Citrate Functionalized Zirconium-Based Metal Organic Framework for the Fluorescent Detection of Ciprofloxacin in Aqueous Media
Abstract
:1. Introduction
2. Materials, Methods, and Experiments
2.1. Synthesis of MOF-808
2.2. Synthesis of Receptor (C-MOF-808)
3. Results and Discussion
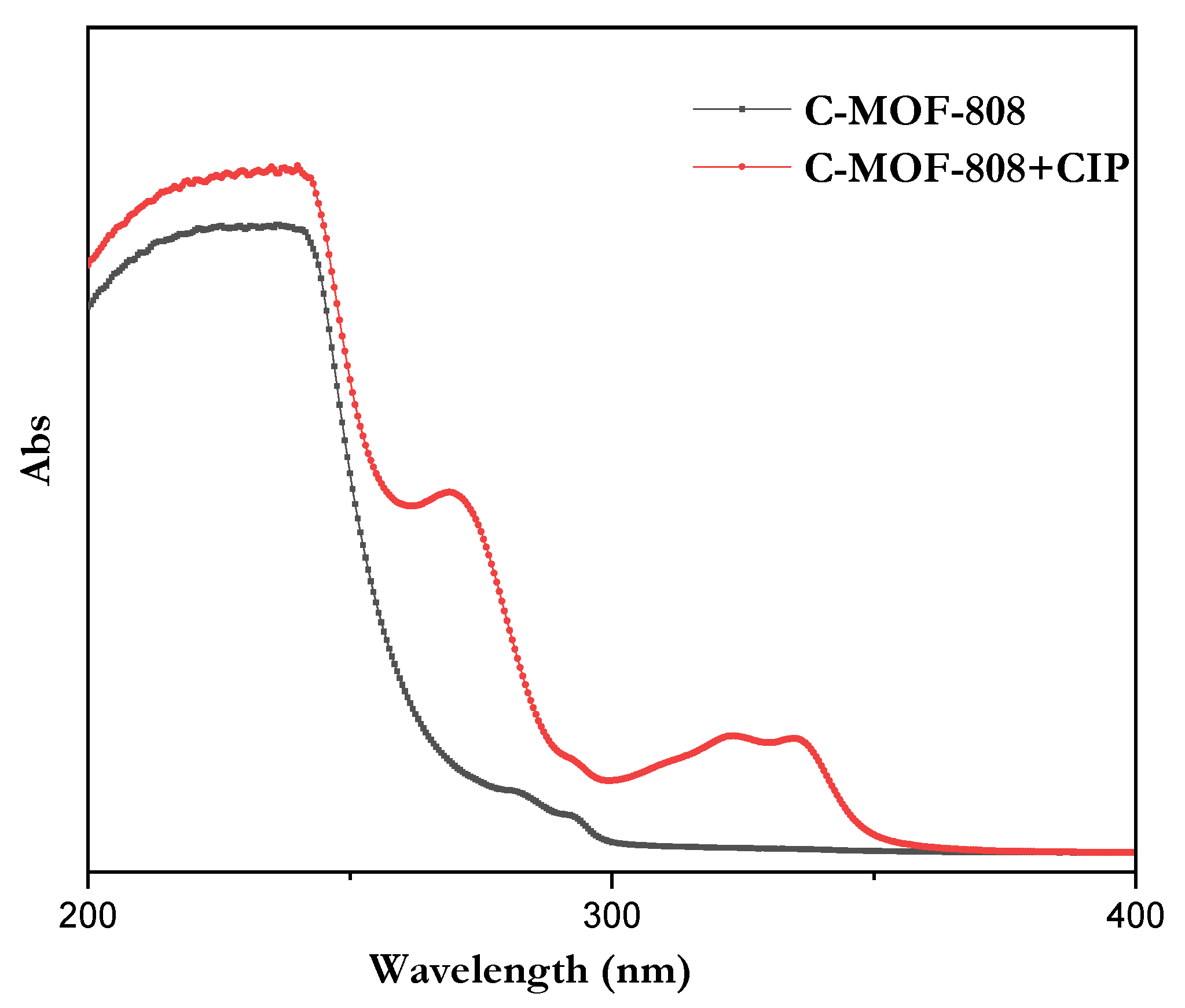
3.1. Characterization of the Synthesized MOF and C-MOF-808
3.2. Detection of Ciprofloxacin (CIP) by C-MOF-808
3.3. Absorption and Emission–Kinetic Studies
3.4. Binding Mechanism of the Receptors towards CIP
4. Findings and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Yang, Y.-W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ye, J.W.; Wang, H.P.; Pan, M.; Yin, S.Y.; Wei, Z.W.; Zhang, L.Y.; Wu, K.; Fan, Y.N.; Su, C.Y. Ultrafast water sensing and thermal imaging by a metal-organic framework with switchable luminescence. Nat. Commun. 2017, 8, 15985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.-J.; Zheng, H.-Q.; Zheng, H.-Y.; Lin, L.-P.; Xin, Q.; Cao, R. Efficient Capture and Effective Sensing of Cr2O72− From Water Using a Zirconium Metal–Organic Framework. Inorg. Chem. 2017, 56, 14178–14188. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Zhang, K.; Wang, Y.; Long, W.W.; Sa, R.J.; Liu, T.F.; Lu, J. Fluorescent Metal–Organic Framework (MOF) as a Highly Sensitive and Quickly Responsive Chemical Sensor for the Detection of Antibiotics in Simulated Wastewater. Inorg. Chem. 2018, 57, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, H.; Zhang, H.; Li, H.; Shi, W.; Cheng, P. A Bimetallic Lanthanide Metal–Organic Material as a Self-Calibrating Color-Gradient Luminescent Sensor. Adv. Mater. 2015, 27, 7072–7077. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Zhao, K.; Niu, Z.; Li, H.; Cheng, P. Highly Selective Sorption and Luminescent Sensing of Small Molecules Demonstrated in a Multifunctional Lanthanide Microporous Metal–Organic Framework Containing 1D Honeycomb-Type Channels. Chem.—Eur. J. 2013, 19, 3358–3365. [Google Scholar] [CrossRef]
- Giménez-Marqués, M.; Hidalgo, T.; Serre, C.; Horcajada, P. Nanostructured metal–organic frameworks and their bio-related applications. Coord. Chem. Rev. 2016, 307, 342–360. [Google Scholar] [CrossRef]
- Zheng, H.-Q.; Liu, C.-Y.; Zeng, X.-Y.; Chen, J.; Lü, J.; Lin, R.-G.; Cao, R.; Lin, Z.-J.; Su, J.-W. MOF-808: A Metal–Organic Framework with Intrinsic Peroxidase-Like Catalytic Activity at Neutral pH for Colorimetric Biosensing. Inorg. Chem. 2018, 57, 9096–9104. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chevreau, H.; Ragon, F.; Southon, P.D.; Peterson, V.K.; D’Alessandro, D.M. Tuning pore size in a zirconium–tricarboxylate metal–organic framework. CrystEngComm 2014, 16, 6530–6533. [Google Scholar] [CrossRef]
- Zheng, H.-Q.; Zeng, Y.-N.; Chen, J.; Lin, R.-G.; Zhuang, W.-E.; Cao, R.; Lin, Z.-J. Zr-Based Metal–Organic Frameworks with Intrinsic Peroxidase-Like Activity for Ultradeep Oxidative Desulfurization: Mechanism of H2O2 Decomposition. Inorg. Chem. 2019, 58, 6983–6992. [Google Scholar] [CrossRef]
- Jun, H.J.; Yoo, D.K.; Jhung, S.H. Metal-organic framework (MOF-808) functionalized with ethyleneamines: Selective adsorbent to capture CO2 under low pressure. J. CO2 Util. 2022, 58, 101932. [Google Scholar] [CrossRef]
- Jiang, J.; Gándara, F.; Zhang, Y.-B.; Na, K.; Yaghi, O.M.; Klemperer, W.G. Superacidity in Sulfated Metal–Organic Framework-808. J. Am. Chem. Soc. 2014, 136, 12844–12847. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Huang, H.; Zhang, Y.; Kang, C.; Chen, S.; Song, L.; Liu, D.; Zhong, C. A versatile MOF-based trap for heavy metal ion capture and dispersion. Nat. Commun. 2018, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Xue, R.; Ma, Y.; Ge, Y.; Wang, Z.; Qiao, X.; Zhou, P. Study on the performance of a MOF-808-based photocatalyst prepared by a microwave-assisted method for the degradation of antibiotics. RSC Adv. 2021, 11, 32955–32964. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Buzo, S.; Bohigues, B.; Lopes, C.W.; Meira, D.M.; Boronat, M.; Moliner, M.; Corma, A. Tailoring Lewis/Brønsted acid properties of MOF nodes via hydrothermal and solvothermal synthesis: Simple approach with exceptional catalytic implications. Chem. Sci. 2021, 12, 10106–10115. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Li, H.; Zhang, X.; Zhang, L. Designed Tb(III)-Functionalized MOF-808 as Visible Fluorescent Probes for Monitoring Bilirubin and Identifying Fingerprints. Inorg. Chem. 2021, 60, 3172–3180. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Sk, M.; Biswas, S. Rapid switch-on fluorescent detection of nanomolar-level hydrazine in water by a diacetoxy-functionalized MOF: Application in paper strips and environmental samples. Dalton Trans. 2020, 49, 12565–12573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, Q.; Liu, X.; Niu, H.; Luo, L.; Li, R.; Feng, X. A turn-off Eu-MOF@Fe2+ sensor for the selective and sensitive fluorescence detection of bromate in wheat flour. Food Chem. 2022, 382, 132379. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Yin, H.-Q.; Yin, X.-B. MOF@COFs with Strong Multiemission for Differentiation and Ratiometric Fluorescence Detection. ACS Appl. Mater. Interfaces 2020, 12, 20973–20981. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Li, R.; Du, L.; Feng, X.; Ding, Y. A highly sensitive and selective “turn off-on” fluorescent sensor based on Sm-MOF for the detection of tertiary butylhydroquinone. Dyes Pigments 2020, 178, 108347. [Google Scholar] [CrossRef]
- Qin, G.; Wang, J.; Li, L.; Yuan, F.; Zha, Q.; Bai, W.; Ni, Y. Highly water-stable Cd-MOF/Tb3+ ultrathin fluorescence nanosheets for ultrasensitive and selective detection of Cefixime. Talanta 2021, 221, 121421. [Google Scholar] [CrossRef]
- Yuphintharakun, N.; Nurerk, P.; Chullasat, K.; Kanatharana, P.; Davis, F.; Sooksawat, D.; Bunkoed, O. A nanocomposite optosensor containing carboxylic functionalized multiwall carbon nanotubes and quantum dots incorporated into a molecularly imprinted polymer for highly selective and sensitive detection of ciprofloxacin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Gayen, P.; Chaplin, B.P. Selective Electrochemical Detection of Ciprofloxacin with a Porous Nafion/Multiwalled Carbon Nanotube Composite Film Electrode. ACS Appl. Mater. Interfaces 2016, 8, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lin, X.; Jia, R.; Liu, Z.; Liu, Z.; Hong, M.; Jia, A.; Li, Y.; Zhang, H. Fabrication of three-component hydrogen-bonded covalent-organic polymers for ciprofloxacin decontamination from water: Adsorption mechanism and modeling. Mater. Today Chem. 2021, 20, 100463. [Google Scholar] [CrossRef]
- Fan, H.; Ma, Y.; Wan, J.; Wang, Y.; Li, Z.; Chen, Y. Adsorption properties and mechanisms of novel biomaterials from banyan aerial roots via simple modification for ciprofloxacin removal. Sci. Total Environ. 2020, 708, 134630. [Google Scholar] [CrossRef] [PubMed]
- Velissariou, I.M. The use of fluoroquinolones in children: Recent advances. Expert Rev. Anti-Infect. Ther. 2006, 4, 853–860. [Google Scholar] [CrossRef]
- Shan, J.; Li, R.; Yan, K.; Zhu, Y.; Zhang, J. In situ anodic stripping of Cd(II) from CdS quantum dots for electrochemical sensing of ciprofloxacin. Sens. Actuators B Chem. 2016, 237, 75–80. [Google Scholar] [CrossRef]
- Bagheri, H.; Khoshsafar, H.; Amidi, S.; Hosseinzadeh Ardakani, Y. Fabrication of an electrochemical sensor based on magnetic multi-walled carbon nanotubes for the determination of ciprofloxacin. Anal. Methods 2016, 8, 3383–3390. [Google Scholar] [CrossRef]
- Locatelli, M.; Ciavarella, M.T.; Paolino, D.; Celia, C.; Fiscarelli, E.; Ricciotti, G.; Pompilio, A.; Di Bonaventura, G.; Grande, R.; Zengin, G.; et al. Determination of ciprofloxacin and levofloxacin in human sputum collected from cystic fibrosis patients using microextraction by packed sorbent-high performance liquid chromatography photodiode array detector. J. Chromatogr. A 2015, 1419, 58–66. [Google Scholar] [CrossRef]
- Chen, B.; Han, J.; Wang, Y.; Sheng, C.; Liu, Y.; Zhang, G.; Yan, Y. Separation, enrichment and determination of ciprofloxacin using thermoseparating polymer aqueous two-phase system combined with high performance liquid chromatography in milk, egg, and shrimp samples. Food Chem. 2014, 148, 105–111. [Google Scholar] [CrossRef]
- Balamurugan, R.; Liu, J.-H.; Liu, B.-T. A review of recent developments in fluorescent sensors for the selective detection of palladium ions. Coord. Chem. Rev. 2018, 376, 196–224. [Google Scholar] [CrossRef]
- Rathinam, B.; Liu, B.-T. Highly efficient probe of dinuclear zinc complex for selective detection of oxalic acid. J. Taiwan Inst. Chem. Eng. 2021, 127, 349–356. [Google Scholar] [CrossRef]
- Lu, Z.; Hu, Y.; Li, G.; Xia, L. Adamantane Three-Dimensional Porous Organic Framework as a Fluorescence Sensor for Rapid Determination of Tetracycline in Aquatic Products. Chemosensors 2022, 10, 457. [Google Scholar] [CrossRef]
- Santonocito, R.; Tuccitto, N.; Pappalardo, A.; Trusso Sfrazzetto, G. Smartphone-Based Dopamine Detection by Fluorescent Supramolecular Sensor. Molecules 2022, 27, 7503. [Google Scholar] [CrossRef]
- Li, Y.; Gu, X.; Zhao, J.; Xi, F. Fabrication of a Ratiometric Fluorescence Sensor Based on Carbon Dots as Both Luminophores and Nanozymes for the Sensitive Detection of Hydrogen Peroxide. Molecules 2022, 27, 7379. [Google Scholar] [CrossRef]
- Noushija, M.K.; Shanmughan, A.; Mohan, B.; Shanmugaraju, S. Selective Recognition and Reversible “Turn-Off” Fluorescence Sensing of Acetate (CH3COO−) Anion at Ppb Level Using a Simple Quinizarin Fluorescent Dye. Chemistry 2022, 4, 1407–1416. [Google Scholar]
- Muchohi, S.N.; Thuo, N.; Karisa, J.; Muturi, A.; Kokwaro, G.O.; Maitland, K. Determination of ciprofloxacin in human plasma using high-performance liquid chromatography coupled with fluorescence detection: Application to a population pharmacokinetics study in children with severe malnutrition. J. Chromatogr. B 2011, 879, 146–152. [Google Scholar] [CrossRef] [Green Version]
- El Kojok, H.; El Darra, N.; Khalil, M.; Capo, A.; Pennacchio, A.; Staiano, M.; Camarca, A.; D’Auria, S.; Varriale, A. Fluorescence polarization assay to detect the presence of traces of ciprofloxacin. Sci. Rep. 2020, 10, 4550. [Google Scholar] [CrossRef] [Green Version]
- An, X.; Zhu, X.; Liu, J.; Zou, L.; Li, G.; Ye, B. Ratiometric fluorescence detection of ciprofloxacin using the terbium-based coordination polymers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 269, 120775. [Google Scholar] [CrossRef]
- Singha, S.; Ahn, K.H. Detection of Ciprofloxacin in Urine through Sensitized Lanthanide Luminescence. Sensors 2016, 16, 2065. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.; Wu, C.; Zhou, Z.; Fu, L.; Bai, Y. Fluorescent Sensing of Ciprofloxacin and Chloramphenicol in Milk Samples via Inner Filter Effect and Photoinduced Electron Transfer Based on Nanosized Rod-Shaped Eu-MOF. Foods 2022, 11, 3138. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Shen, Q.; Zhu, X.; Hao, Y.; Qu, P.; Xu, M. Turn-on fluorescence detection of ciprofloxacin in tablets based on lanthanide coordination polymer nanoparticles. RSC Adv. 2016, 6, 100743–100747. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.-B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal–Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef]
- Feng, J.; Zhong, Y.; Xie, M.; Li, M.; Jiang, S. Using MOF-808 as a Promising Support to Immobilize Ru for Selective Hydrogenation of Levulinic Acid to γ-Valerolactone. Catal. Lett. 2021, 151, 86–94. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Li, Z.; Wang, X.; Xu, Y.; Chen, S.; Wang, Z. Optimized synthesis of Zr(iv) metal organic frameworks (MOFs-808) for efficient hydrogen storage. New J. Chem. 2019, 43, 4092–4099. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.; Chen, J.; Niu, H. Excellent Catalytic Performance of Ce–MOF with Abundant Oxygen Vacancies Supported Noble Metal Pt in the Oxidation of Toluene. Catalysts 2022, 12, 775. [Google Scholar]
- Healey, K.; Liang, W.; Southon, P.D.; Church, T.L.; D’Alessandro, D.M. Photoresponsive spiropyran-functionalised MOF-808: Postsynthetic incorporation and light dependent gas adsorption properties. J. Mater. Chem. A 2016, 4, 10816–10819. [Google Scholar] [CrossRef]
- Hadi Al-Kadhemy, M.F.; Abbas, K.N.; Abdalmuhdi, W.B. Physical Properties of Rhodamine 6G Laser Dye Combined in Polyvinyl Alcohol films as Heat Sensor. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 072126. [Google Scholar] [CrossRef]
- Goswami, S.; Chakraborty, S.; Paul, S.; Halder, S.; Panja, S.; Mukhopadhyay, S.K. A new pyrene based highly sensitive fluorescence probe for copper(ii) and fluoride with living cell application. Org. Biomol. Chem. 2014, 12, 3037–3044. [Google Scholar] [CrossRef]
- Xing, X.; Feng, J.; Lv, G.; Song, K.; Mei, L.; Liao, L.; Wang, X.; Xu, B. Adsorption Mechanism of Ciprofloxacin from Water by Synthesized Birnessite. Adv. Mater. Sci. Eng. 2015, 2015, 148423. [Google Scholar] [CrossRef] [Green Version]
- Gu, C.; Karthikeyan, K.G. Sorption of the Antimicrobial Ciprofloxacin To Aluminum and Iron Hydrous Oxides. Environ. Sci. Technol. 2005, 39, 9166–9173. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Vasudevan, D. Spectroscopic Investigation of Ciprofloxacin Speciation at the Goethite−Water Interface. Environ. Sci. Technol. 2007, 41, 3153–3158. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, J.; Li, Z.; Xiao, L.; Aryee, A.A.; Sun, Y.; Yang, R.; Meng, H.; Qu, L.; Lin, Y.; et al. Hydrogen-Bond-Induced Emission of Carbon Dots for Wash-Free Nucleus Imaging. Anal. Chem. 2019, 91, 9259–9265. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.-T.; Nagarajan, D.; Kaliyamoorthy, S.; Rathinam, B. Citrate Functionalized Zirconium-Based Metal Organic Framework for the Fluorescent Detection of Ciprofloxacin in Aqueous Media. Micromachines 2022, 13, 2097. https://doi.org/10.3390/mi13122097
Liu B-T, Nagarajan D, Kaliyamoorthy S, Rathinam B. Citrate Functionalized Zirconium-Based Metal Organic Framework for the Fluorescent Detection of Ciprofloxacin in Aqueous Media. Micromachines. 2022; 13(12):2097. https://doi.org/10.3390/mi13122097
Chicago/Turabian StyleLiu, Bo-Tau, Dillirani Nagarajan, Selvam Kaliyamoorthy, and Balamurugan Rathinam. 2022. "Citrate Functionalized Zirconium-Based Metal Organic Framework for the Fluorescent Detection of Ciprofloxacin in Aqueous Media" Micromachines 13, no. 12: 2097. https://doi.org/10.3390/mi13122097
APA StyleLiu, B.-T., Nagarajan, D., Kaliyamoorthy, S., & Rathinam, B. (2022). Citrate Functionalized Zirconium-Based Metal Organic Framework for the Fluorescent Detection of Ciprofloxacin in Aqueous Media. Micromachines, 13(12), 2097. https://doi.org/10.3390/mi13122097







