Battery-Free Tattooing Mechanism-Based Functional Active Capsule Endoscopy
Abstract
:1. Introduction
- First, the robot utilizes the advantages of a WCE that can move actively in the GI tract. The capsule should be able to direct the injection needle toward the target, which is the area surrounding the organs. The magnetic force from the controlled system should be enough for the needle to penetrate the tissue. During locomotion, the injection mechanism should be disabled. Upon reaching the target, it will be triggered to eject ink beneath the mucosa layer.
- Second, the capsule can regulate the needle’s condition to address safety concerns. To prevent damage, and even perforation, during movement, the tattooing needle should not be exposed to the exterior of the capsule body. The capsule can protrude the needle at the target to pierce the mucosa layer of the organ and withdraw once the tattooing is complete.
- Third, the ejection mechanism should generate an adequate force to expel the ink from the container into the tissue. In this study, we focus on using chemical reactions that can create pressure to push a piston. The resultant force is controllable due to the reactant content. Gas-generation chemical reactions can lead to high pressure with a low volume of reacting substances. Therefore, we can minimize the size of the tattooing module and the capsule robot.
- Fourth, a battery-free triggering mechanism is desired. Since the capacity of a battery embedded inside a capsule body is limited to the energy for the camera and wireless communication, the battery-free design is a perfect solution to the long-term operation of the capsule robot.
2. Materials and Methods
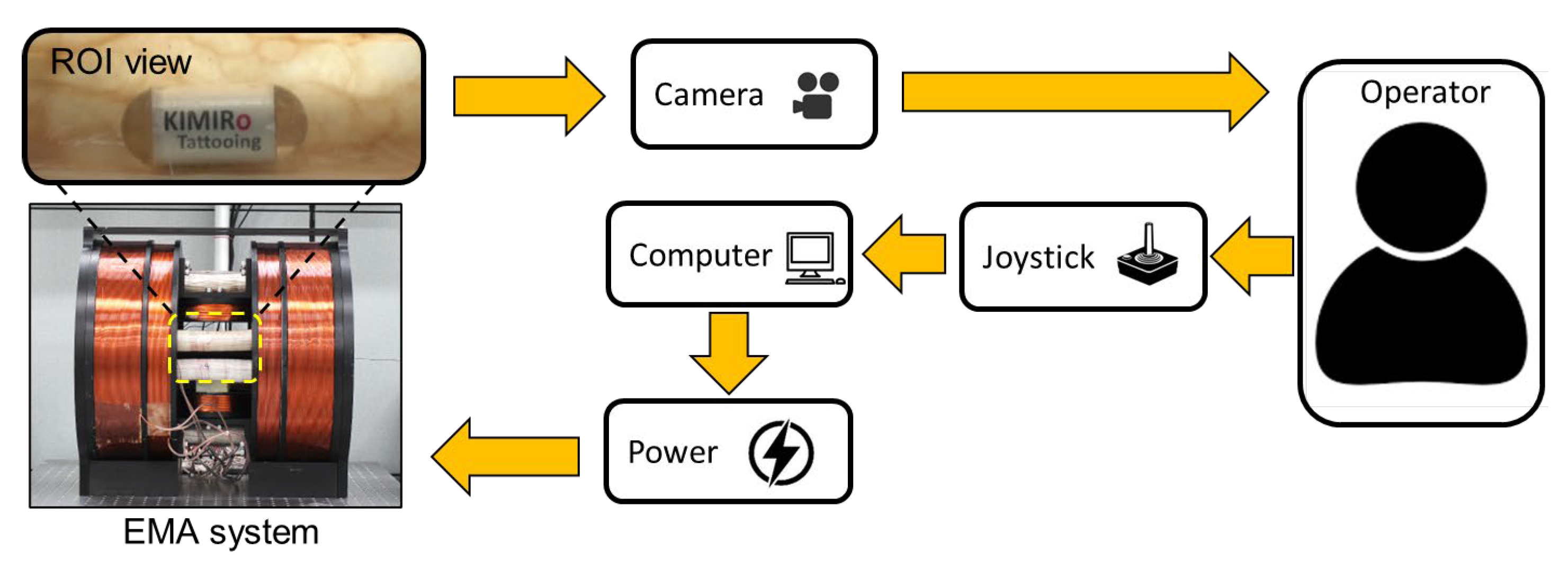
2.1. Overall System and Design of TCE
2.2. Design of the Tattooing Module
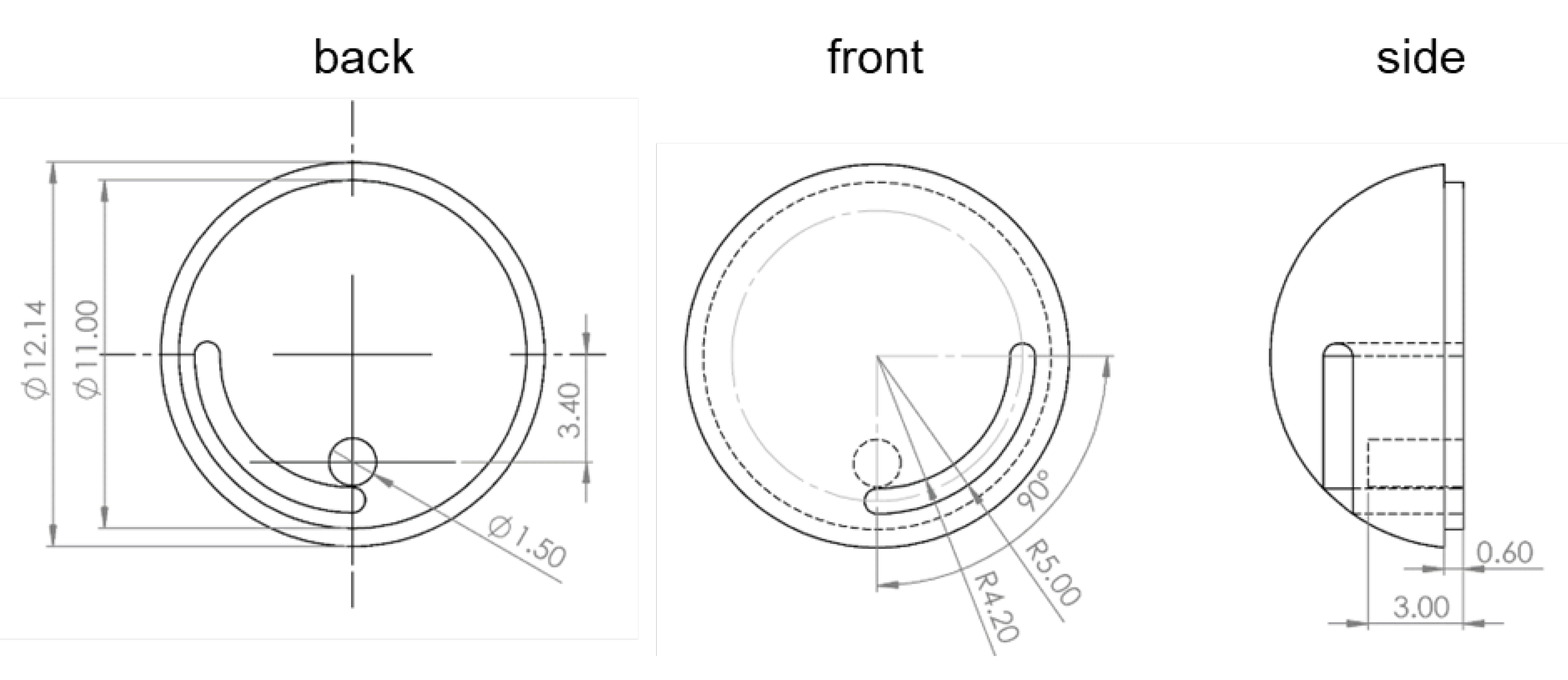
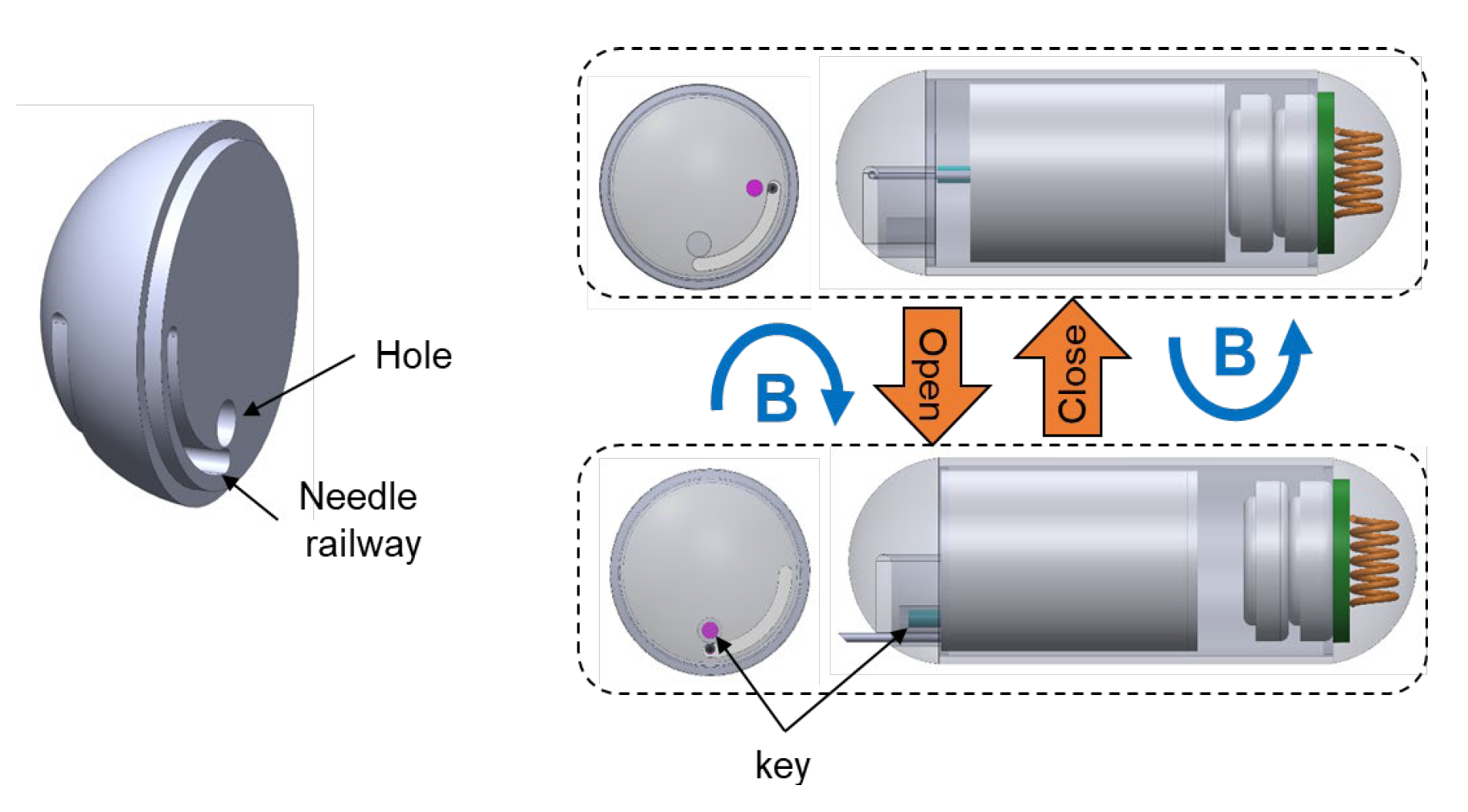
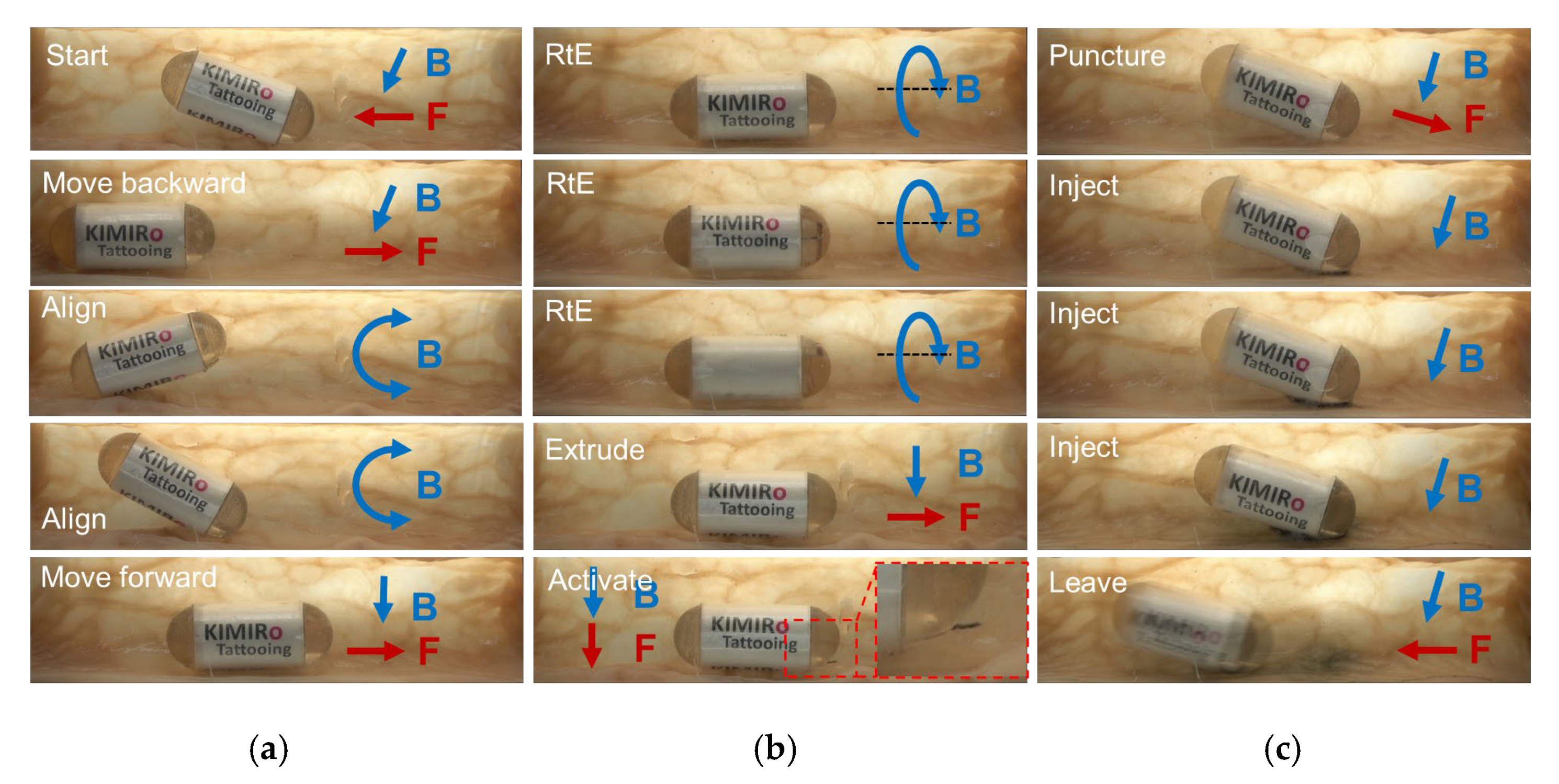
2.3. Design of the Needle Management Module
- Travel to the destination;
- Rotate clockwise two loops to enable needle extrusion;
- Command a forward movement to expose the needle;
- Insert the needle into the tissue;
- Trigger the chemical reaction using magnetic force;
- Wait for ink delivery;
- Withdraw the needle and roll the TCE counterclockwise to lock the needle’s linear motion;
- Travel to visual of the other area.
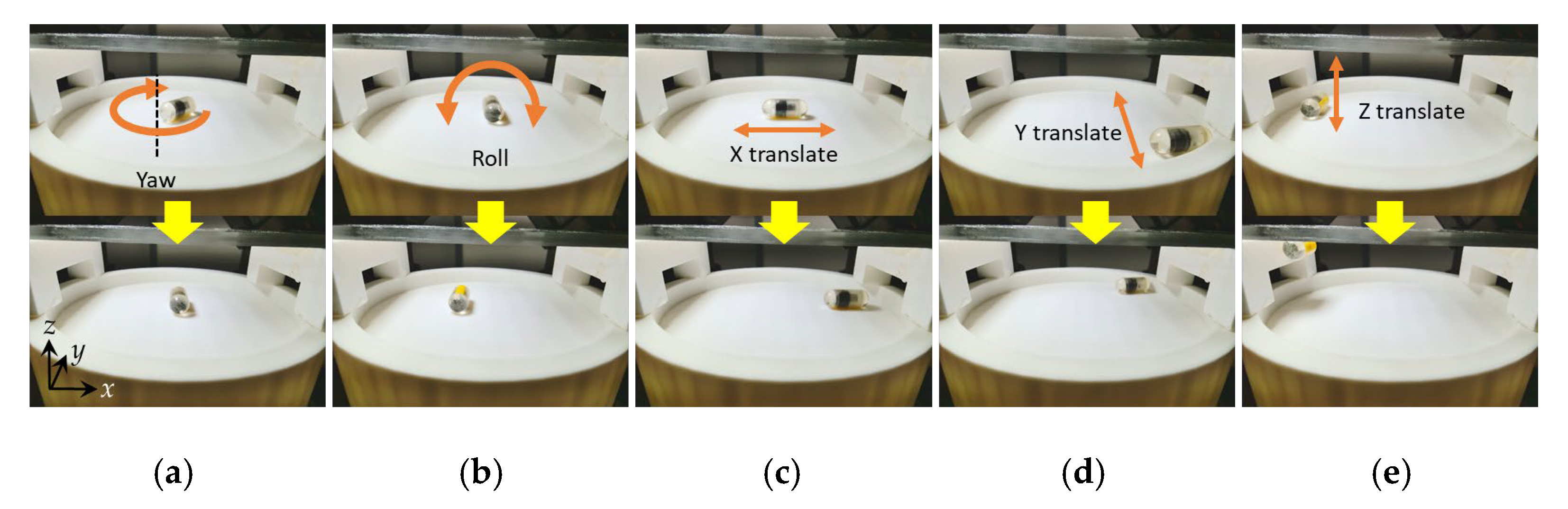
2.4. External Magnetic Field Control with 5 DOF Locomotion Motion
3. Results
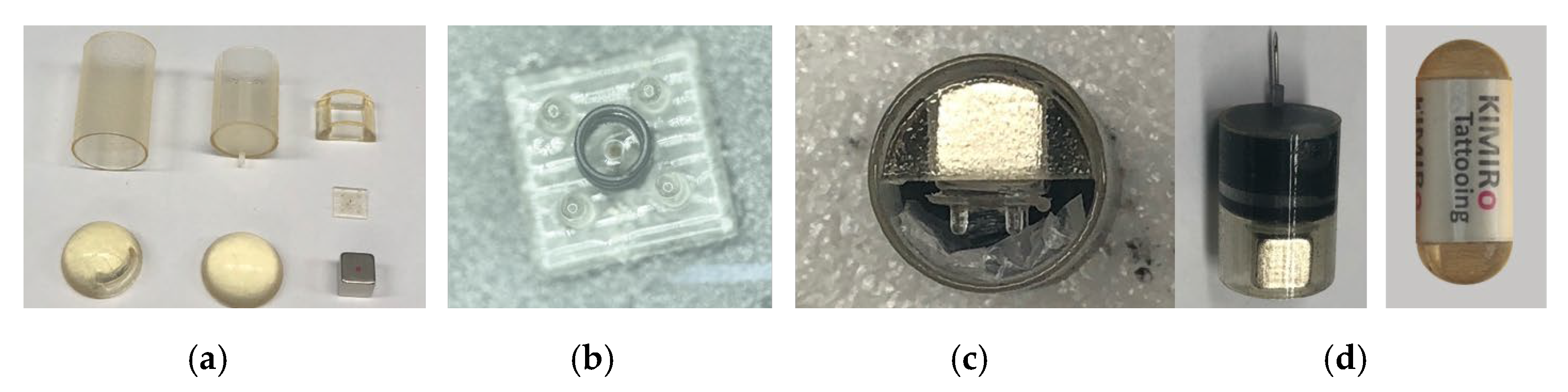
3.1. Prototype Fabrication and Experiment Setup
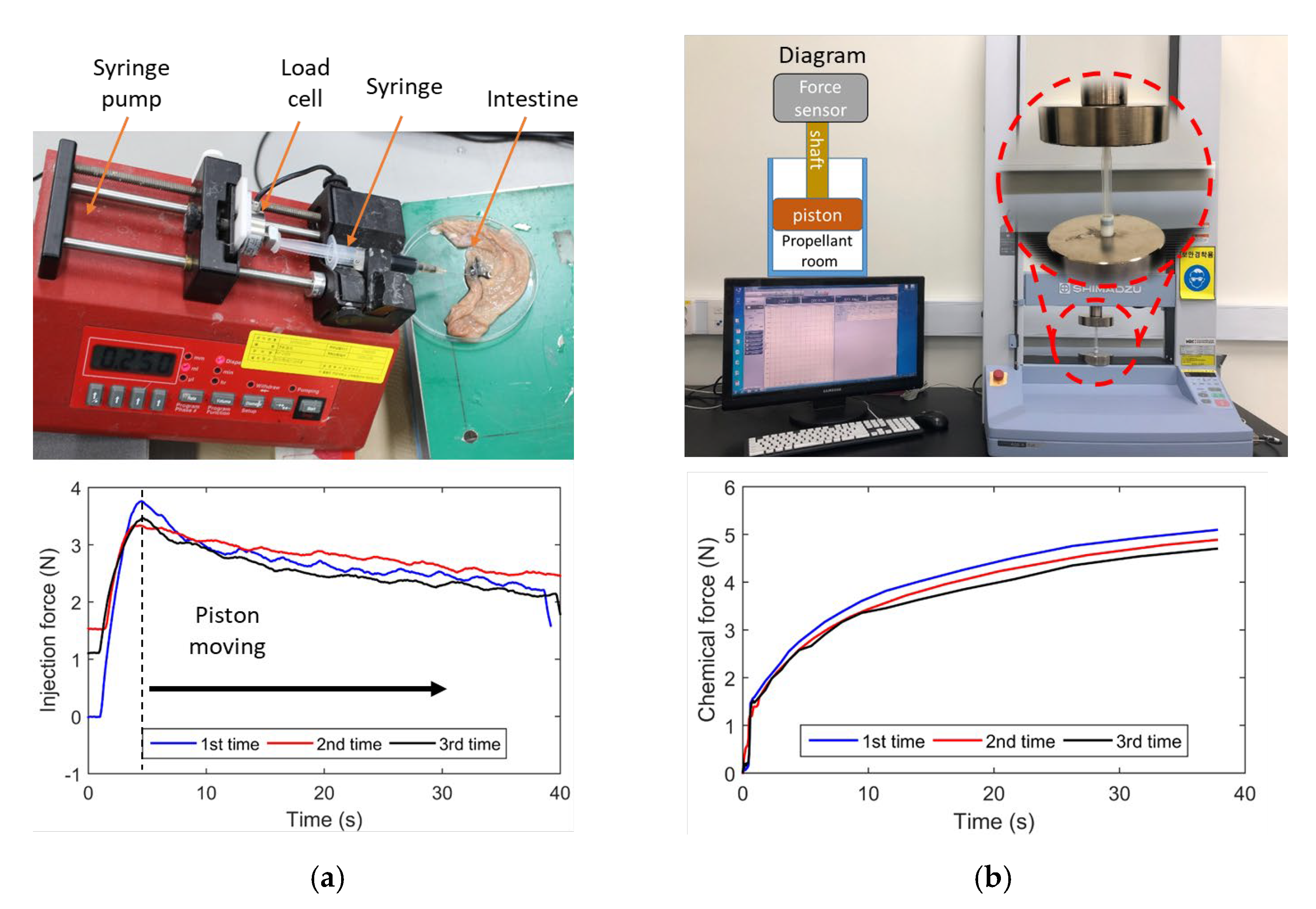
3.2. Injection Force
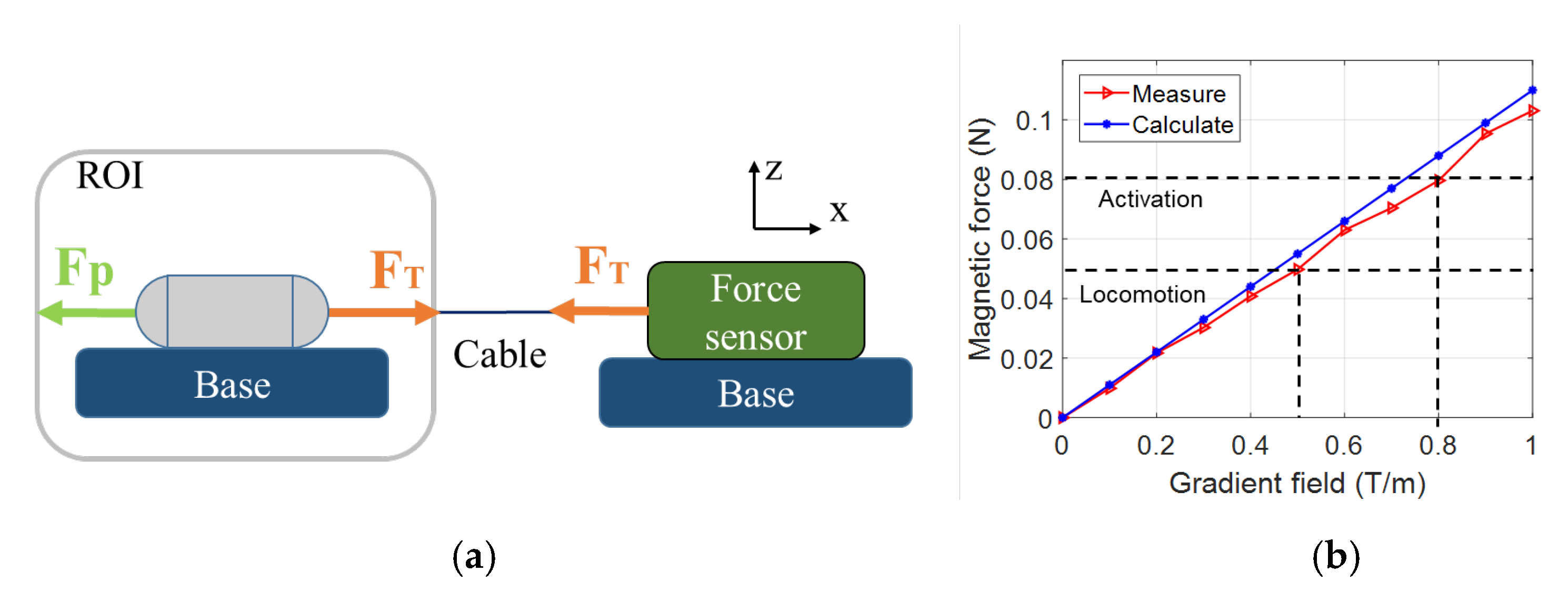
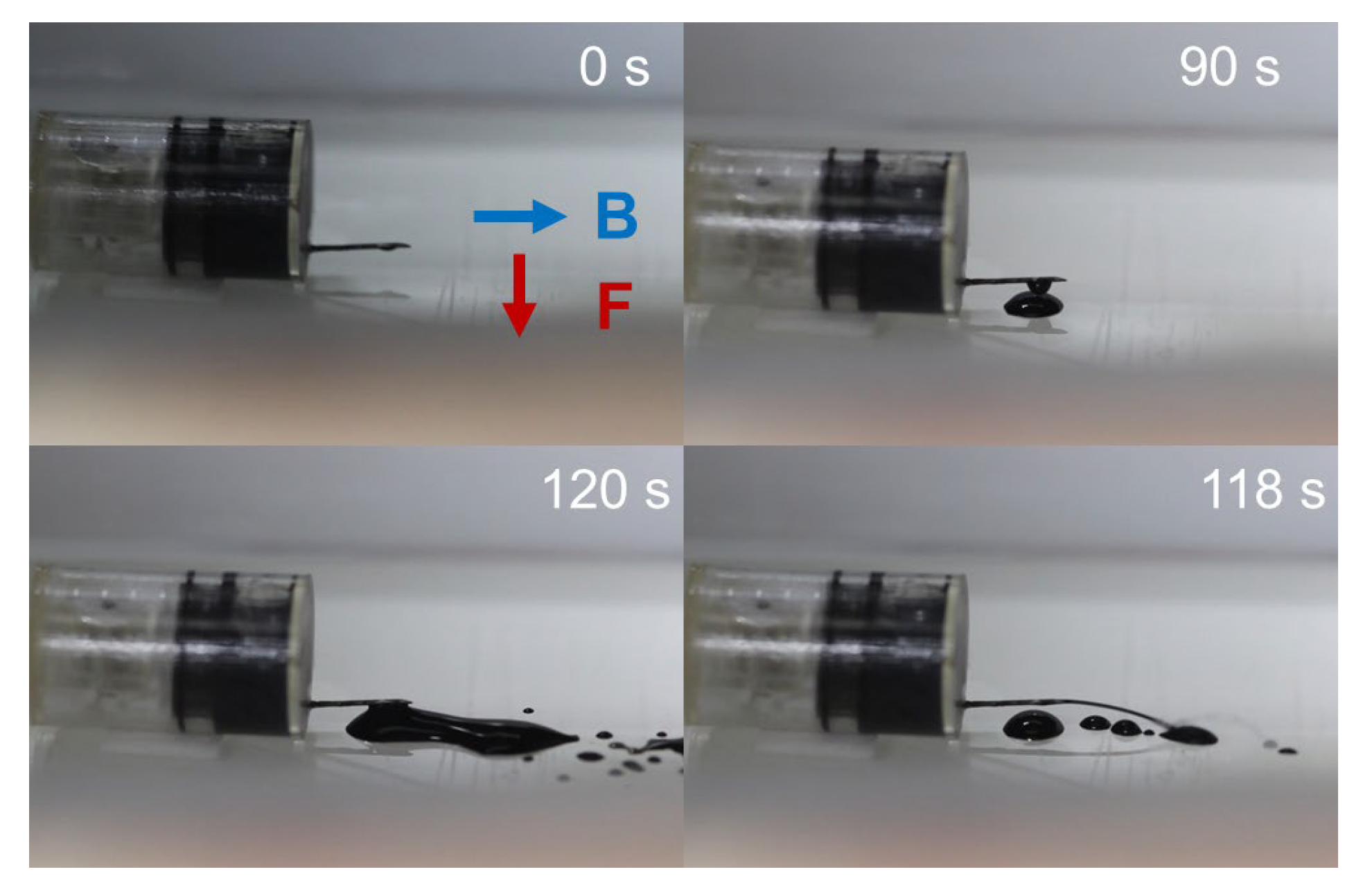
3.3. Triggering Mechanism Validation
3.4. The 5 DOF Locomotion
3.5. Ex Vivo Experiment in a Pig Small Intestine
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Chatenoud, L.; Levi, F.; Praud, D.; Ferlay, J.; Negri, E.; Malvezzi, M.; Vecchia, C. La Recent patterns in gastric cancer: A global overview. Int. J. Cancer 2009, 125, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.W.; Wei, J.T.; Thiny, M.T.; Gangarosa, L.M.; Brown, A.; Ringel, Y.; Shaheen, N.J.; Sandler, R.S. Digestive and Liver Diseases Statistics, 2004. Gastroenterology 2004, 126, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Ferro, A.; Peleteiro, B.; Malvezzi, M.; Bosetti, C.; Bertuccio, P.; Levi, F.; Negri, E.; La Vecchia, C.; Lunet, N. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur. J. Cancer 2014, 50, 1330–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.D.; Ortiz, A.P.; Pinheiro, P.S.; Bandi, P.; Minihan, A.; Fuchs, H.E.; Martinez Tyson, D.; Tortolero-Luna, G.; Fedewa, S.A.; Jemal, A.M.; et al. Cancer statistics for the US Hispanic/Latino population, 2021. CA Cancer J. Clin. 2021, 71, 466–487. [Google Scholar] [CrossRef]
- Wexner, S.D.; Cohen, S.M.; Ulrich, A.; Reissman, P.; Surgery, C.; Florida, C.C.; Lauderdale, F. Laparoscopic Colorectal Surgery-Are We Being Honest with Our Patients? Dis. Colon Rectum 1994, 38, 723–727. [Google Scholar] [CrossRef]
- Civello, I.M.; Brisinda, G.; Brandara, F.; Marniga, G.; Mazzeo, P.; Giacchi, F.; Vanella, S. Laparoscopic rectal resection with intraoperative radiotherapy in locally advanced cancer: Preliminary results. Surg. Oncol. 2007, 16, 97–100. [Google Scholar] [CrossRef]
- Frasson, M.; Braga, M.; Vignali, A.; Zuliani, W.; Di Carlo, V. Benefits of laparoscopic colorectal resection are more pronounced in elderly patients. Dis. Colon Rectum 2008, 51, 296–300. [Google Scholar] [CrossRef]
- Cho, Y.B.; Lee, W.Y.; Yun, H.R.; Lee, W.S.; Yun, S.H.; Chun, H.K. Tumor Localization for Laparoscopic Colorectal Surgery. Dis. Colon Rectum 2007, 31, 1491–1495. [Google Scholar] [CrossRef]
- Botoman, V.A.; Pietro, M.; Thirlby, R.C. Localization of Colonic Lesions with Endoscopic Tattoo. Dis. Colon Rectum 1994, 37, 775–776. [Google Scholar] [CrossRef]
- Status, T.; Report, E. Endoscopic tattooing. Gastrointest. Endosc. 2010, 72, 681–685. [Google Scholar] [CrossRef]
- Fu, K.I.; Fujii, T.; Kato, S.; Sano, Y.; Koba, I.; Mera, K.; Saito, H.; Yoshino, T.; Sugito, M.; Yoshida, S. A new endoscopic tattooing technique for identifying the location of colonic lesions during laparoscopic surgery: A comparison with the conventional technique. Endoscopy 2001, 33, 687–691. [Google Scholar] [CrossRef]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef]
- Fernandez-urien, I.; Carretero, C.; Armendariz, R.; Muñoz-navas, M.; Fernandez-urien, I.; Carretero, C. Esophageal capsule endoscopy. World J. Gastroenterol. 2008, 14, 5254–5260. [Google Scholar] [CrossRef]
- OMOM. OMOM Robotic Capsule Endoscopy. Available online: https://www.jinshangroup.com/en/solutions/omomrobotic.html (accessed on 20 May 2021).
- Bang, S.; Park, J.Y.; Jeong, S.; Kim, Y.H.; Shim, H.B. First clinical trial of the “MiRo” capsule endoscope by using a novel transmission technology: Electric-field propagation. Gastrointest. Endosc. 2009, 69, 253–259. [Google Scholar] [CrossRef]
- Kim, H.M.; Yang, S.; Kim, J.; Park, S.; Cho, J.H.; Park, J.Y.; Kim, T.S.; Yoon, E.S.; Song, S.Y.; Bang, S. Active locomotion of a paddling-based capsule endoscope in an in vitro and in vivo experiment (with videos). Gastrointest. Endosc. 2010, 72, 381–387. [Google Scholar] [CrossRef]
- Carpi, F.; Pappone, C. Magnetic Maneuvering of Endoscopic Capsules by Means of a Robotic Navigation System. IEEE Trans. Biomed. Eng. 2009, 56, 1482–1490. [Google Scholar] [CrossRef]
- Kong, K.; Yim, S.; Choi, S.; Jeon, D. A Robotic Biopsy Device for Capsule Endoscopy. J. Med. Device 2012, 6, 031004. [Google Scholar] [CrossRef]
- Simi, M.; Valdastri, P. Magnetic Torsion Spring Mechanism for a Wireless Biopsy Capsule. J. Med. Device 2014, 7, 041009. [Google Scholar] [CrossRef]
- Le, V.H.; Jin, Z.; Leon-Rodriguez, H.; Lee, C.; Choi, H.; Nguyen, V.D.; Go, G.; Ko, S.Y.; Park, J.O.; Park, S. Electromagnetic field intensity triggered micro-biopsy device for active locomotive capsule endoscope. Mechatronics 2016, 36, 112–118. [Google Scholar] [CrossRef]
- Aiello, G.; Momi, E.D.; Völgyesi, P.; Lédeczi, Á.; Valdastri, P. Component based design of a drug delivery capsule robot. Sens. Actuators A Phys. 2016, 245, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Munoz, F.; Alici, G.; Li, W. A review of drug delivery systems for capsule endoscopy. Adv. Drug Deliv. Rev. 2014, 71, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Beccani, M.; Di Natali, C.; Aiello, G.; Benjamin, C.; Susilo, E.; Valdastri, P. A Magnetic drug delivery Capsule based on a coil actuation mechanism. Procedia Eng. 2015, 120, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Le, V.H.; Rodriguez, H.L.; Lee, C.; Go, G.; Zhen, J.; Nguyen, V.D.; Choi, H.; Ko, S.Y.; Park, J.O.; Park, S. A soft-magnet-based drug-delivery module for active locomotive intestinal capsule endoscopy using an electromagnetic actuation system. Sens. Actuators A Phys. 2016, 243, 81–89. [Google Scholar] [CrossRef]
- Joe, S.; Lee, D.; Kang, H.; Kang, B.; Park, J.O.; Kim, B. A micro-tattooing device for capsule endoscope using a Wood’s metal triggering mechanism. Mechatronics 2019, 62, 102259. [Google Scholar] [CrossRef]
- Joe, S.; Lee, D.; Kang, B.; Park, J.O.; Kim, B. Design and dynamics analysis of a compact tattooing mechanism for a capsule endoscope. In Proceedings of the 2017 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS), Montreal, QC, Canada, 17–21 July 2017; pp. 1–4. [Google Scholar]
- Hoang, M.C.; Le, V.H.; Kim, J.; Choi, E.; Kang, B.; Park, J.O.; Kim, C.S. A wireless tattooing capsule endoscope using external electromagnetic actuation and chemical reaction pressure. PLoS ONE 2019, 14, e0219740. [Google Scholar] [CrossRef] [Green Version]
- Hoang, M.C.; Le, V.H.; Kim, J.; Choi, E.; Kang, B.; Park, J.O.; Kim, C.S. Intestinal Tattooing Mechanism Integrated with Active Wireless Capsule Endoscope. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, The Netherlands, 26–29 August 2018; pp. 1254–1259. [Google Scholar] [CrossRef]
- Valdivia, P.C.; Robertson, A.R.; De Boer, N.K.H.; Marlicz, W.; Koulaouzidis, A. An overview of robotic capsules for drug delivery to the gastrointestinal tract. J. Clin. Med. 2021, 10, 5791. [Google Scholar] [CrossRef]
- Gröning, R.; Bensmann, H.; Müller, R.S. Control of drug release from capsules using high frequency energy transmission systems. Int. J. Pharm. 2008, 364, 9–13. [Google Scholar] [CrossRef]
- Groening, R.; Bensmann, H. High frequency controlled capsules with integrated gas producing cells. Eur. J. Pharm. Biopharm. 2009, 72, 282–284. [Google Scholar] [CrossRef]
- Pi, X.; Lin, Y.; Wei, K.; Liu, H.; Wang, G.; Zheng, X.; Wen, Z.; Li, D. A novel micro-fabricated thruster for drug release in remote controlled capsule. Sensors Actuators A Phys. 2010, 159, 227–232. [Google Scholar] [CrossRef]
- Dhalla, A.K.; Al-Shamsie, Z.; Beraki, S.; Dasari, A.; Fung, L.C.; Fusaro, L.; Garapaty, A.; Gutierrez, B.; Gratta, D.; Hashim, M.; et al. A robotic pill for oral delivery of biotherapeutics: Safety, tolerability, and performance in healthy subjects. Drug Deliv. Transl. Res. 2022, 12, 294–305. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Hoang, M.C.; Choi, E.; Kang, B.; Park, J.O.; Kim, C.S. Medical Microrobot—A Drug Delivery Capsule Endoscope with Active Locomotion and Drug Release Mechanism: Proof of Concept. Int. J. Control. Autom. Syst. 2020, 18, 65–75. [Google Scholar] [CrossRef]
- Dijksman, J.F.; Wanke, C.; Siersema, P.D.; Iordanov, V.; Shimizu, J.; Zou, H.; Broekhuizen-de Gast, H.; van der Schaar, P.J.; van Lelyveld, N. A novel ingestible electronic drug delivery and monitoring device. Gastrointest. Endosc. 2013, 78, 520–528. [Google Scholar] [CrossRef]
- Wanke, C.; Kulmatycki, K.; Heimbach, T.; Penland, R.C.; Zhang, J.; Becker, D.; Shimizu, J. Novel Orally Swallowable IntelliCap® Device to Quantify Regional Drug Absorption in Human GI Tract Using Diltiazem as Model Drug. AAPS PharmSciTech 2014, 15, 1490–1497. [Google Scholar] [CrossRef] [Green Version]
- Hoang, M.C.; Choi, E.; Kang, B.; Park, J.; Kim, C. A Miniaturized Capsule Endoscope Equipped a Marking Module for Intestinal Tumor Localization. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 3712–3715. [Google Scholar]
- Hoang, M.C.; Kim, J.; Park, J.; Kim, C. Optimized magnetic field control of an electromagnetic actuation system for enhanced microrobot manipulation. Mechatronics 2022, 85, 102830. [Google Scholar] [CrossRef]
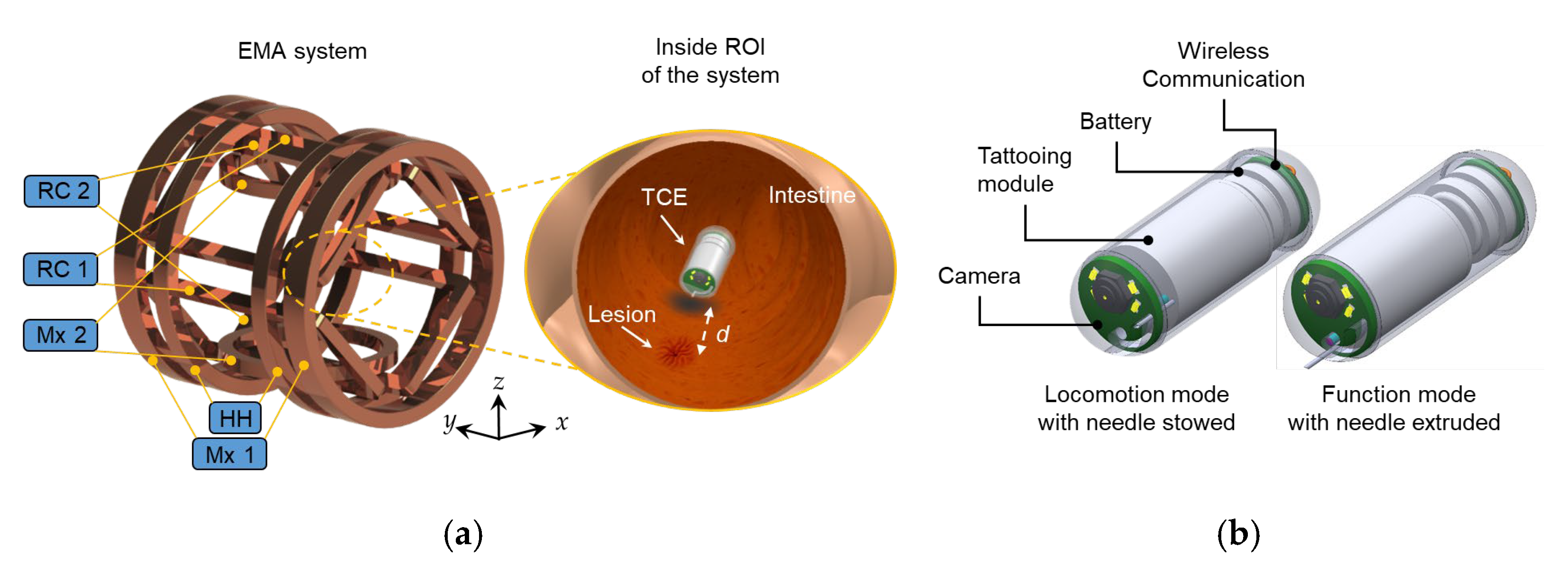

| Coil | Mx 1 | Mx 2 | RC 1, RC 2 | HH |
|---|---|---|---|---|
| Type | Maxwell | Maxwell | Rectangular | Helmholtz |
| Radius (mm) | 195 | 100 | n/a | 195 |
| Width × Length (mm) | n/a | n/a | 156 × 337 | n/a |
| Distance (mm) | 337.75 | 173.2 | 200 | 195 |
| Coil turns | 1426 | 660 | 600 | 710 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, M.-C.; Park, J.-O.; Kim, J. Battery-Free Tattooing Mechanism-Based Functional Active Capsule Endoscopy. Micromachines 2022, 13, 2111. https://doi.org/10.3390/mi13122111
Hoang M-C, Park J-O, Kim J. Battery-Free Tattooing Mechanism-Based Functional Active Capsule Endoscopy. Micromachines. 2022; 13(12):2111. https://doi.org/10.3390/mi13122111
Chicago/Turabian StyleHoang, Manh-Cuong, Jong-Oh Park, and Jayoung Kim. 2022. "Battery-Free Tattooing Mechanism-Based Functional Active Capsule Endoscopy" Micromachines 13, no. 12: 2111. https://doi.org/10.3390/mi13122111






