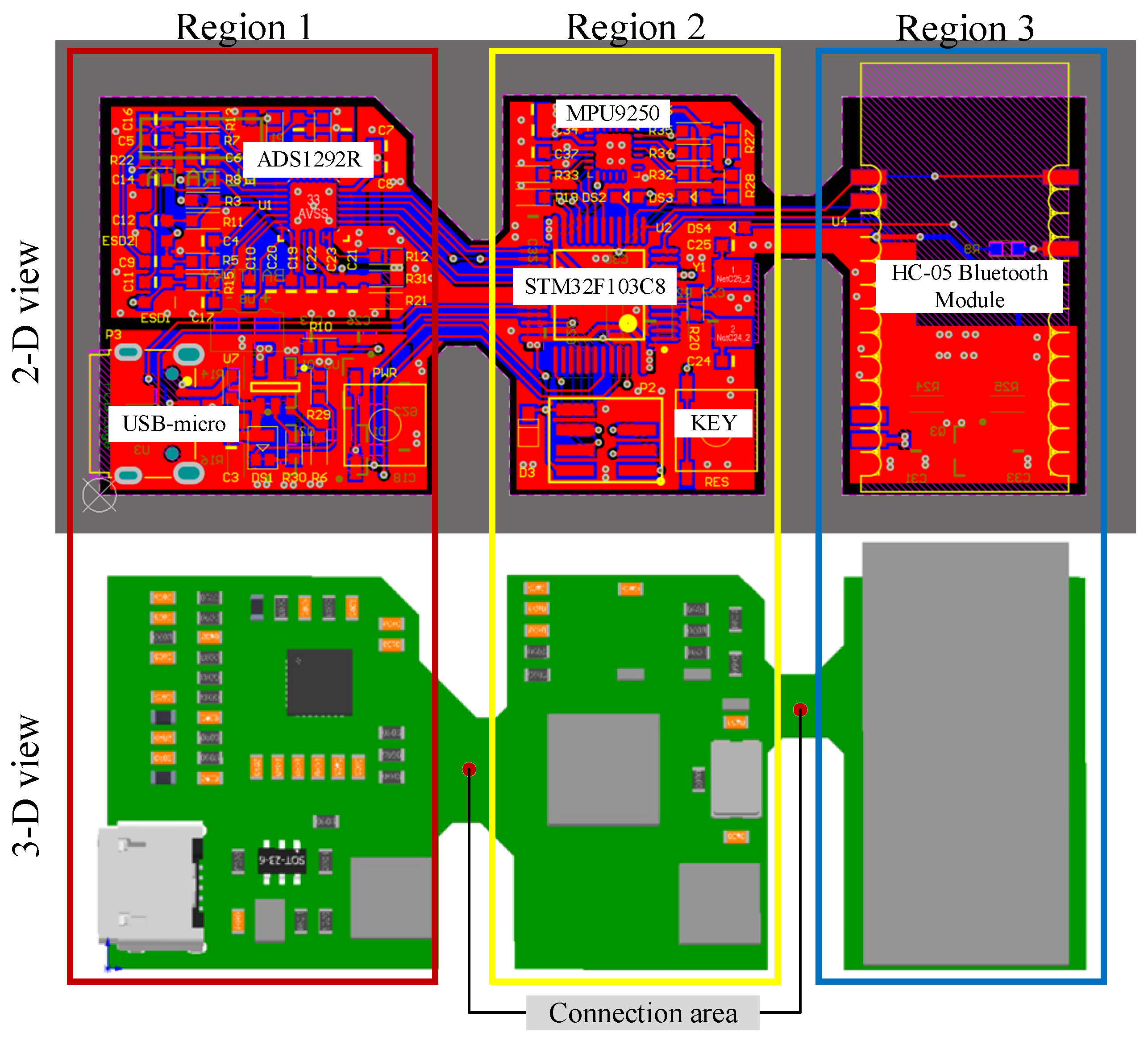
2.2.1. Flexible sEMG Acquisition Hardware Preparation Process
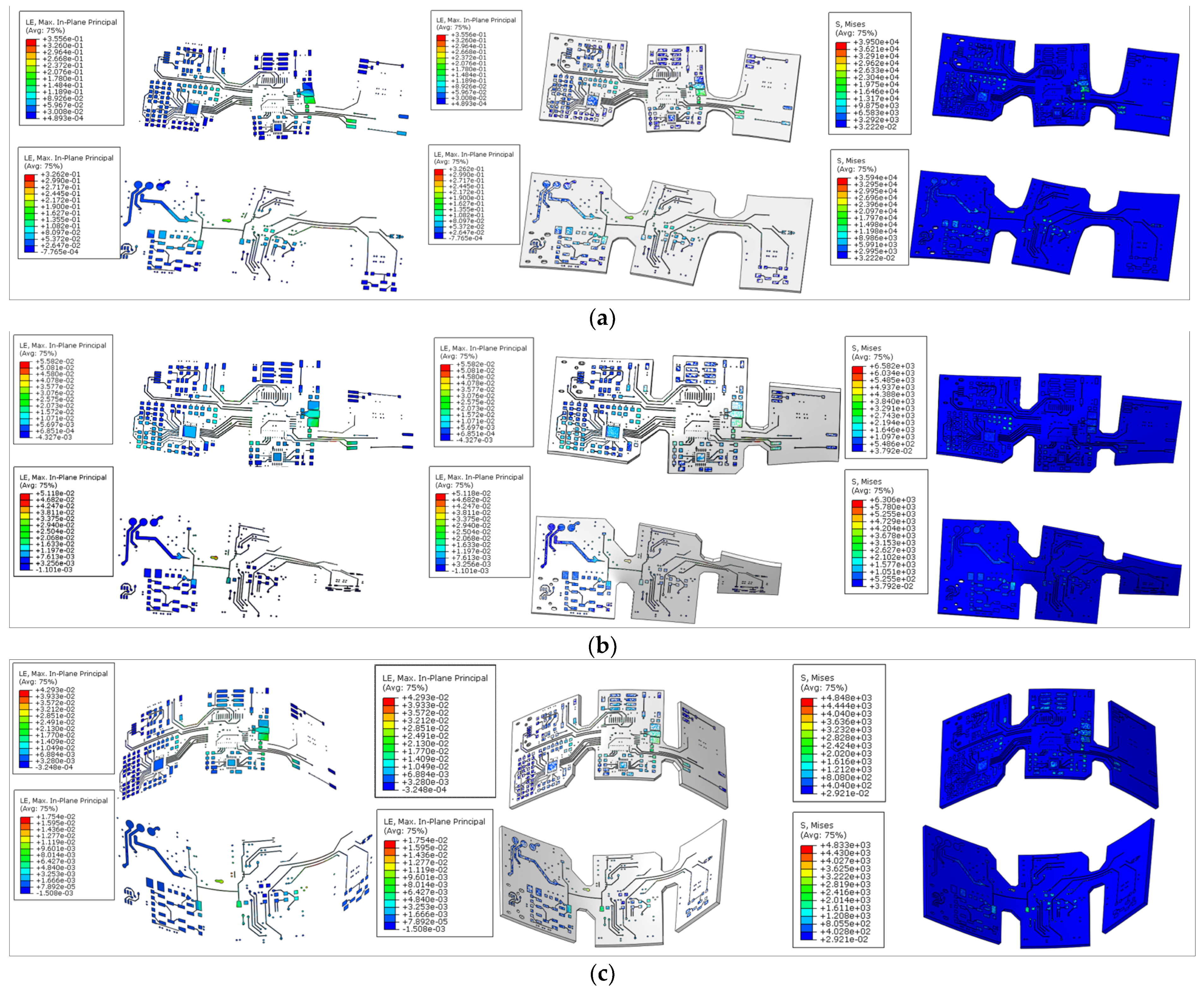
2.2.2. Flexible sEMG Hardware Mechanical Performance Testing
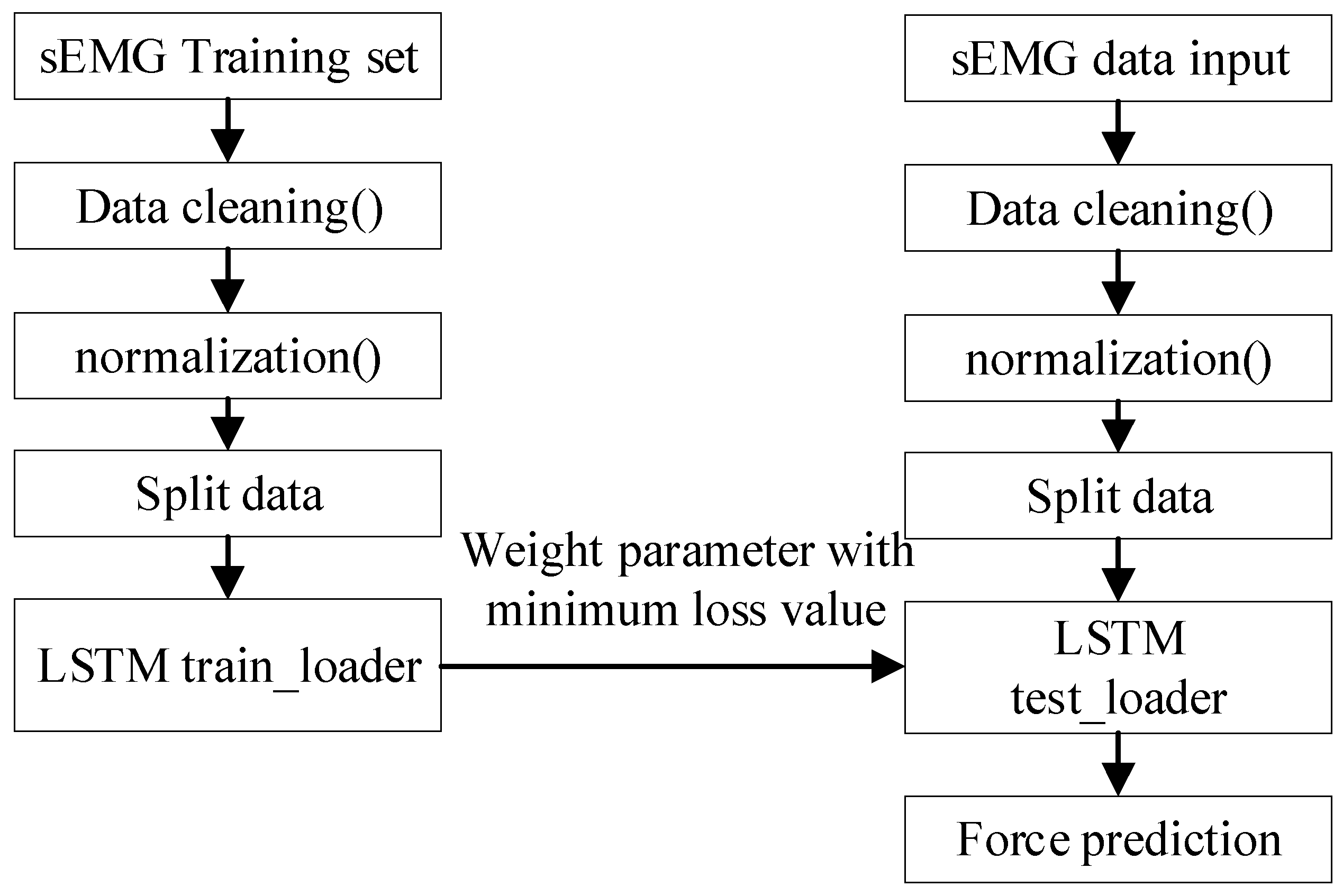
2.3. Grip Force Prediction Algorithm and Hand Rehabilitation Control Method Based on Flexible sEMG Sensing System
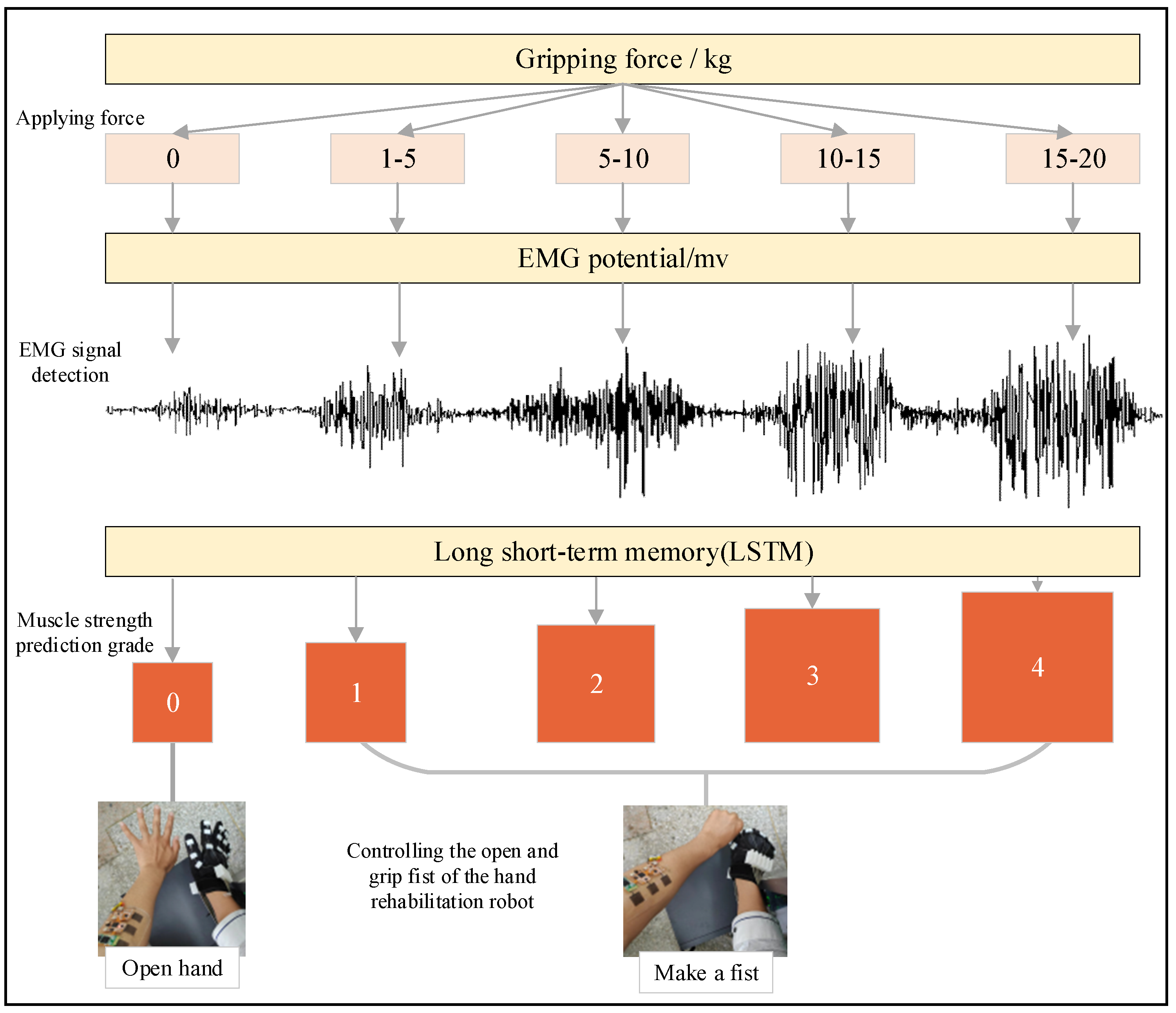
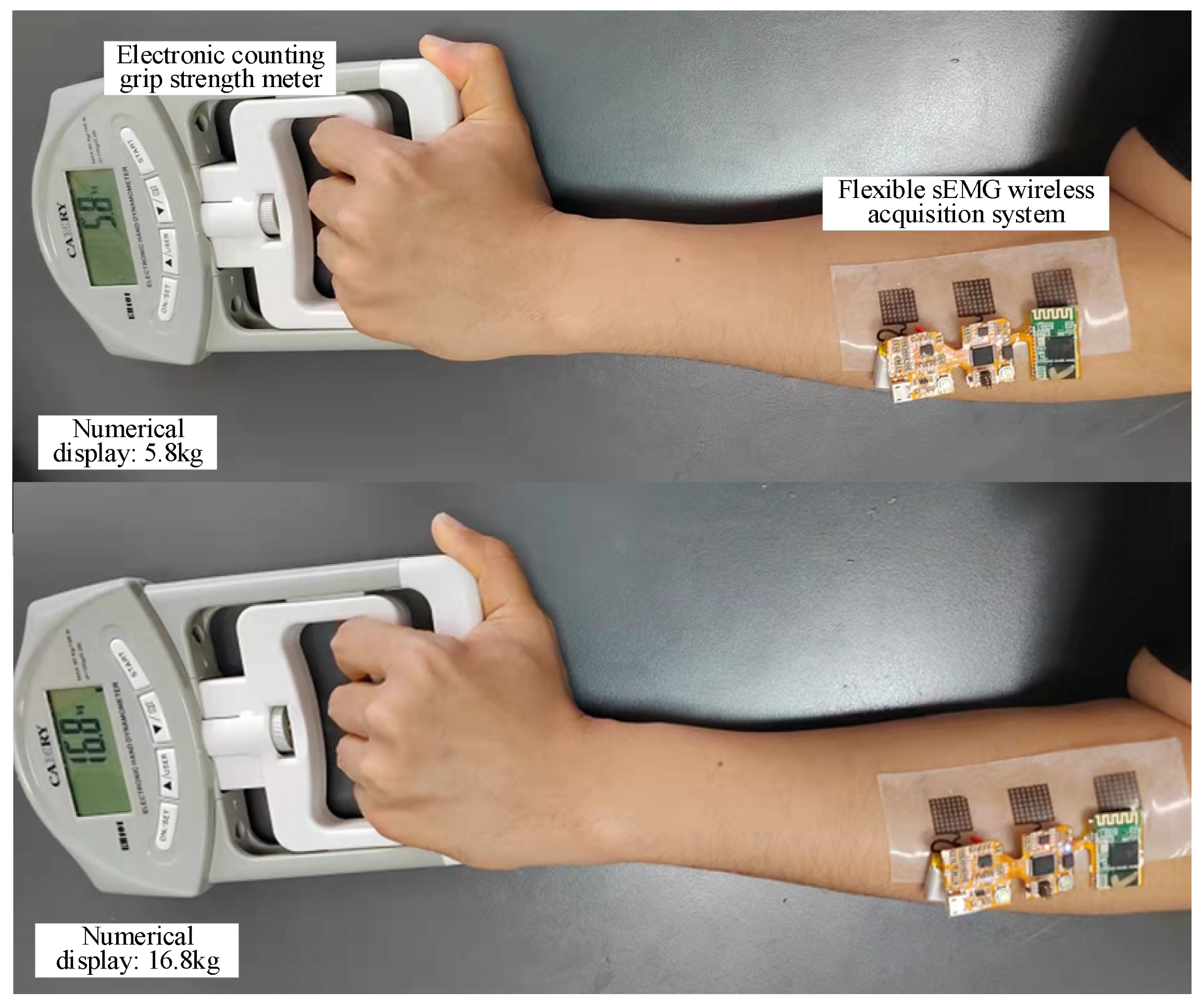
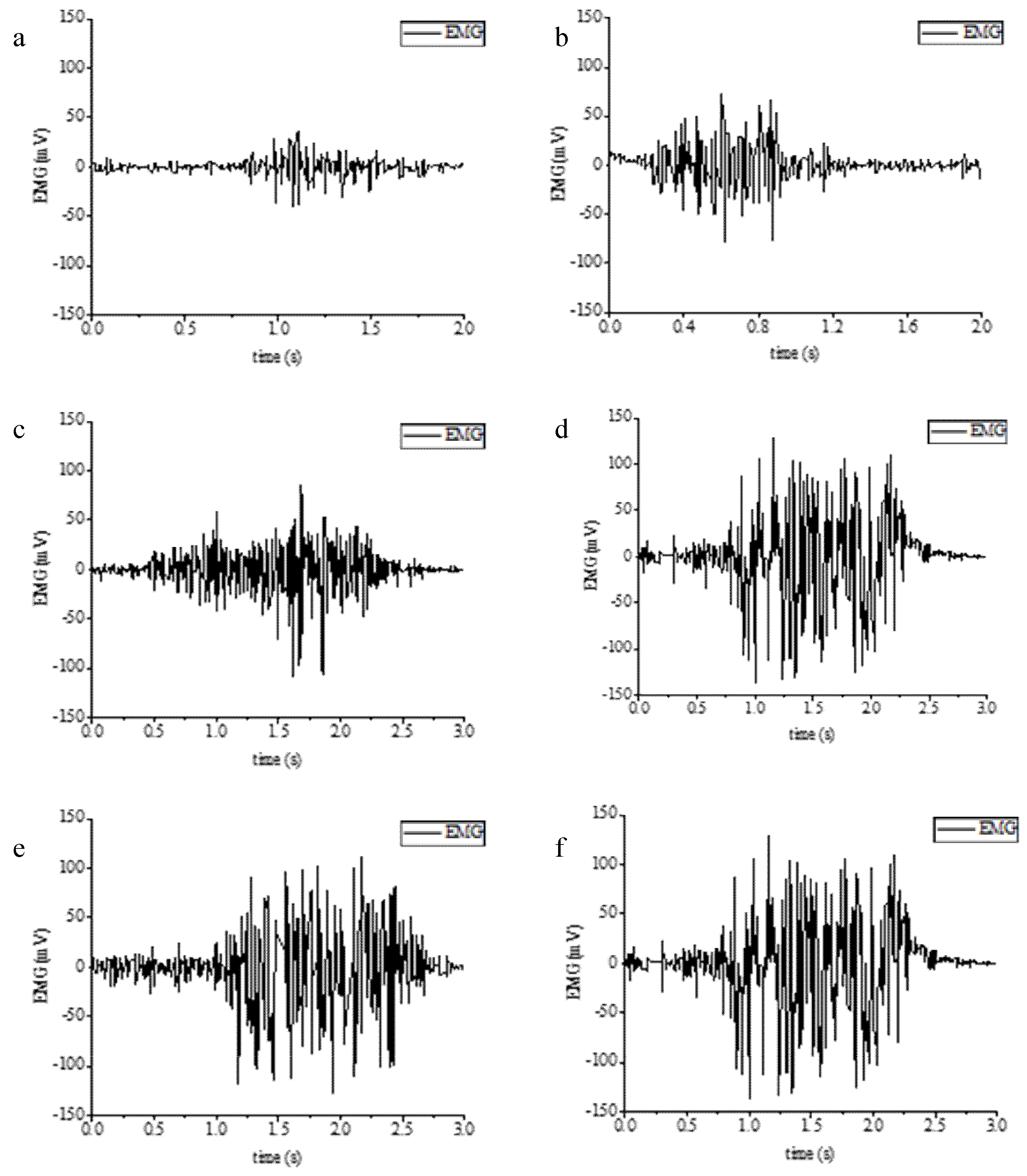
2.4. Scenario Setting of Muscle Strength Prediction Experiment and sEMG Signal Processing Process
3. Results
3.1. Flexible sEMG Hardware Mechanical Performance Test Results
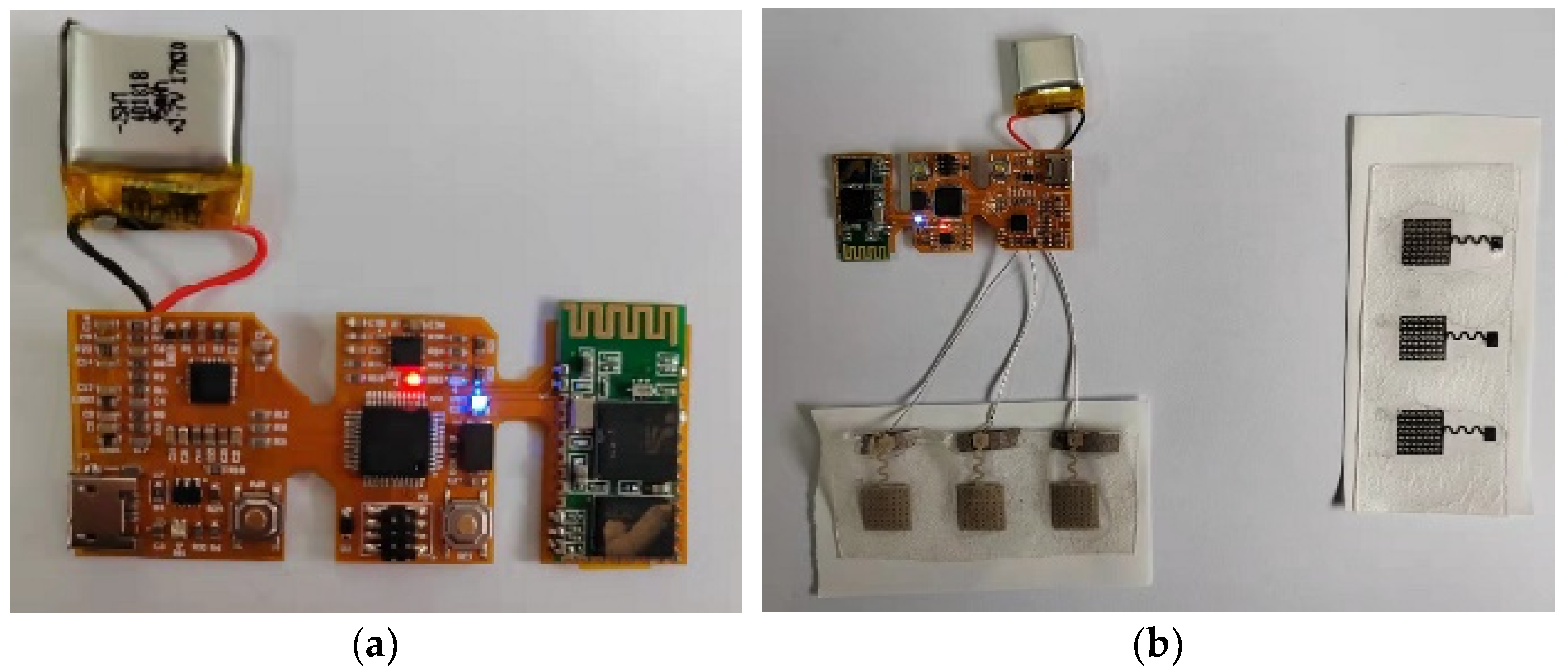
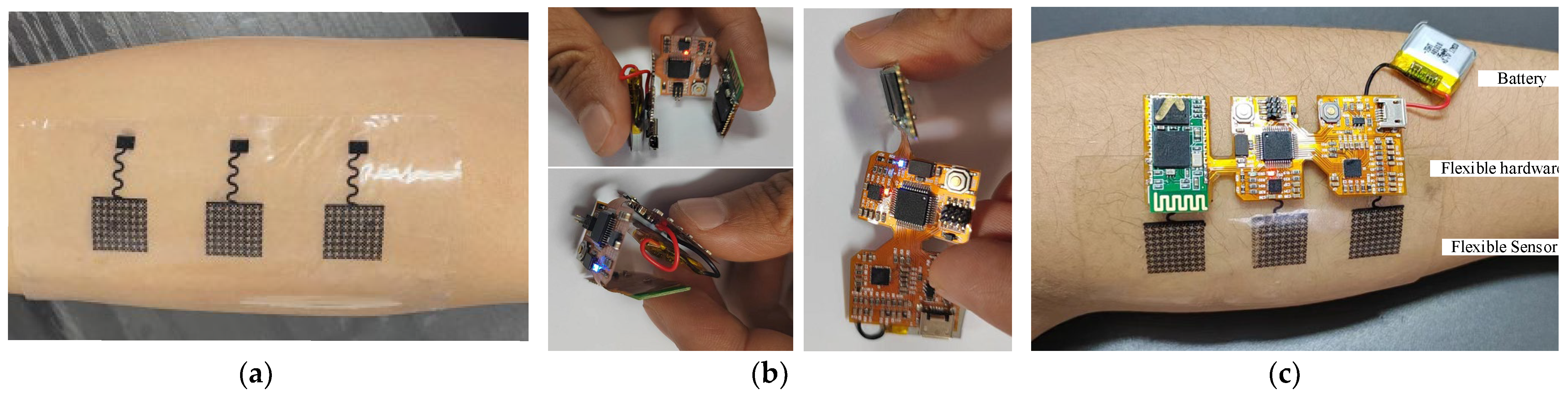
3.2. Overall Display of Flexible sEMG Collection System
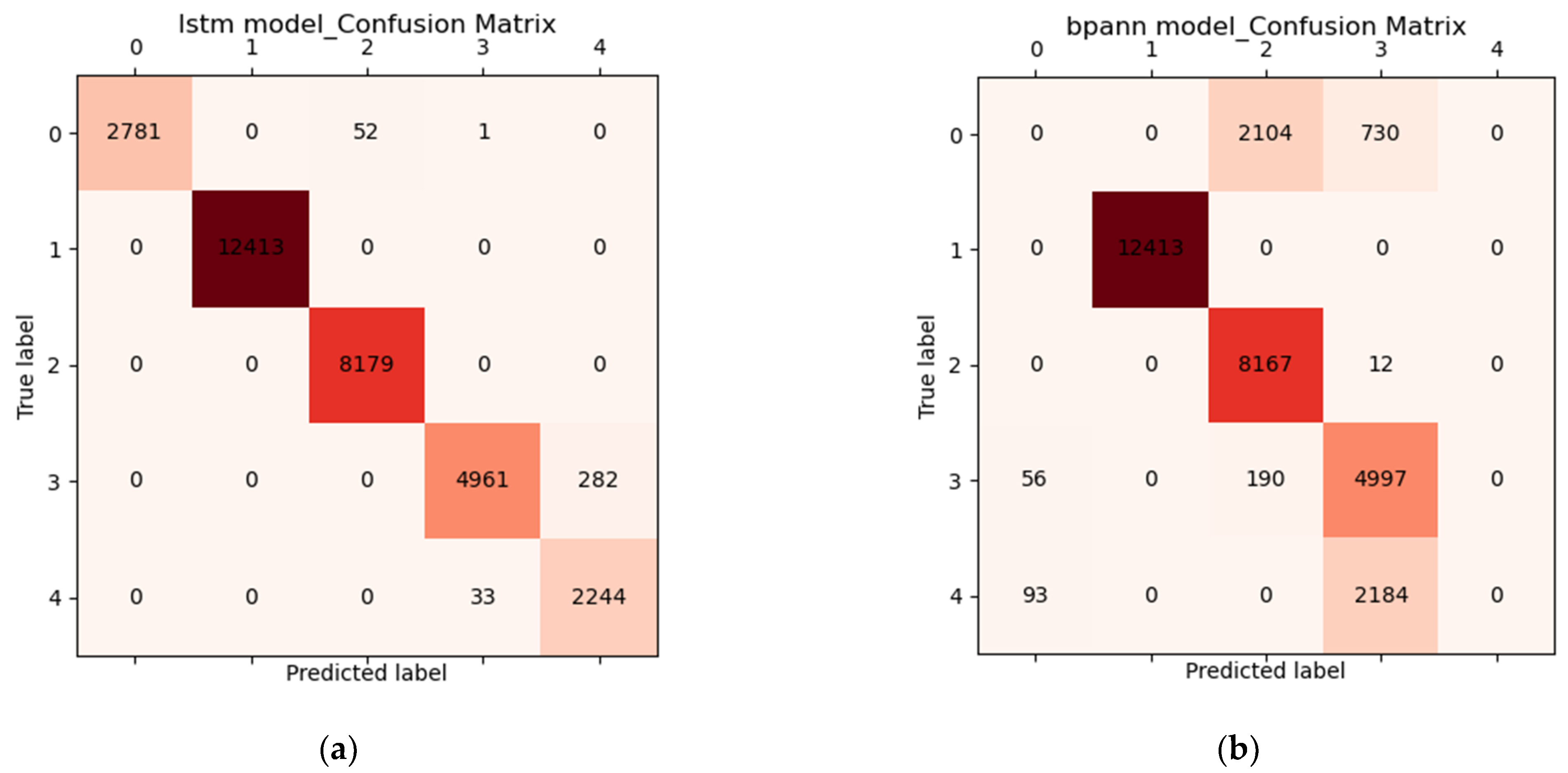
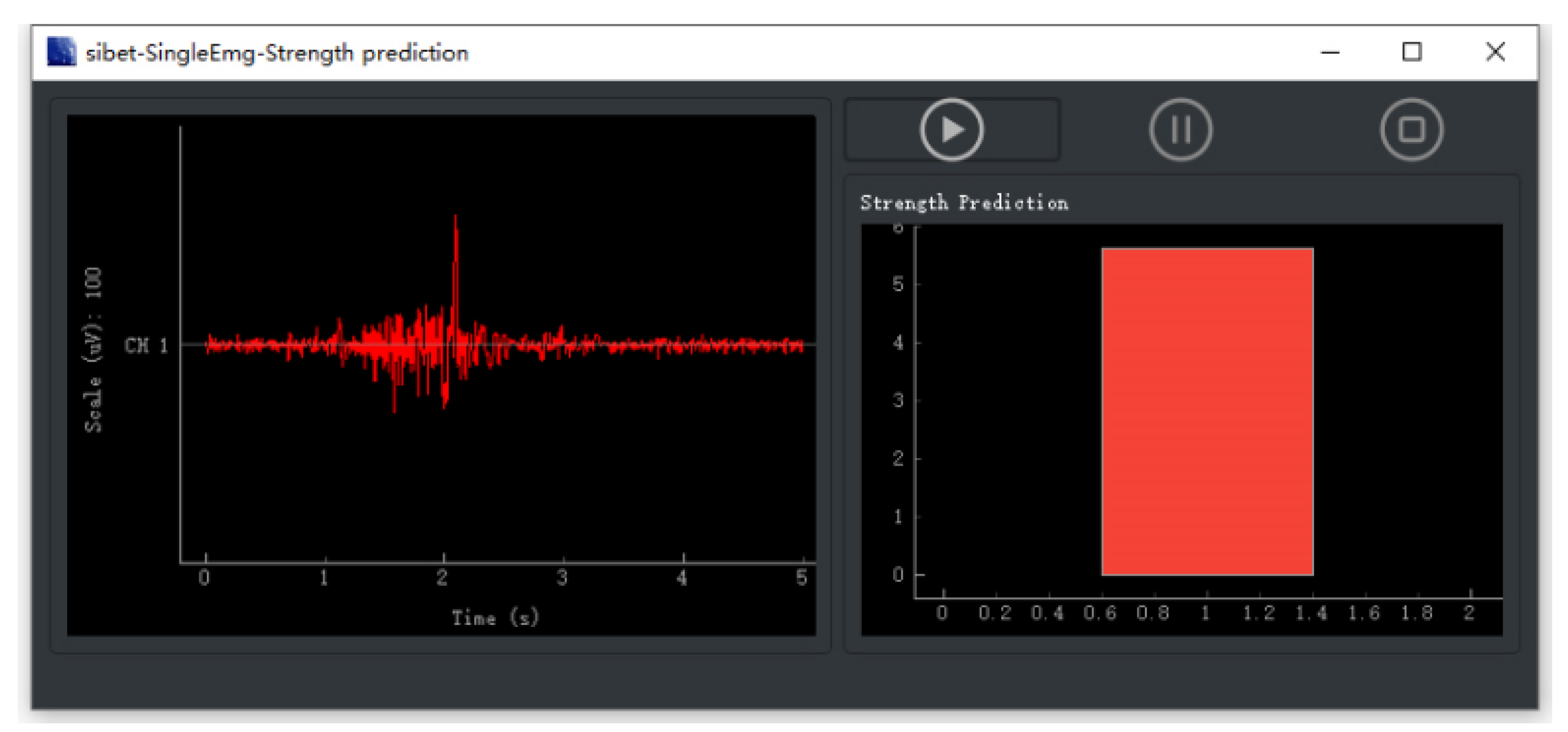
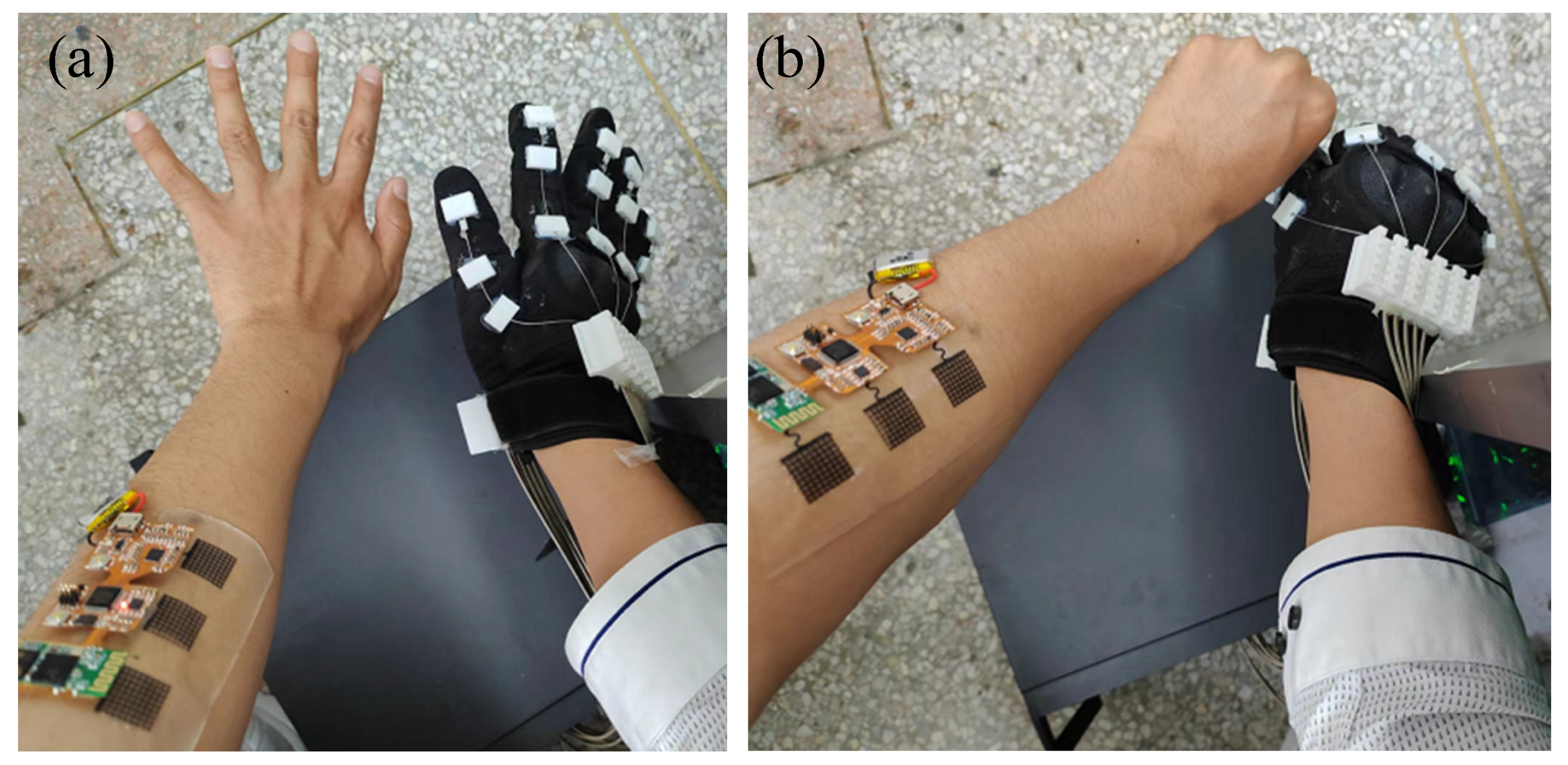
3.3. Results of Grip Force Prediction Algorithm and Hand Rehabilitation Control Method Based on Flexible sEMG Acquisition System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.; Zhao, J.; Dong, Y.; Wang, X. Dry Electrodes for Human Bioelectrical Signal Monitoring. Sensors 2020, 20, 3651. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, Y.; Zhao, Y.; Ren, Z.; Guo, C.F. Flexible electronics: Stretchable electrodes and their future. Adv. Funct. Mater. 2019, 29, 1805924. [Google Scholar] [CrossRef]
- You, I.; Mackanic, D.G.; Matsuhisa, N.; Kang, J.; Kwon, J.; Beker, L.; Mun, J.; Suh, W.; Kim, T.Y.; Tok, J.B.H.; et al. Artificial multimodal receptors based on ion relaxation dynamics. Science 2020, 370, 961. [Google Scholar] [CrossRef] [PubMed]
- Song, E.M.; Li, J.H.; Won, S.M.; Bai, W.B.; Rogers, J.A. Materials for flexible bioelectronic systems as chronic neural interfaces. Nat. Mater. 2020, 19, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Kim, D.H.; Price, J.T.; Lee, J.Y.; Chung, H.U.; Allen, E.; Walter, J.R.; Jeong, H.; Cao, J.Y.; Kulikova, E.; et al. Comprehensive pregnancy monitoring with a network of wireless, soft, and flexible sensors in high- and low-resource health settings. Proc. Natl. Acad. Sci. USA 2021, 118, e2100466118. [Google Scholar] [CrossRef]
- Kang, J.; Tok, J.B.H.; Bao, Z.A. Self-healing soft electronics. Nat. Electron. 2019, 2, 144–150. [Google Scholar] [CrossRef]
- Zhou, W.; Yao, S.; Wang, H.; Du, Q.; Ma, Y.; Zhu, Y. Gas-Permeable, Ultrathin, Stretchable Epidermal Electronics with Porous Electrodes. ACS Nano 2020, 14, 5798–5805. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Y.Z.; Lai, W.Y.; Huang, W. Stretchable Thin-Film Electrodes for Flexible Electronics with High Deformability and Stretchability. Adv. Mater. 2015, 27, 3349–3376. [Google Scholar] [CrossRef]
- Wu, H.; Guo, H.; Su, Z.; Shi, M.; Chen, X.; Cheng, X.; Han, M.; Zhang, H. Fabric-based self-powered noncontact smart gloves for gesture recognition. J. Mater. Chem. A 2018, 6, 20277–20288. [Google Scholar] [CrossRef]
- Sierotowicz, M.; Lotti, N.; Nell, L.; Missiroli, F.; Alicea, R.; Zhang, X.; Xiloyannis, M.; Rupp, R.; Papp, E.; Krzywinski, J.; et al. EMG-Driven Machine Learning Control of a Soft Glove for Grasping Assistance and Rehabilitation. IEEE Robot. Autom. Lett. 2022, 7, 1566–1573. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Zhang, Y.; Liu, H.; Chen, Z.a.; Lu, Y.; Dai, Y.; Xu, L.; Gao, S. Flexible and Wearable EMG and PSD Sensors Enabled Locomotion Mode Recognition for IoHT-Based In-Home Rehabilitation. IEEE Sens. J. 2021, 21, 26311–26319. [Google Scholar] [CrossRef]
- Bouteraa, Y.; Ben Abdallah, I.; Elmogy, A.M. Training of Hand Rehabilitation Using Low Cost Exoskeleton and Vision-Based Game Interface. J. Intell. Robot. Syst. 2019, 96, 31–47. [Google Scholar] [CrossRef]
- Cochran, R.; Rosen, T. Contact dermatitis caused by ECG electrode paste. South. Med. J. 1980, 73, 1667–1668. [Google Scholar] [CrossRef]
- Toral, V.; Castillo, E.; Albretch, A.; Romero, F.J.; Garcia, A.; Rodriguez, N.; Lugli, P.; Morales, D.P.; Rivadeneyra, A. Cost-Effective Printed Electrodes Based on Emerging Materials Applied to Biosignal Acquisition. IEEE Access 2020, 8, 127789–127800. [Google Scholar] [CrossRef]
- Cisnal, A.; Perez-Turiel, J.; Fraile, J.-C.; Sierra, D.; de la Fuente, E. RobHand: A Hand Exoskeleton With Real-Time EMG-Driven Embedded Control. Quantifying Hand Gesture Recognition Delays for Bilateral Rehabilitation. IEEE Access 2021, 9, 137809–137823. [Google Scholar] [CrossRef]
- Li, K.; Zhang, J.; Wang, L.; Zhang, M.; Li, J.; Bao, S. A review of the key technologies for sEMG-based human-robot interaction systems. Biomed. Signal Process. Control 2020, 62, 102074. [Google Scholar] [CrossRef]
- Tang, Z.Q.; Heung, H.L.; Shi, X.Q.; Tong, R.K.Y.; Li, Z. Probabilistic Model-Based Learning Control of a Soft Pneumatic Glove for Hand Rehabilitation. IEEE Trans. Biomed. Eng. 2022, 69, 1016–1028. [Google Scholar] [CrossRef]
- Palumbo, A.; Vizza, P.; Calabrese, B.; Ielpo, N. Biopotential Signal Monitoring Systems in Rehabilitation: A Review. Sensors 2021, 21, 7172. [Google Scholar] [CrossRef]
- Gomez-Donoso, F.; Escalona, F.; Nasri, N.; Cazorla, M. A Hand Motor Skills Rehabilitation for the Injured Implemented on a Social Robot. Appl. Sci. 2021, 11, 2943. [Google Scholar] [CrossRef]
- Liu, H.; Dong, W.; Li, Y.; Li, F.; Geng, J.; Zhu, M.; Chen, T.; Zhang, H.; Sun, L.; Lee, C. An epidermal sEMG tattoo-like patch as a new human–machine interface for patients with loss of voice. Microsyst. Nanoeng. 2020, 6, 16. [Google Scholar] [CrossRef]
- Chandra, S.; Li, J.; Afsharipour, B.; Cardona, A.F.; Suresh, N.L.; Tian, L.; Deng, Y.; Zhong, Y.; Xie, Z.; Shen, H.; et al. Performance Evaluation of a Wearable Tattoo Electrode Suitable for High-Resolution Surface Electromyogram Recording. IEEE Trans. Biomed. Eng. 2021, 68, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Fialkoff, B.; Hadad, H.; Santos, D.; Simini, F.; David, M. Hand grip force estimation via EMG imaging. Biomed. Signal Process. Control 2022, 74, 103550. [Google Scholar] [CrossRef]
- Tan, P.; Wang, H.; Xiao, F.; Lu, X.; Shang, W.; Deng, X.; Song, H.; Xu, Z.; Cao, J.; Gan, T.; et al. Solution-processable, soft, self-adhesive, and conductive polymer composites for soft electronics. Nat. Commun. 2022, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Kouzaki, M.; Ogawa, M.; Akima, H.; Moritani, T. Relationships between muscle strength and multi-channel surface EMG parameters in eighty-eight elderly. Eur. Rev. Aging Phys. Act. 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Shahid, T.; Gouwanda, D.; Nurzaman, S.G.; Gopalai, A.A. Moving toward Soft Robotics: A Decade Review of the Design of Hand Exoskeletons. Biomimetics 2018, 3, 17. [Google Scholar] [CrossRef]
- Quan-Sheng, X.U.; Shi-Ming, L.I.; Shu-Mei, J.I.; University, Y. Estimation of Human Dynamic Knee Torque Using Surface Electromyography Signals Based on BP Network. China Sport Sci. Technol. 2018, 40, 335–342. [Google Scholar]
- De Lee, G.; Wang, W.-W.; Lee, K.-W.; Lin, S.-Y.; Fu, L.-C.; Lai, J.-S.; Chen, W.-S.; Luh, J.-J. Arm exoskeleton rehabilitation robot with assistive system for patient after stroke. In Proceedings of the 2012 12th International Conference on Control, Automation and Systems, Guangzhou, China, 26–29 March 2012; pp. 1943–1948. [Google Scholar]
- Duque, J.; Masset, D.; Malchaire, J. Evaluation of handgrip force from EMG measurements. Appl. Ergon. 1995, 26, 61–66. [Google Scholar] [CrossRef]
- Disselhorst-Klug, C.; Schmitz-Rode, T.; Rau, G. Surface electromyography and muscle force: Limits in sEMG–force relationship and new approaches for applications. Clin. Biomech. 2009, 24, 225–235. [Google Scholar] [CrossRef]
- Ibitoye, M.O.; Hamzaid, N.A.; Zuniga, J.M.; Wahab, A.K.A. Mechanomyography and muscle function assessment: A review of current state and prospects. Clin. Biomech. 2014, 29, 691–704. [Google Scholar] [CrossRef]
- Li, Z.B.; Gao, L.F.; Lu, W.; Wang, D.Q.; Cao, H.B.; Zhang, G. Estimation of Knee Extension Force Using Mechanomyography Signals Based on GRA and ICS-SVR. Sensors 2022, 22, 4651. [Google Scholar] [CrossRef]
- Li, H.H.; Zhao, G.R.; Zhou, Y.J.; Chen, X.; Ji, Z.; Wang, L. Relationship of EMG/SMG features and muscle strength level: An exploratory study on tibialis anterior muscles during plantar-flexion among hemiplegia patients. Biomed. Eng. Online 2014, 13, 5. [Google Scholar] [CrossRef]
- Mokri, C.; Bamdad, M.; Abolghasemi, V. Muscle force estimation from lower limb EMG signals using novel optimised machine learning techniques. Med. Biol. Eng. Comput. 2022, 60, 683–699. [Google Scholar] [CrossRef]
- Wininger, M.; Kim, N.-H.; Craelius, W. Pressure signature of forearm as predictor of grip force. J. Rehabil. Res. Dev. 2008, 45. [Google Scholar] [CrossRef]
- Esposito, D.; Gargiulo, G.D.; Parajuli, N.; Cesarelli, G.; Andreozzi, E.; Bifulco, P. Measurement of muscle contraction timing for prosthesis control: A comparison between electromyography and force-myography. In Proceedings of the 2020 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Virtual, 1 July 2020; pp. 1–6. [Google Scholar]
- Li, Z.; Wang, B.; Sun, F.; Yang, C.; Xie, Q.; Zhang, W. sEMG-Based Joint Force Control for an Upper-Limb Power-Assist Exoskeleton Robot. Biomed. Health Inform. 2014, 18, 1043–1050. [Google Scholar]
- Bai, F.; Chew, C.-M. Muscle force estimation with surface EMG during dynamic muscle contractions: A wavelet and ANN based approach. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4589–4592. [Google Scholar]
- Huang, C.; Chen, X.; Cao, S.; Qiu, B.; Zhang, X. An isometric muscle force estimation framework based on a high-density surface EMG array and an NMF algorithm. J. Neural Eng. 2017, 14, 046005. [Google Scholar] [CrossRef]
- Liang, F.; Li, C.; Gao, Y.; Zhang, C.; Chen, J. Study on pattern recognition of hand motion modes based on wavelet packet and SVM. In Computational Intelligence, Networked Systems and Their Applications; Springer: Berlin/Heidelberg, Germany, 2014; pp. 180–188. [Google Scholar]
- Jalaludin, N.A.; Na’im Sidek, S.; Shamsudin, A.U.; Aibinu, A.M. Electromyography (EMG)-based thump-tip force estimation for prosthetic thumb. In Proceedings of the 2012 International Conference on Computer and Communication Engineering (ICCCE), Kuala Lumpur, Malaysia, 3–5 July 2012; pp. 783–786. [Google Scholar]
- Esposito, D.; Andreozzi, E.; Fratini, A.; Gargiulo, G.D.; Savino, S.; Niola, V.; Bifulco, P. A Piezoresistive Sensor to Measure Muscle Contraction and Mechanomyography. Sensors 2018, 18, 2553. [Google Scholar] [CrossRef]
- Fukuhara, S.; Oka, H. Displacement Mechanomyography Reflects Mechanical Pedaling Force of Muscle Associated with Changes in Cadence and Work Rate During Pedaling. J. Med. Biol. Eng. 2022. [Google Scholar] [CrossRef]
- Esposito, D.; Andreozzi, E.; Gargiulo, G.D.; Fratini, A.; D’Addio, G.; Naik, G.R.; Bifulco, P. A Piezoresistive Array Armband With Reduced Number of Sensors for Hand Gesture Recognition. Front. Neurorobot. 2020, 13, 114. [Google Scholar] [CrossRef]
- Jiang, X.T.; Merhi, L.K.; Xiao, Z.G.; Menon, C. Exploration of Force Myography and surface Electromyography in hand gesture classification. Med. Eng. Phys. 2017, 41, 63–73. [Google Scholar] [CrossRef]
- Basmajian, J.V. Muscles alive. Their functions revealed by electromyography. Acad. Med. 1962, 37, 802. [Google Scholar]
- Zhou, C.; Yang, L.; Liao, H.; Liang, B.; Ye, X. Ankle foot motion recognition based on wireless wearable sEMG and acceleration sensors for smart AFO. Sens. Actuators A-Phys. 2021, 331, 113025. [Google Scholar] [CrossRef]
- Stefanou, T.; Guiraud, D.; Fattal, C.; Azevedo-Coste, C.; Fonseca, L. Frequency-Domain sEMG Classification Using a Single Sensor. Sensors 2022, 22, 1939. [Google Scholar] [CrossRef] [PubMed]
- Unanyan, N.N.; Belov, A.A. Design of upper limb prosthesis using real-time motion detection method based on EMG signal processing. Biomed. Signal Process. Control 2021, 70, 103062. [Google Scholar] [CrossRef]
- de Oliveira de Souza, J.O.; Bloedow, M.D.; Rubo, F.C.; de Figueiredo, R.M.; Pessin, G.; Rigo, S.J. Investigation of Different Approaches to Real-Time Control of Prosthetic Hands With Electromyography Signals. IEEE Sens. J. 2021, 21, 20674–20684. [Google Scholar] [CrossRef]
- Li, Y.; Bai, K.; Wang, H.; Chen, S.; Liu, X.; Xu, H. Research on improved FAWT signal denoising method in evaluation of firefighter training efficacy based on sEMG. Biomed. Signal Process. Control 2022, 72, 103336. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; He, D.; Wang, R.; Guo, Y. sEMG Signals Characterization and Identification of Hand Movements by Machine Learning Considering Sex Differences. Appl. Sci. 2022, 12, 2962. [Google Scholar] [CrossRef]
- Pancholi, S.; Joshi, A.M. Electromyography-based hand gesture recognition system for upper limb amputees. IEEE Sens. Lett. 2019, 3, 1–4. [Google Scholar] [CrossRef]
- Moin, A.; Zhou, A.; Rahimi, A.; Menon, A.; Benatti, S.; Alexandrov, G.; Tamakloe, S.; Ting, J.; Yamamoto, N.; Khan, Y.; et al. A wearable biosensing system with in-sensor adaptive machine learning for hand gesture recognition. Nat. Electron. 2021, 4, 54–63. [Google Scholar] [CrossRef]
- Xiao, F.; Gu, L.; Ma, W.; Zhu, Y.; Zhang, Z.; Wang, Y. Real time motion intention recognition method with limited number of surface electromyography sensors for A 7-DOF hand/wrist rehabilitation exoskeleton. Mechatronics 2021, 79, 102642. [Google Scholar] [CrossRef]
- Ogul, O.E.; Coskunsu, D.K.; Akcay, S.; Akyol, K.; Hanoglu, L.; Ozturk, N. The effect of Electromyography (EMG)-driven Robotic Treatment on the recovery of the hand Nine years after stroke. J. Hand Ther. Off. J. Am. Soc. Hand Ther. 2021, in press. [Google Scholar] [CrossRef]
- Prakash, A.; Sharma, N.; Sharma, S. An affordable transradial prosthesis based on force myography sensor. Sens. Actuators A-Phys. 2021, 325, 112699. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, L.; Fu, Y. Development and Test of a Spasm Sensor for Hand Rehabilitation Exoskeleton. IEEE Trans. Instrum. Meas. 2022, 71, 3127628. [Google Scholar] [CrossRef]
- Ghildiyal, S.; Mani, G.; Nersisson, R. Electromyography pattern-recognition based prosthetic limb control using various machine learning techniques. J. Med. Eng. Technol. 2022, 46, 370–377. [Google Scholar] [CrossRef]
- Phutane, U.; Liphardt, A.-M.; Braeunig, J.; Penner, J.; Klebl, M.; Tascilar, K.; Vossiek, M.; Kleyer, A.; Schett, G.; Leyendecker, S. Evaluation of Optical and Radar Based Motion Capturing Technologies for Characterizing Hand Movement in Rheumatoid Arthritis-A Pilot Study. Sensors 2021, 21, 1208. [Google Scholar] [CrossRef]
- Shin, Y.S.; Cho, K.; Lim, S.H.; Chung, S.; Park, S.-J.; Chung, C.; Han, D.-C.; Chang, J.K. PDMS-based micro PCR chip with parylene coating. J. Micromech. Microeng. 2003, 13, 768. [Google Scholar] [CrossRef]
- De Luca, C.J.; Gilmore, L.D.; Kuznetsov, M.; Roy, S.H. Filtering the surface EMG signal: Movement artifact and baseline noise contamination. J. Biomech. 2010, 43, 1573–1579. [Google Scholar] [CrossRef]
- Merletti, R.; Botter, A.; Troiano, A.; Merlo, E.; Minetto, M.A. Technology and instrumentation for detection and conditioning of the surface electromyographic signal: State of the art. Clin. Biomech. (Bristol Avon) 2009, 24, 122–134. [Google Scholar] [CrossRef]
- Chen, L.-F.; Hu, N.; Liu, N.; Guo, B.; Yao, J.; Xia, L.; Zheng, X.; Hou, W.; Yin, Z.Q. The design and preparation of a flexible bio-chip for use as a visual prosthesis, and evaluation of its biological features. Cell Tissue Res. 2010, 340, 421–426. [Google Scholar] [CrossRef]
- Hochreiter, S.; Schmidhuber, J. Long short-term memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Sun, T.; Hu, Q.; Gulati, P.; Atashzar, S.F. Temporal dilation of deep LSTM for agile decoding of sEMG: Application in prediction of Upper-Limb motor intention in NeuroRobotics. IEEE Robot. Autom. Lett. 2021, 6, 6212–6219. [Google Scholar] [CrossRef]
- Liu, T.-P.; Kao, M.-F.; Jang, T.-R.; Wang, C.-W.; Chuang, C.-L.; Chen, J.; Chen, Y.-Y.; Hsieh, K.-C. New Application of Bioelectrical Impedance Analysis by the Back Propagation Artificial Neural Network Mathematically Predictive Model of Tissue Composition in the Lower Limbs of Elderly People. Int. J. Gerontol. 2012, 6, 20–26. [Google Scholar] [CrossRef][Green Version]
- Guo, K.; Zhang, S.; Zhao, S.; Yang, H. Design and Manufacture of Data Gloves for Rehabilitation Training and Gesture Recognition Based on Flexible Sensors. J. Healthc. Eng. 2021, 2021, 6359403. [Google Scholar] [CrossRef] [PubMed]
- Nazmi, N.; Rahman, M.A.A.; Yamamoto, S.-I.; Ahmad, S.A. Walking gait event detection based on electromyography signals using artificial neural network. Biomed. Signal Process. Control 2019, 47, 334–343. [Google Scholar] [CrossRef]
- Margenau, H. Van der Waals forces. Rev. Mod. Phys. 1939, 11, 1. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Li, J.; Zhang, S.; Yang, H.; Guo, K. Study on Flexible sEMG Acquisition System and Its Application in Muscle Strength Evaluation and Hand Rehabilitation. Micromachines 2022, 13, 2047. https://doi.org/10.3390/mi13122047
Liu C, Li J, Zhang S, Yang H, Guo K. Study on Flexible sEMG Acquisition System and Its Application in Muscle Strength Evaluation and Hand Rehabilitation. Micromachines. 2022; 13(12):2047. https://doi.org/10.3390/mi13122047
Chicago/Turabian StyleLiu, Chang, Jiuqiang Li, Senhao Zhang, Hongbo Yang, and Kai Guo. 2022. "Study on Flexible sEMG Acquisition System and Its Application in Muscle Strength Evaluation and Hand Rehabilitation" Micromachines 13, no. 12: 2047. https://doi.org/10.3390/mi13122047
APA StyleLiu, C., Li, J., Zhang, S., Yang, H., & Guo, K. (2022). Study on Flexible sEMG Acquisition System and Its Application in Muscle Strength Evaluation and Hand Rehabilitation. Micromachines, 13(12), 2047. https://doi.org/10.3390/mi13122047









