A Novel Cortisol Immunosensor Based on a Hafnium Oxide/Silicon Structure for Heart Failure Diagnosis
Abstract
1. Introduction
2. Materials and Methods
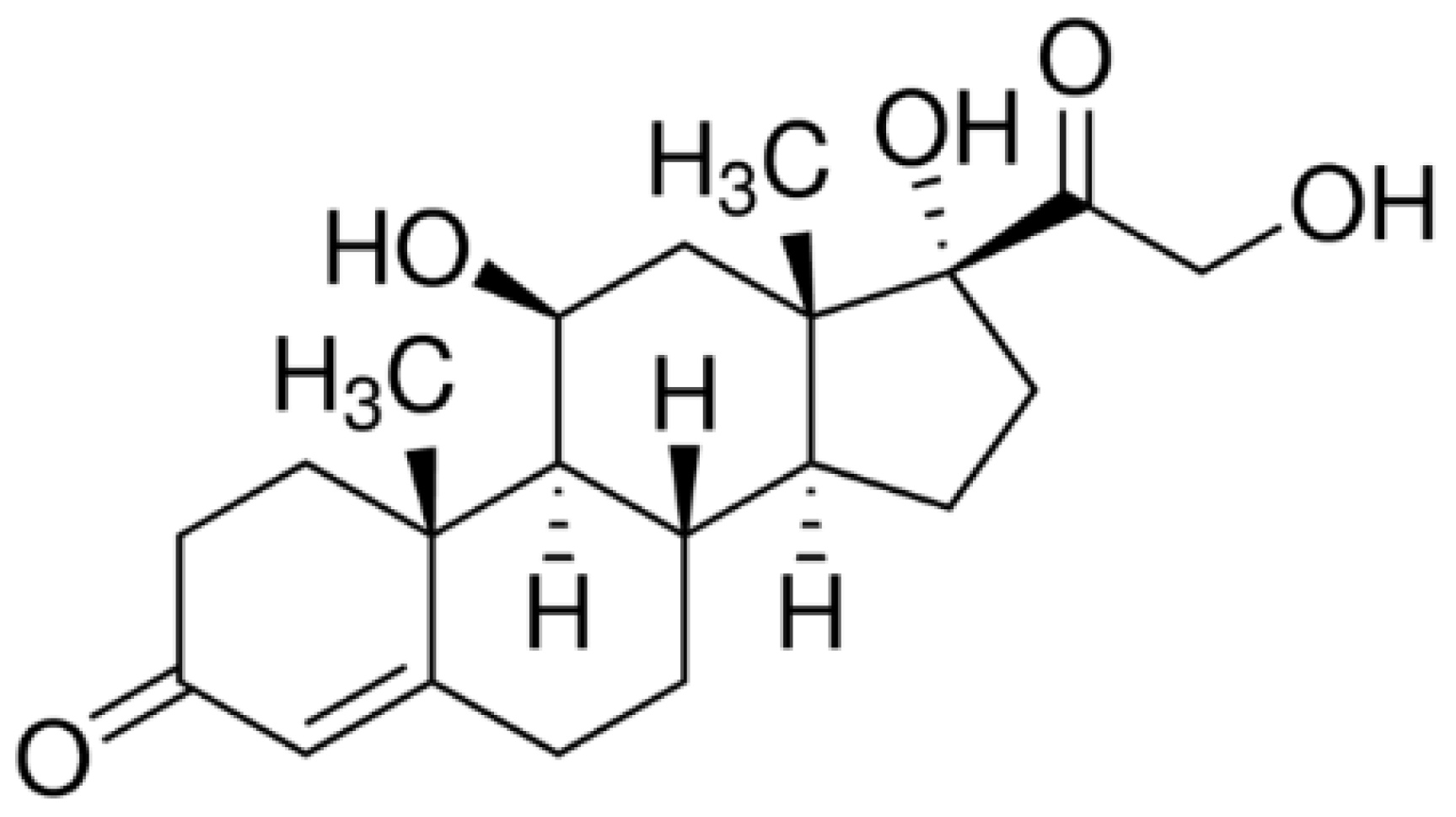
2.1. Materials and Chemicals
2.2. Fabrication Technology of HfO2/Silicon Structures
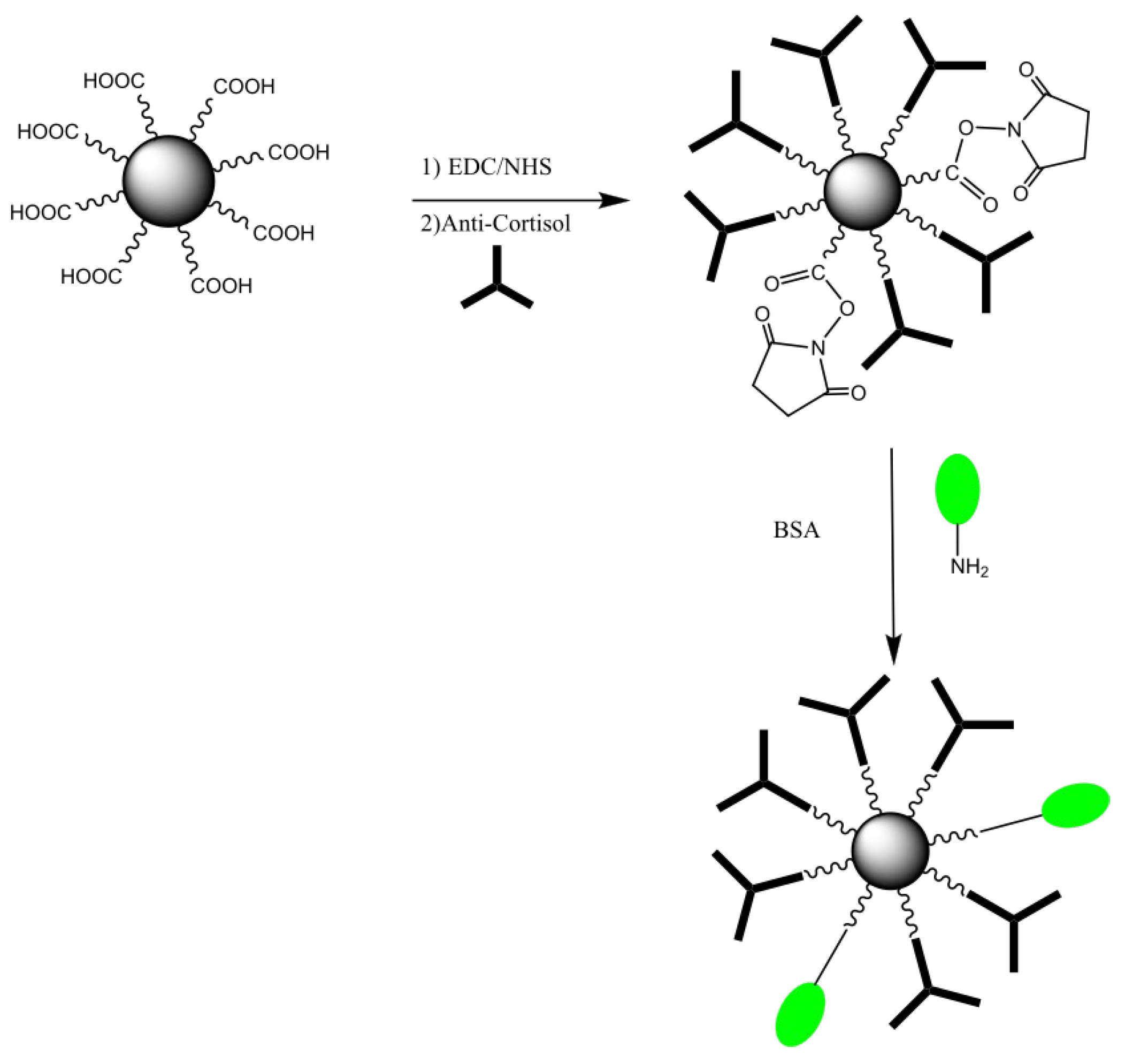
2.3. Functionalization of HfO2 Surface with Antibodies
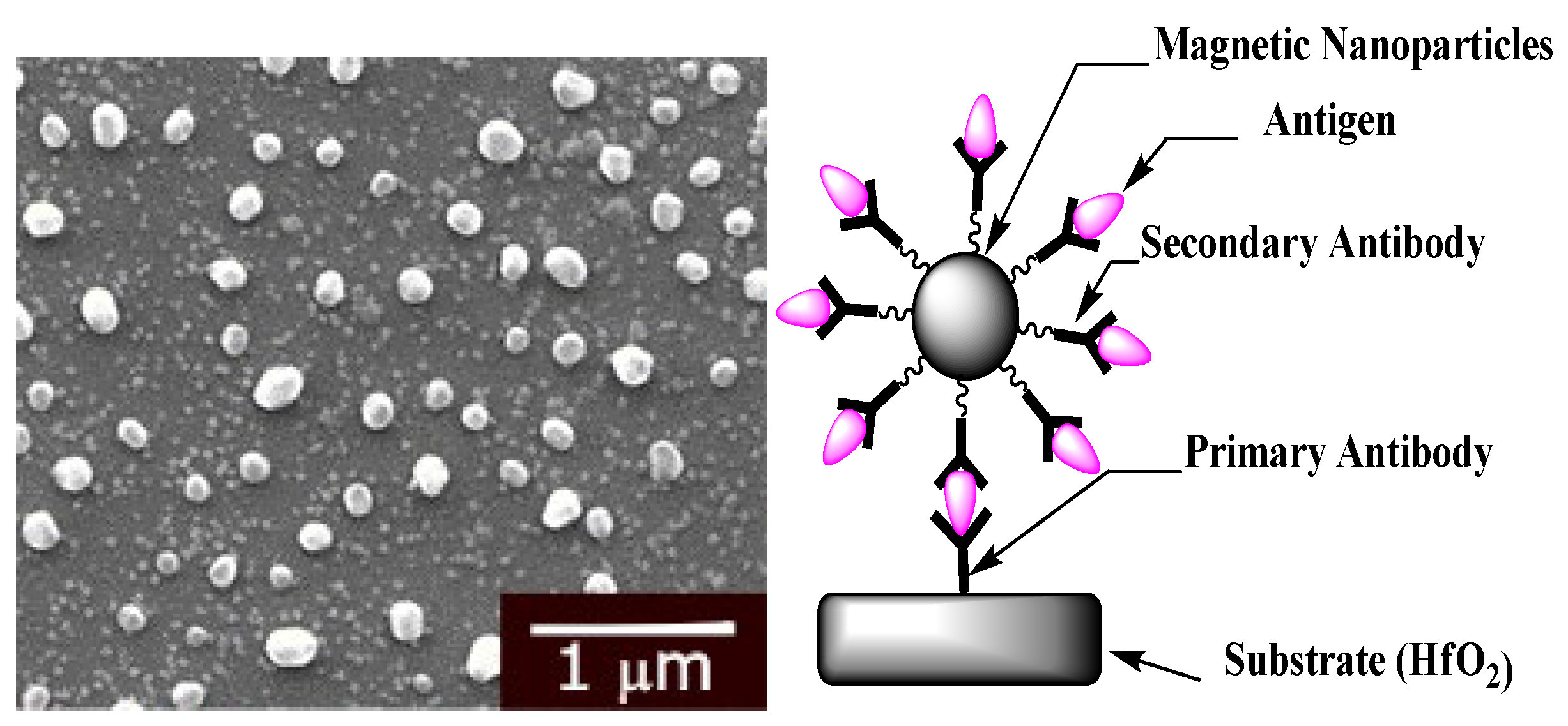
2.4. Magnetic Nanoparticles Biofunctionalization
2.5. Characterization of HfO2 Surface Using Contact Angle Measurement (CAM)
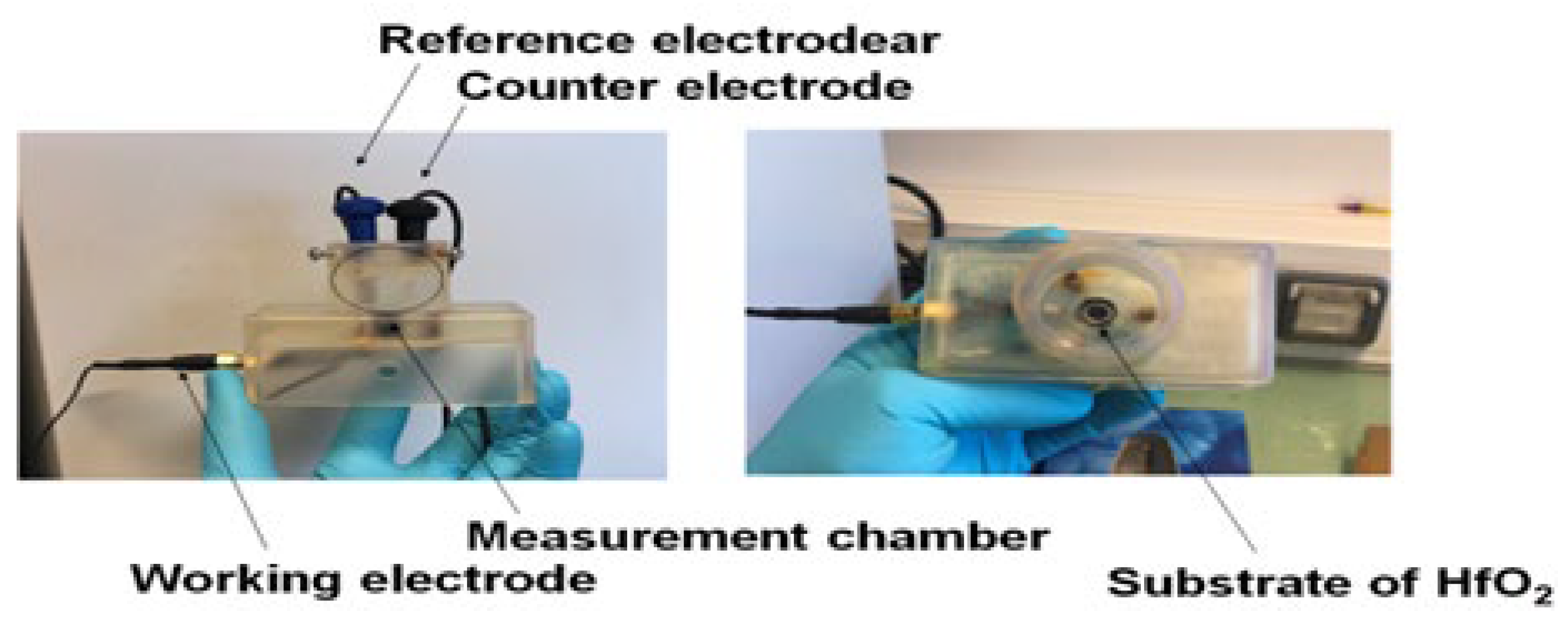
2.6. Electrochemical Measurements with HfO2/Silicon Structures
3. Results
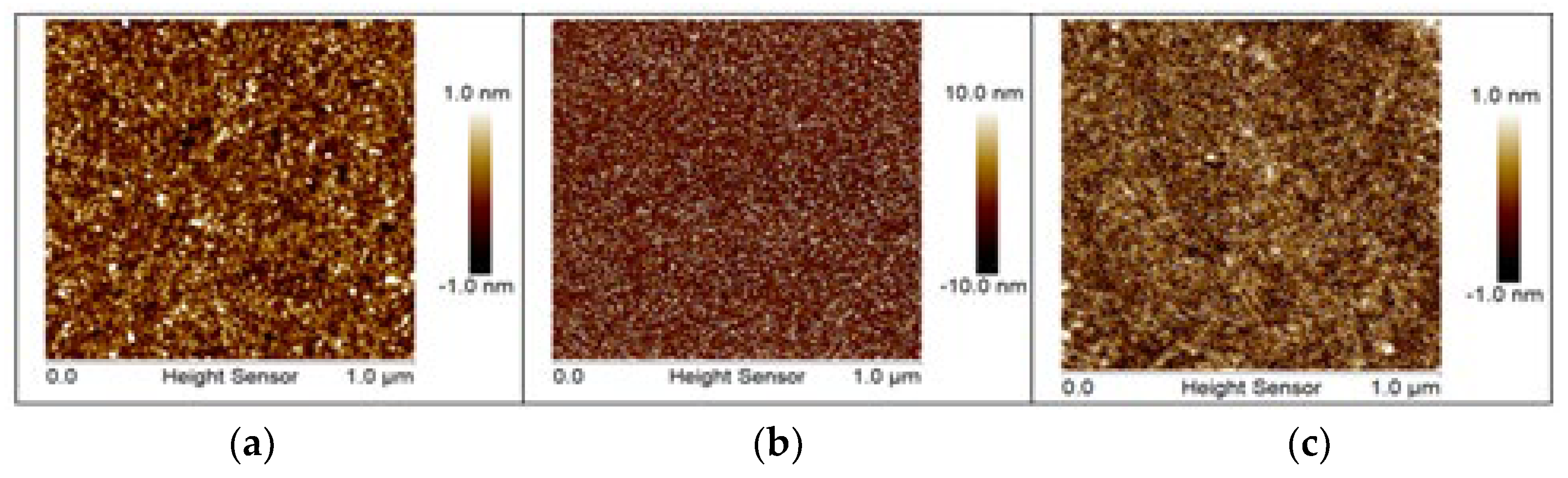
3.1. Surface Characterization
3.2. Biosensor Calibration by EIS Measurements
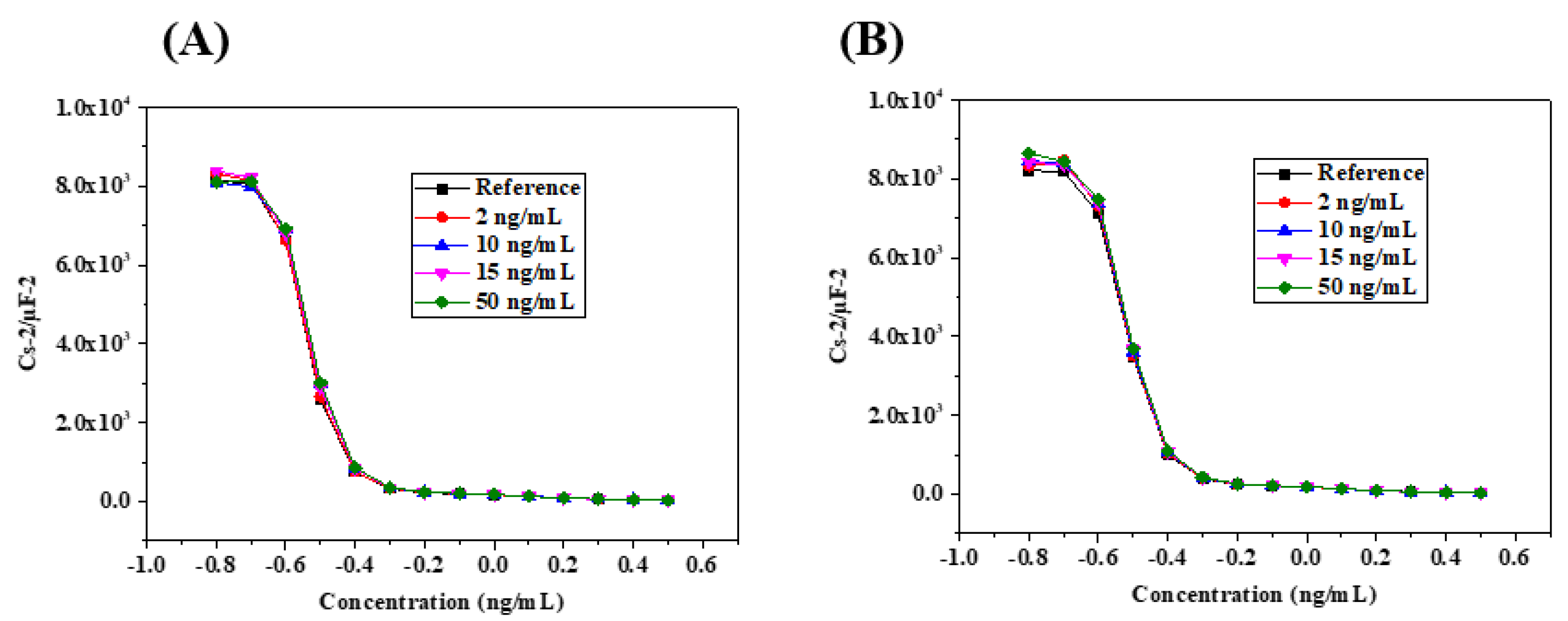
3.3. Biosensor Calibration by Capacitive Measurements
3.4. Cortisol Quantification in Saliva Samples by Standard Addition Method (SAM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jessup, M.; Brozena, S. Medical Progress. Heart Failure. N. Engl. J. Med. 2003, 348, 2007–2018. [Google Scholar]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Zea, M.; Bellagambi, F.G.; Ben Halima, H.; Zine, N.; Jaffrezic-Renault, N.; Villa, R.; Gabriel, G.; Errachid, A. Electrochemical Sensors for Cortisol Detections: Almost There. TrAC Trends Anal. Chem. 2020, 132, 116058. [Google Scholar] [CrossRef]
- Raff, H. Utility of Salivary Cortisol Measurements in Cushing’s Syndrome and Adrenal Insufficiency. J. Clin. Endocrinol. Metab. 2009, 94, 3647–3655. [Google Scholar] [CrossRef]
- Whitworth, J.A.; Williamson, P.M.; Mangos, G.; Kelly, J.J. Cardiovascular Consequences of Cortisol Excess. Vasc. Health Risk Manag. 2005, 1, 291–299. [Google Scholar] [CrossRef]
- Güder, G.; Bauersach, J.; Frantz, S.; Weismann, D.; Allolio, B.; Ertl, G.; Angermann, C.E.; Störk, S. Mortality Risk Prediction by Cortisol and Aldosterone in Chronic Heart Failure. Exp. Clin. Endocrinol. Diabetes 2007, 115, OR09_5. [Google Scholar] [CrossRef]
- Morey, J.N.; Boggero, I.A.; Scott, A.B.; Segerstrom, S.C. Current Directions in Stress and Human Immune Function. Curr. Opin. Psychol. 2015, 5, 13–17. [Google Scholar] [CrossRef]
- Turpeinen, U.; Hämäläinen, E. Determination of Cortisol in Serum, Saliva and Urine. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 795–801. [Google Scholar] [CrossRef]
- Stephens, M.A.C.; Wand, G. Stress and the HPA Axi: Role of Glucocorticoids in Alcohol Dependence. Alcohol Res. Curr. Rev. 2012, 34, 468–483. [Google Scholar]
- De Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the Brain: From Adaptation to Disease. Nat. Rev. Neurosci. 2005, 6, 463. [Google Scholar] [CrossRef]
- Taves, M.D.; Gomez-Sanchez, C.E.; Soma, K.K. Extra-Adrenal Glucocorticoids and Mineralocorticoids: Evidence for Local Synthesis, Regulation, and Function. Am. J. Physiol.-Endocrinol. Metab. 2011, 301, E11–E24. [Google Scholar] [CrossRef]
- Gecaite-Stonciene, J.; Hughes, B.M.; Kazukauskiene, N.; Bunevicius, A.; Burkauskas, J.; Neverauskas, J.; Bellani, M.; Mickuviene, N. Cortisol Response to Psychosocial Stress, Mental Distress, Fatigue and Quality of Life in Coronary Artery Disease Patients. Sci. Rep. 2022, 12, 19373. [Google Scholar] [CrossRef]
- Zubarev, A.; Cuzminschi, M.; Iordache, A.-M.; Iordache, S.-M.; Rizea, C.; Grigorescu, C.E.A.; Giuglea, C. Graphene-Based Sensor for the Detection of Cortisol for Stress Level Monitoring and Diagnostics. Diagnostics 2022, 12, 2593. [Google Scholar] [CrossRef]
- Eto, K.; Mazilu-Brown, J.K.; Henderson-MacLennan, N.; Dipple, K.M.; McCabe, E.R.B. Development of Catecholamine and Cortisol Stress Responses in Zebrafish. Mol. Genet. Metab. Rep. 2014, 1, 373–377. [Google Scholar] [CrossRef]
- Kaur, K.; Dhingra, S.; Slezak, J.; Sharma, A.K.; Bajaj, A.; Singal, P.K. Biology of TNFα and IL-10, and Their Imbalance in Heart Failure. Heart Fail. Rev. 2009, 14, 113–123. [Google Scholar] [CrossRef]
- James, G.D.; Alfarano, A.S.; van Berge-Landry, H.M. Differential Circadian Catecholamine and Cortisol Responses between Healthy Women with and without a Parental History of Hypertension. Am. J. Hum. Biol. 2014, 26, 753–759. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. CIA 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A.; Fatima, N. Increased Reactive Oxygen Species Formation and Oxidative Stress in Rheumatoid Arthritis. PLoS ONE 2016, 11, e0152925. [Google Scholar] [CrossRef]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxidative Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Byrne, J.A.; Grieve, D.J.; Cave, A.C.; Shah, A.M. Oxidative Stress and Heart Failure. Arch. Mal. Coeur. Vaiss. 2003, 96, 214–221. [Google Scholar]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative Stress and Heart Failure. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef]
- Grieve, D. Oxidative Stress in Heart Failure More than Just Damage. Eur. Heart J. 2003, 24, 2161–2163. [Google Scholar] [CrossRef]
- Sawyer, D.B. Oxidative Stress in Heart Failure: What Are We Missing? Am. J. Med. Sci. 2011, 342, 120–124. [Google Scholar] [CrossRef]
- Yamaji, M.; Tsutamoto, T.; Kawahara, C.; Nishiyama, K.; Yamamoto, T.; Fujii, M.; Horie, M. Serum Cortisol as a Useful Predictor of Cardiac Events in Patients with Chronic Heart Failure: The Impact of Oxidative Stress. Circ. Heart Fail. 2009, 2, 608–615. [Google Scholar] [CrossRef]
- Tzanis, G.; Dimopoulos, S.; Agapitou, V.; Nanas, S. Exercise Intolerance in Chronic Heart Failure: The Role of Cortisol and the Catabolic State. Curr. Heart Fail. Rep. 2014, 11, 70–79. [Google Scholar] [CrossRef]
- Güder, G.; Bauersachs, J.; Frantz, S.; Weismann, D.; Allolio, B.; Ertl, G.; Angermann, C.E.; Störk, S. Complementary and Incremental Mortality Risk Prediction by Cortisol and Aldosterone in Chronic Heart Failure. Circulation 2007, 115, 1754–1761. [Google Scholar] [CrossRef]
- Braun, T.P.; Marks, D.L. The Regulation of Muscle Mass by Endogenous Glucocorticoids. Front. Physiol. 2015, 6, 12. [Google Scholar] [CrossRef]
- Canalis, E.; Mazziotti, G.; Giustina, A.; Bilezikian, J.P. Glucocorticoid-Induced Osteoporosis: Pathophysiology and Therapy. Osteoporos. Int. 2007, 18, 1319–1328. [Google Scholar] [CrossRef]
- Ebrecht, M.; Hextall, J.; Kirtley, L.-G.; Taylor, A.; Dyson, M.; Weinman, J. Perceived Stress and Cortisol Levels Predict Speed of Wound Healing in Healthy Male Adults. Psychoneuroendocrinology 2004, 29, 798–809. [Google Scholar] [CrossRef]
- Sephton, S.E.; Lush, E.; Dedert, E.A.; Floyd, A.R.; Rebholz, W.N.; Dhabhar, F.S.; Spiegel, D.; Salmon, P. Diurnal Cortisol Rhythm as a Predictor of Lung Cancer Survival. Brain Behav. Immun. 2013, 30, S163–S170. [Google Scholar] [CrossRef]
- Chan, S.; Debono, M. Review: Replication of Cortisol Circadian Rhythm: New Advances in Hydrocortisone Replacement Therapy. Ther. Adv. Endocrinol. 2010, 1, 129–138. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Degano, I.; Ghimenti, S.; Lomonaco, T.; Dini, V.; Romanelli, M.; Mastorci, F.; Gemignani, A.; Salvo, P.; Fuoco, R.; et al. Determination of Salivary α-Amylase and Cortisol in Psoriatic Subjects Undergoing the Trier Social Stress Test. Microchem. J. 2018, 136, 177–184. [Google Scholar] [CrossRef]
- Aas, M.; Ueland, T.; Inova, A.; Melle, I.; Andreassen, O.A.; Steen, N.E. Childhood Trauma Is Nominally Associated with Elevated Cortisol Metabolism in Severe Mental Disorder. Front. Psychiatry 2020, 11, 391. [Google Scholar] [CrossRef]
- Hamzavi, M.; Tadbir, A.A.; Rezvani, G.; Ashraf, M.J.; Fattahi, M.J.; Khademi, B.; Sardari, Y.; Jeirudi, N. Tissue Expression, Serum and Salivary Levels of IL-10 in Patients with Head and Neck Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2013, 14, 1681–1685. [Google Scholar] [CrossRef]
- King, A.; Munisamy, G.; de Wit, H.; Lin, S. Attenuated Cortisol Response to Alcohol in Heavy Social Drinkers. Int. J. Psychophysiol. 2006, 59, 203–209. [Google Scholar] [CrossRef]
- Cinar, V.; Mogulkoc, R.; Baltaci, A.K.; Polat, Y. Adrenocorticotropic Hormone and Cortisol Levels in Athletes and Sedentary Subjects at Rest and Exhaustion: Effects of Magnesium Supplementation. Biol. Trace Elem. Res. 2008, 121, 215–220. [Google Scholar] [CrossRef]
- Noreen, E.E.; Sass, M.J.; Crowe, M.L.; Pabon, V.A.; Brandauer, J.; Averill, L.K. Effects of Supplemental Fish Oil on Resting Metabolic Rate, Body Composition, and Salivary Cortisol in Healthy Adults. J. Int. Soc. Sport. Nutr. 2010, 7, 31. [Google Scholar] [CrossRef]
- Uedo, N.; Ishikawa, H.; Morimoto, K.; Ishihara, R.; Narahara, H.; Akedo, I.; Ioka, T.; Kaji, I.; Fukuda, S. Reduction in Salivary Cortisol Level by Music Therapy during Colonoscopic Examination. Hepatogastroenterology 2004, 51, 451–453. [Google Scholar]
- Field, T.; Hernandez-Reif, M.; Diego, M.; Schanberg, S.; Kuhn, C. Cortisol decreases and serotonin and dopamine increase following massage therapy. Int. J. Neurosci. 2005, 115, 1397–1413. [Google Scholar] [CrossRef]
- Berk, L.S.; Tan, S.A.; Berk, D. Cortisol and Catecholamine Stress Hormone Decrease Is Associated with the Behavior of Perceptual Anticipation of Mirthful Laughter. FASEB J. 2008, 22, 946.11. [Google Scholar] [CrossRef]
- Quiroga Murcia, C.; Bongard, S.; Kreutz, G. Emotional and Neurohumoral Responses to Dancing Tango Argentino: The Effects of Music and Partner. Music. Med. 2009, 1, 14–21. [Google Scholar] [CrossRef]
- Kalman, B.A.; Grahn, R.E. Measuring Salivary Cortisol in the Behavioral Neuroscience Laboratory. J. Undergrad. Neurosci. Educ. 2004, 2, A41–A49. [Google Scholar]
- Gozansky, W.S.; Lynn, J.S.; Laudenslager, M.L.; Kohrt, W.M. Salivary Cortisol Determined by Enzyme Immunoassay Is Preferable to Serum Total Cortisol for Assessment of Dynamic Hypothalamic–Pituitary–Adrenal Axis Activity. Clin. Endocrinol. 2005, 63, 336–341. [Google Scholar] [CrossRef]
- Inder, W.J.; Dimeski, G.; Russell, A. Measurement of Salivary Cortisol in 2012—Laboratory Techniques and Clinical Indications. Clin. Endocrinol. 2012, 77, 645–651. [Google Scholar] [CrossRef]
- Kataoka, H.; Matsuura, E.; Mitani, K. Determination of Cortisol in Human Saliva by Automated In-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography–Mass Spectrometry. J. Pharm. Biomed. Anal. 2007, 44, 160–165. [Google Scholar] [CrossRef]
- Matsui, F.; Koh, E.; Yamamoto, K.; Sugimoto, K.; Sin, H.-S.; Maeda, Y.; Honma, S.; Namiki, M. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Assay for Simultaneous Measurement of Salivary Testosterone and Cortisol in Healthy Men for Utilization in the Diagnosis of Late-Onset Hypogonadism in Males. Endocr. J. 2009, 56, 1083–1093. [Google Scholar] [CrossRef]
- Turpeinen, U.; Välimäki, M.J.; Hämäläinen, E. Determination of Salivary Cortisol by Liquid Chromatography-Tandem Mass Spectrometry. Scand. J. Clin. Lab. Investig. 2009, 69, 592–597. [Google Scholar] [CrossRef]
- Ben Halima, H.; Bellagambi, F.G.; Alcacer, A.; Pfeiffer, N.; Heuberger, A.; Hangouët, M.; Zine, N.; Bausells, J.; Elaissari, A.; Errachid, A. A Silicon Nitride ISFET Based Immunosensor for Tumor Necrosis Factor-Alpha Detection in Saliva. A Promising Tool for Heart Failure Monitoring. Anal. Chim. Acta 2021, 1161, 338468. [Google Scholar] [CrossRef]
- Ben Halima, H.; Bellagambi, F.G.; Brunon, F.; Alcacer, A.; Pfeiffer, N.; Heuberger, A.; Hangouët, M.; Zine, N.; Bausells, J.; Errachid, A. Immuno Field-Effect Transistor (ImmunoFET) for Detection of Salivary Cortisol Using Potentiometric and Impedance Spectroscopy for Monitoring Heart Failure. Talanta 2022, 123802. [Google Scholar] [CrossRef]
- Ben Halima, H.; Bellagambi, F.G.; Hangouët, M.; Alcacer, A.; Pfeiffer, N.; Heuberger, A.; Zine, N.; Bausells, J.; Errachid, A. A Novel IMFET Biosensor Strategy for Interleukin-10 Quantification for Early Screening Heart Failure Disease in Saliva. Electroanalysis 2022, elan.202200141, early view. [Google Scholar] [CrossRef]
- Bellagambi, F.G.; Petersen, C.; Salvo, P.; Ghimenti, S.; Franzini, M.; Biagini, D.; Hangouët, M.; Trivella, M.G.; Di Francesco, F.; Paolicchi, A.; et al. Determination and Stability of N-Terminal pro-Brain Natriuretic Peptide in Saliva Samples for Monitoring Heart Failure. Sci. Rep. 2021, 11, 13088. [Google Scholar] [CrossRef]
- Lee, M.; Zine, N.; Baraket, A.; Zabala, M.; Campabadal, F.; Caruso, R.; Trivella, M.G.; Jaffrezic-Renault, N.; Errachid, A. A Novel Biosensor Based on Hafnium Oxide: Application for Early Stage Detection of Human Interleukin-10. Sens. Actuators B Chem. 2012, 175, 201–207. [Google Scholar] [CrossRef]
- Ben Halima, H.; Bellagambi, F.G.; Hangouët, M.; Alcacer, A.; Pfeiffer, N.; Heuberger, A.; Zine, N.; Bausells, J.; Elaissari, A.; Errachid, A. A Novel Electrochemical Strategy for NT-ProBNP Detection Using IMFET for Monitoring Heart Failure by Saliva Analysis. Talanta 2023, 251, 123759. [Google Scholar] [CrossRef]
- Jamshaid, T.; Tenório-Neto, E.T.; Eissa, M.; Zine, N.; El-Salhi, A.E.; Kunita, M.H.; Elaissari, A. Preparation and Characterization of Submicron Hybrid Magnetic Latex Particles. Polym. Adv. Technol. 2015, 26, 1102–1108. [Google Scholar] [CrossRef]
- Cardinell, B.A.; Spano, M.L.; La Belle, J.T. Toward a Label-Free Electrochemical Impedance Immunosensor Design for Quantifying Cortisol in Tears. Crit. Rev. Biomed. Eng. 2019, 47, 207–215. [Google Scholar] [CrossRef]
- Kämäräinen, S.; Mäki, M.; Tolonen, T.; Palleschi, G.; Virtanen, V.; Micheli, L.; Sesay, A.M. Disposable Electrochemical Immunosensor for Cortisol Determination in Human Saliva. Talanta 2018, 188, 50–57. [Google Scholar] [CrossRef]
- Tuteja, S.K.; Ormsby, C.; Neethirajan, S. Noninvasive Label-Free Detection of Cortisol and Lactate Using Graphene Embedded Screen-Printed Electrode. Nano-Micro. Lett. 2018, 10, 41. [Google Scholar] [CrossRef]
- Pali, M.; Garvey, J.E.; Small, B.; Suni, I.I. Detection of Fish Hormones by Electrochemical Impedance Spectroscopy and Quartz Crystal Microbalance. Sens. Bio-Sens. Res. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Kinnamon, D.; Ghanta, R.; Lin, K.-C.; Muthukumar, S.; Prasad, S. Portable Biosensor for Monitoring Cortisol in Low-Volume Perspired Human Sweat. Sci. Rep. 2017, 7, 13312. [Google Scholar] [CrossRef]


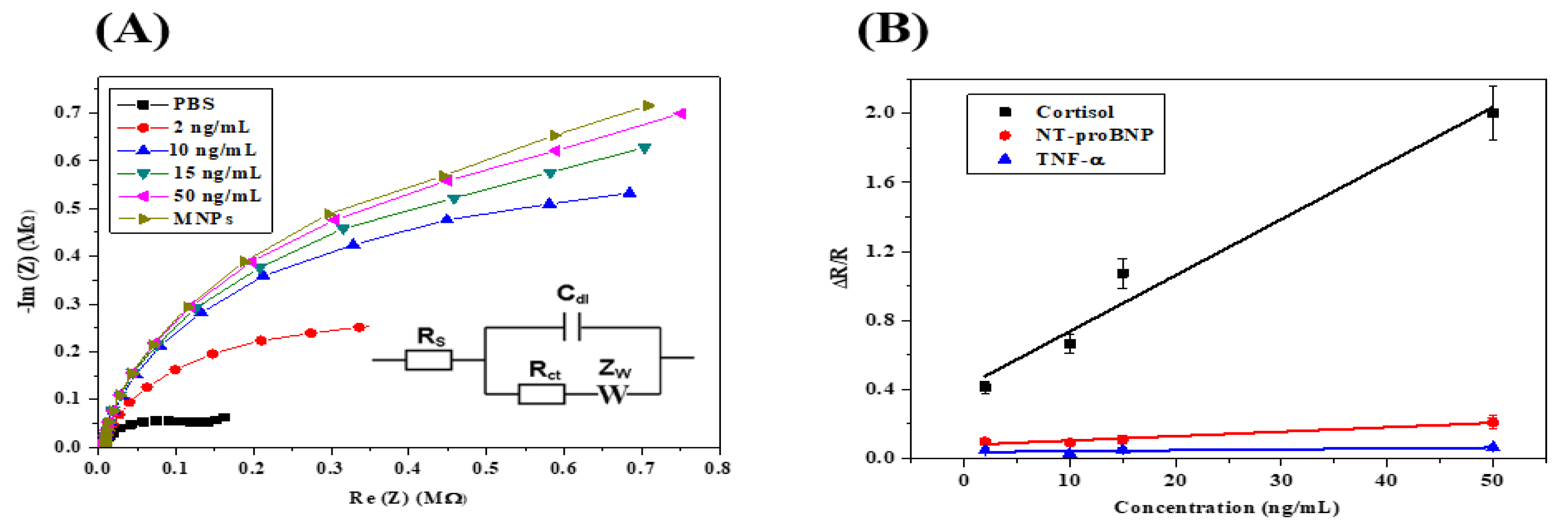
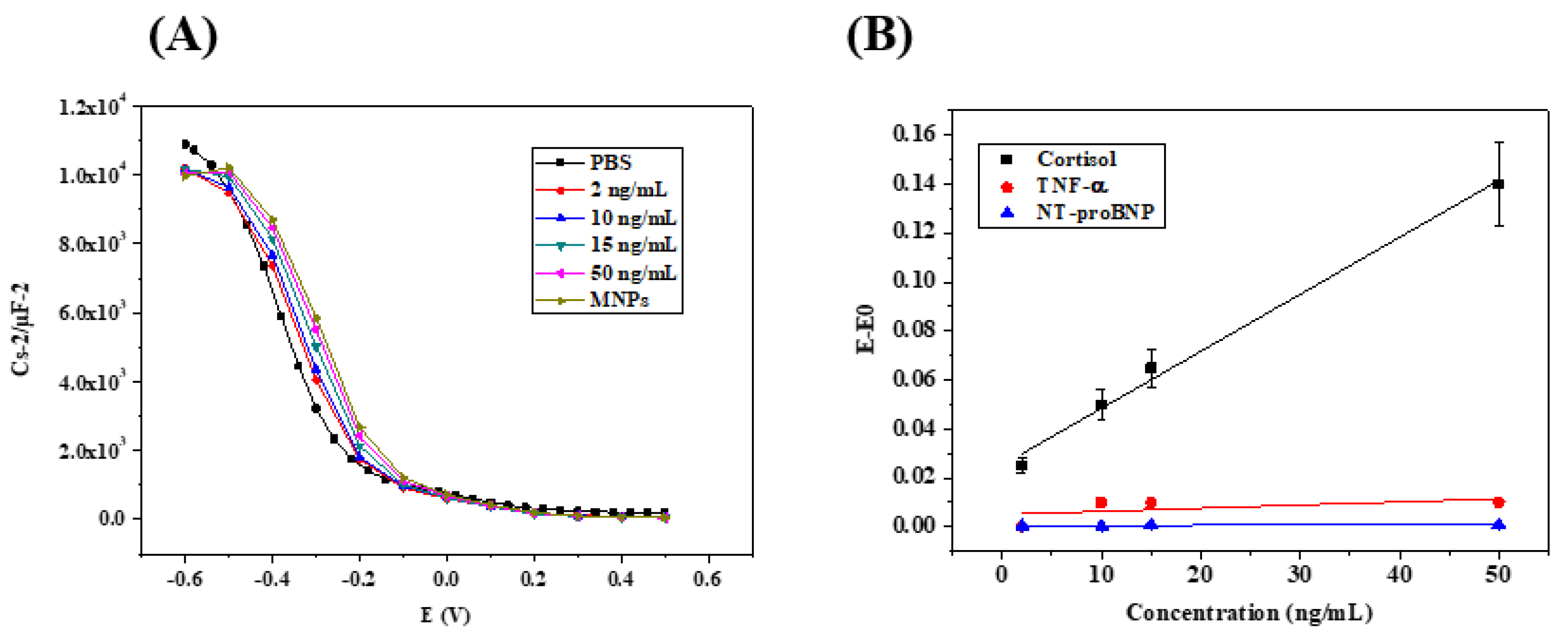
| Cortisol Concentration (ng mL−1) | Rs (Ω) | Q2 (nF.s (a−1)) | Rct (MΩ) | s2 (kΩ.s−1/2) | χ2 |
|---|---|---|---|---|---|
| 0 | 9181 | 10.91 | 0.206 | 0.547 | 1.754 × 10−3 |
| 2 | 27,031 | 10.69 | 0.302 | 1.88 | 0.514 × 10−3 |
| 10 | 29,128 | 10.87 | 0.370 | 2.925 | 1.845 × 10−3 |
| 15 | 33,652 | 10.84 | 0.450 | 4.419 | 1.050 × 10−3 |
| 50 | 44,199 | 12.3 | 0.620 | 3.633 | 1.436 × 10−3 |
| Technique | Electrode | Immobilizing Biomolecules | Analyte | Linear Range (ng mL−1) | Limit of Detection (LOD) (ng mL−1) | References |
|---|---|---|---|---|---|---|
| EIS | - | Anti-cortisol antibody | Human Tears | 0.05–200 | 21.66 | [56] |
| SWV | Graphite | Anti-cortisol antibody | Human saliva | 0.5–55.5 | 1.7 | [57] |
| Amperometry | Reduced graphene oxide | Anti-cortisol antibody | Human saliva and sweat | 0.1–200 | 0.1 | [58] |
| EIS | Au | Anti-cortisol antibody + BSA | Fish plasma | 1440–2170 | 2750 | [59] |
| EIS | Palladium + MoS2 | Anti-cortisol antibody | Human sweat | 1–500 | 1 | [60] |
| EIS/capacitance | HfO2 | Anti-cortisol antibody | Real saliva | 2–50 | 0.66 | This work |
| Sample Name | Added Concentration (ng/mL) | Dilution Factor | Calculated Concentration | Bias |
|---|---|---|---|---|
| Sample A | 2 | 19.9 | 2.4 ± 0.3 ng mL−1 (CV = 1%) | 14% |
| Sample B | 5 | 19.5 | 4.7 ± 0.7 ng mL−1 (CV = 5%) | 18% |
| Sample C | 7 | 19.7 | 7.1 ± 0.2 ng mL−1 (CV = 1%) | 15% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Halima, H.; Zine, N.; Bausells, J.; Jaffrezic-Renault, N.; Errachid, A. A Novel Cortisol Immunosensor Based on a Hafnium Oxide/Silicon Structure for Heart Failure Diagnosis. Micromachines 2022, 13, 2235. https://doi.org/10.3390/mi13122235
Ben Halima H, Zine N, Bausells J, Jaffrezic-Renault N, Errachid A. A Novel Cortisol Immunosensor Based on a Hafnium Oxide/Silicon Structure for Heart Failure Diagnosis. Micromachines. 2022; 13(12):2235. https://doi.org/10.3390/mi13122235
Chicago/Turabian StyleBen Halima, Hamdi, Nadia Zine, Joan Bausells, Nicole Jaffrezic-Renault, and Abdelhamid Errachid. 2022. "A Novel Cortisol Immunosensor Based on a Hafnium Oxide/Silicon Structure for Heart Failure Diagnosis" Micromachines 13, no. 12: 2235. https://doi.org/10.3390/mi13122235
APA StyleBen Halima, H., Zine, N., Bausells, J., Jaffrezic-Renault, N., & Errachid, A. (2022). A Novel Cortisol Immunosensor Based on a Hafnium Oxide/Silicon Structure for Heart Failure Diagnosis. Micromachines, 13(12), 2235. https://doi.org/10.3390/mi13122235










