Fast Electrochemical Actuator with Ti Electrodes in the Current Stabilization Regime
Abstract
:1. Introduction
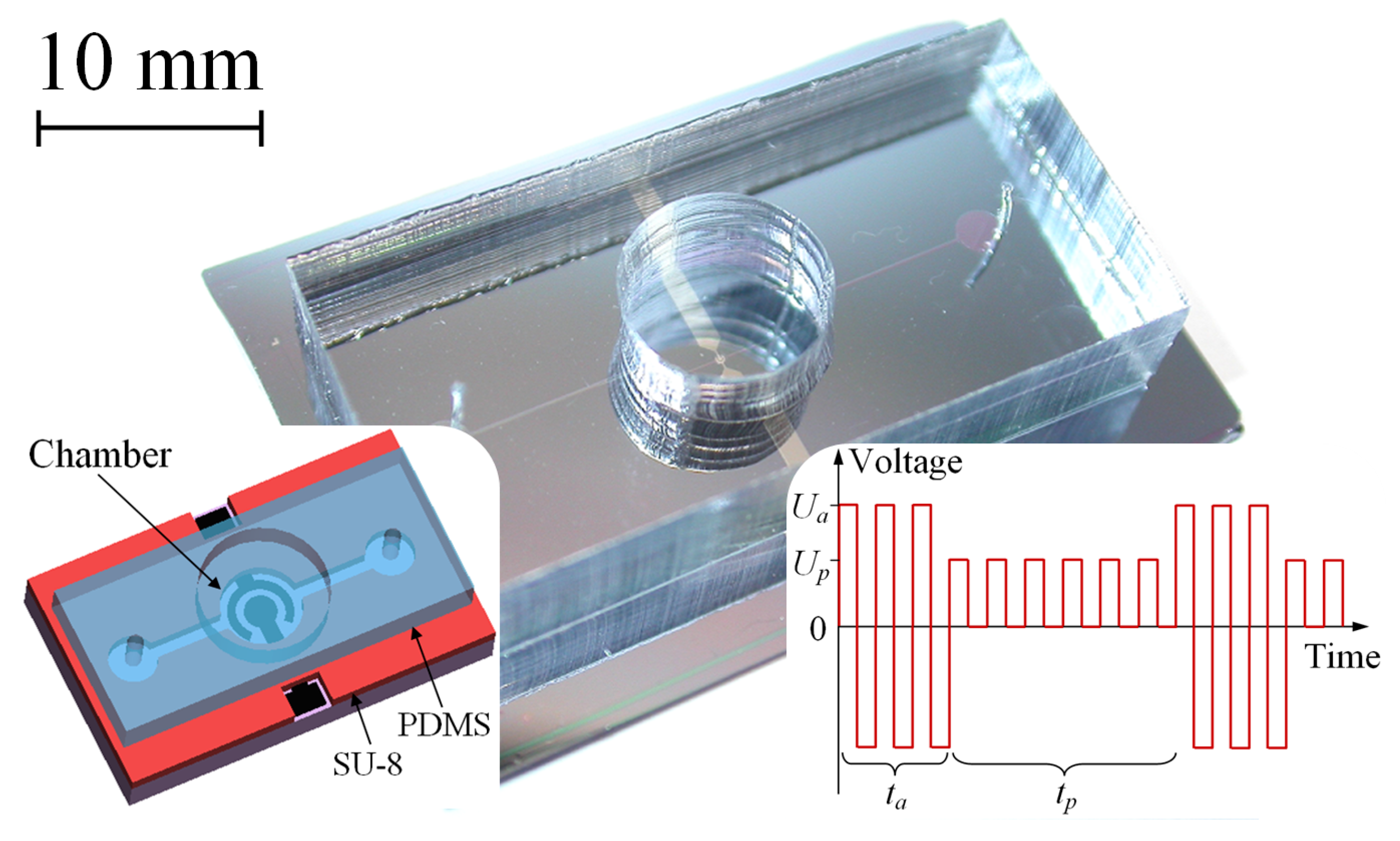
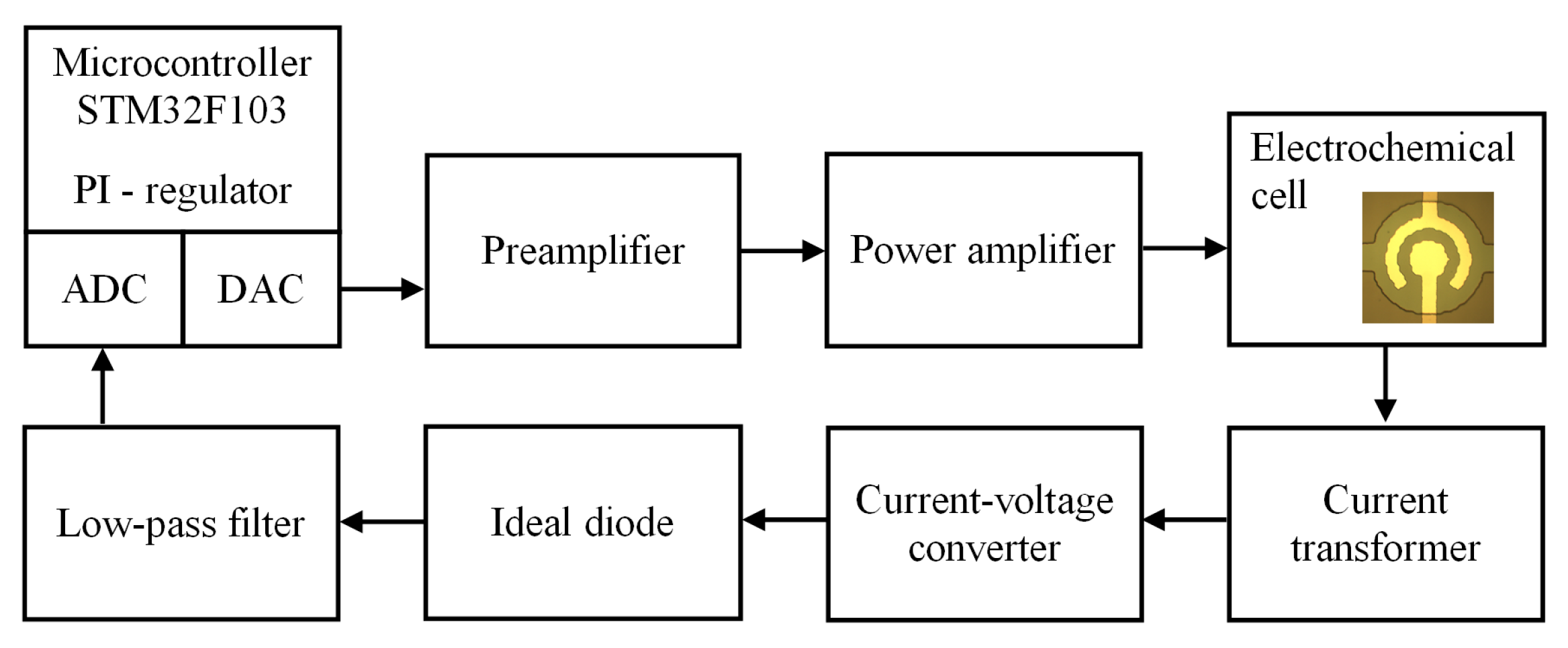
2. Materials and Methods
3. Results and Discussion
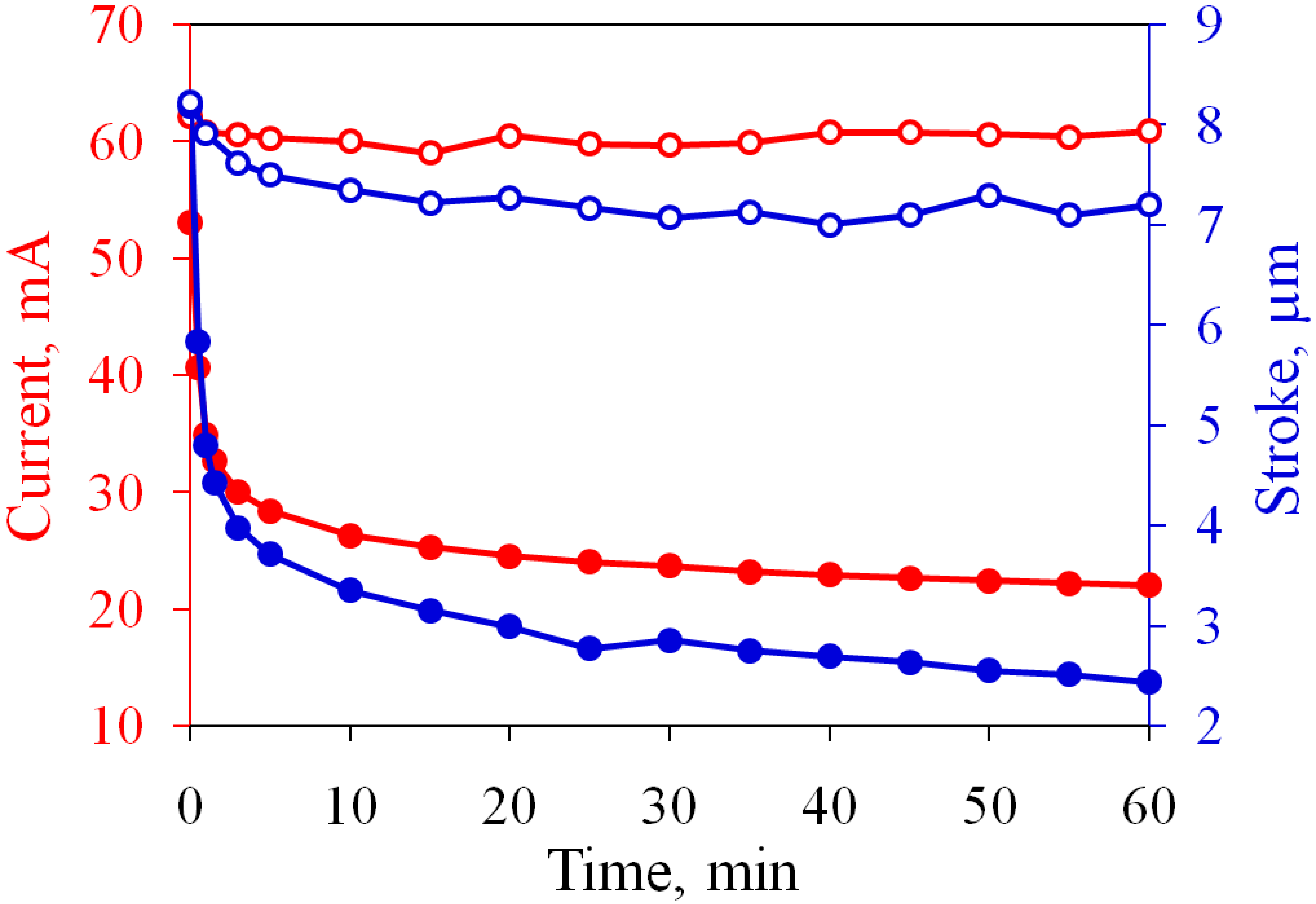
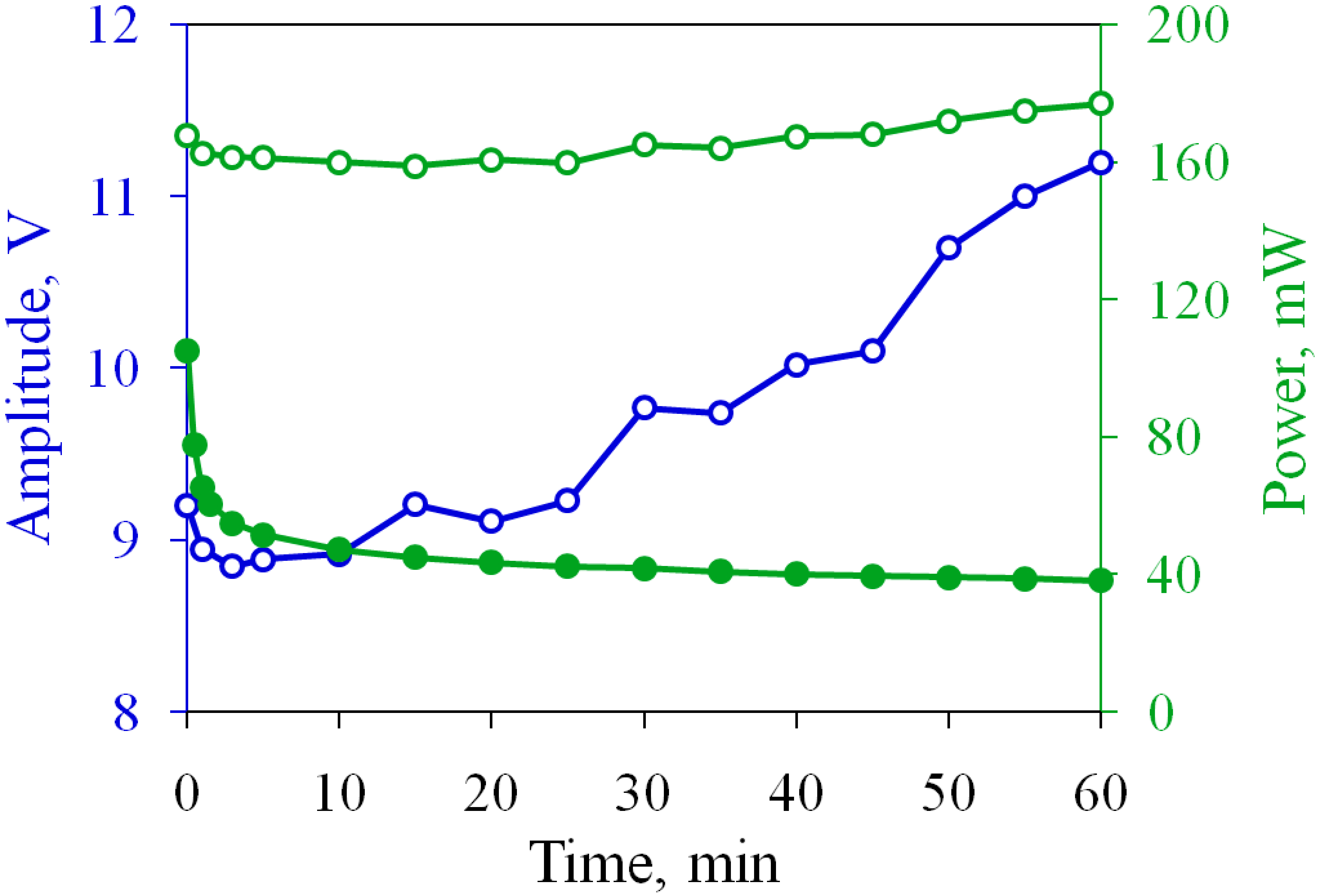
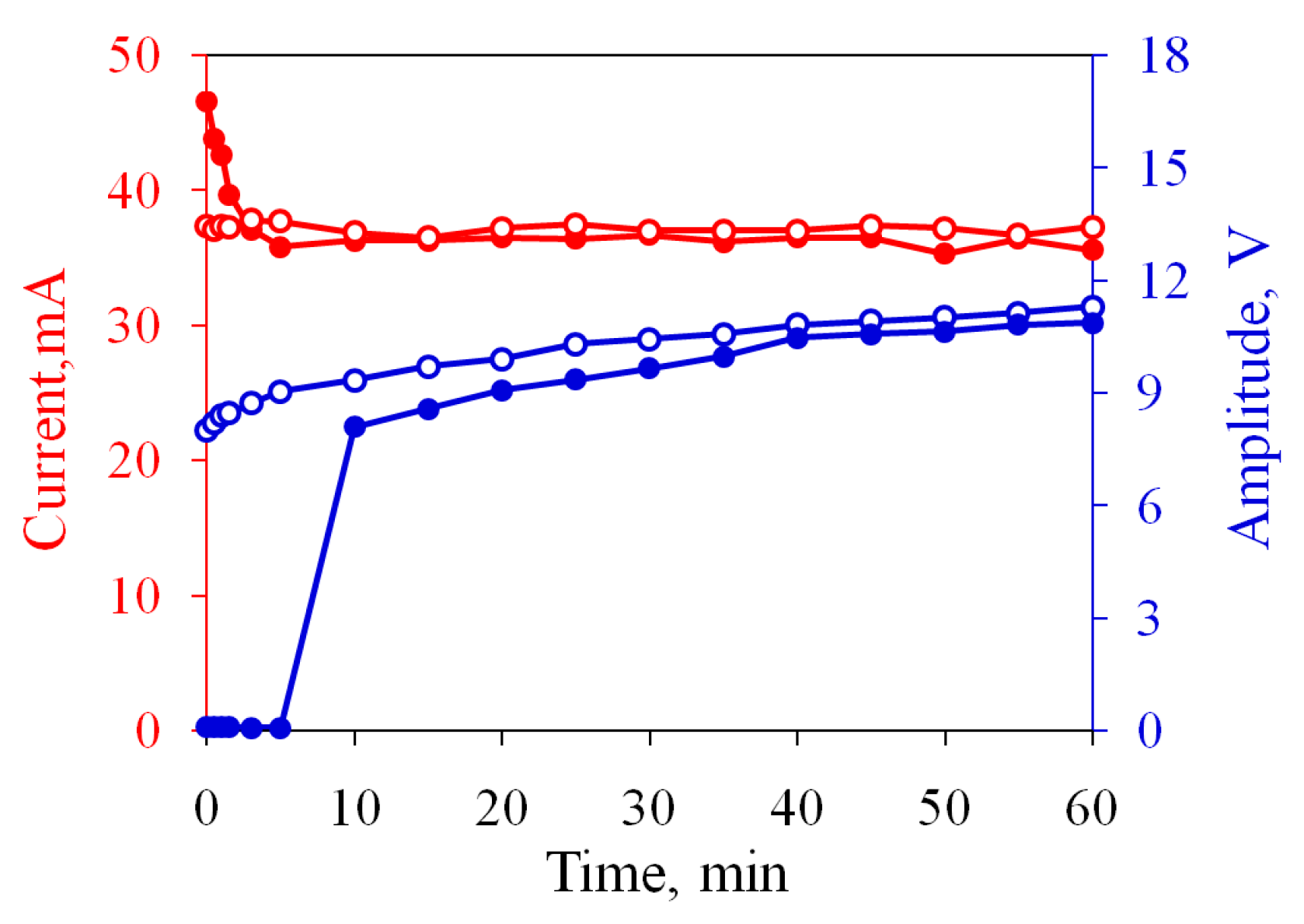
3.1. Restoring the Performance of Oxidized Electrodes
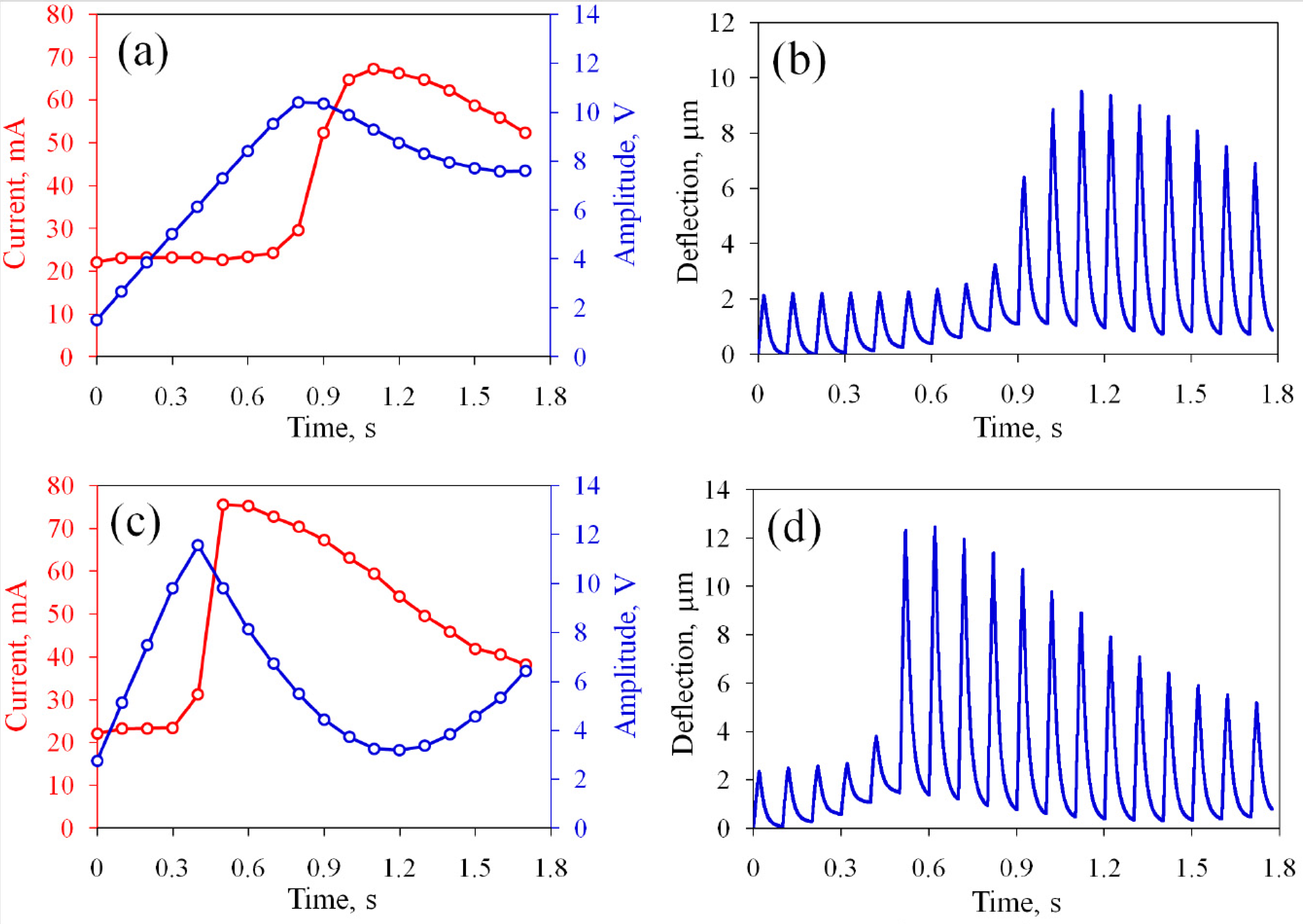
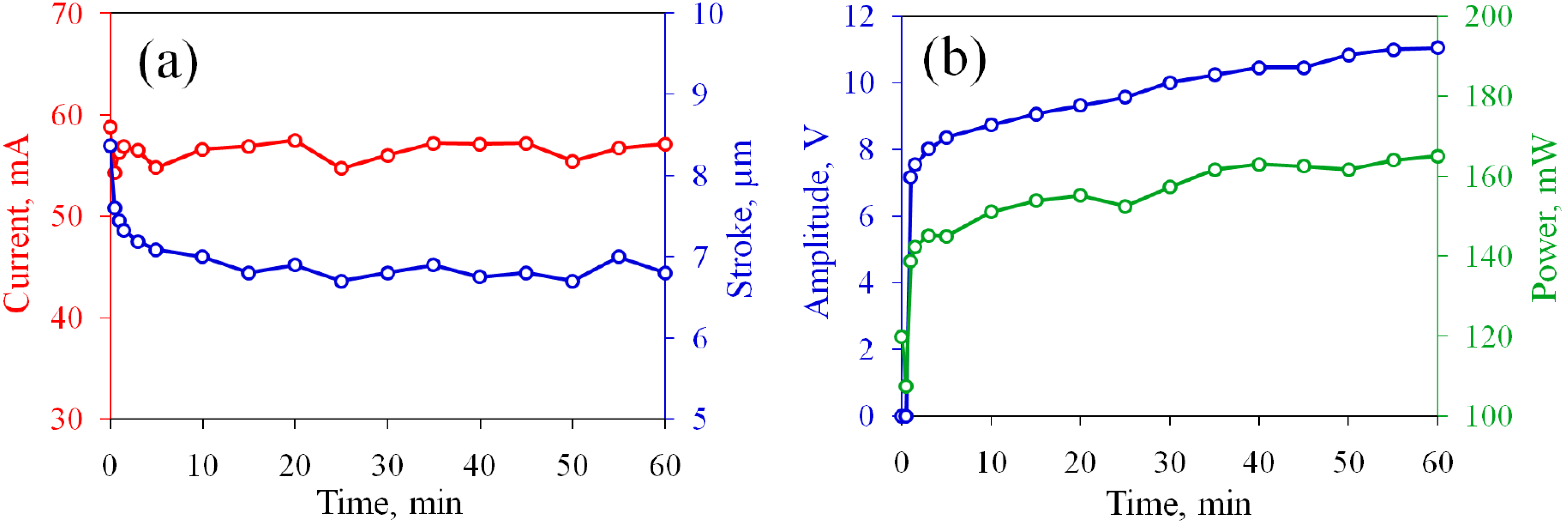
3.2. The Actuator with Non-Oxidized Electrodes
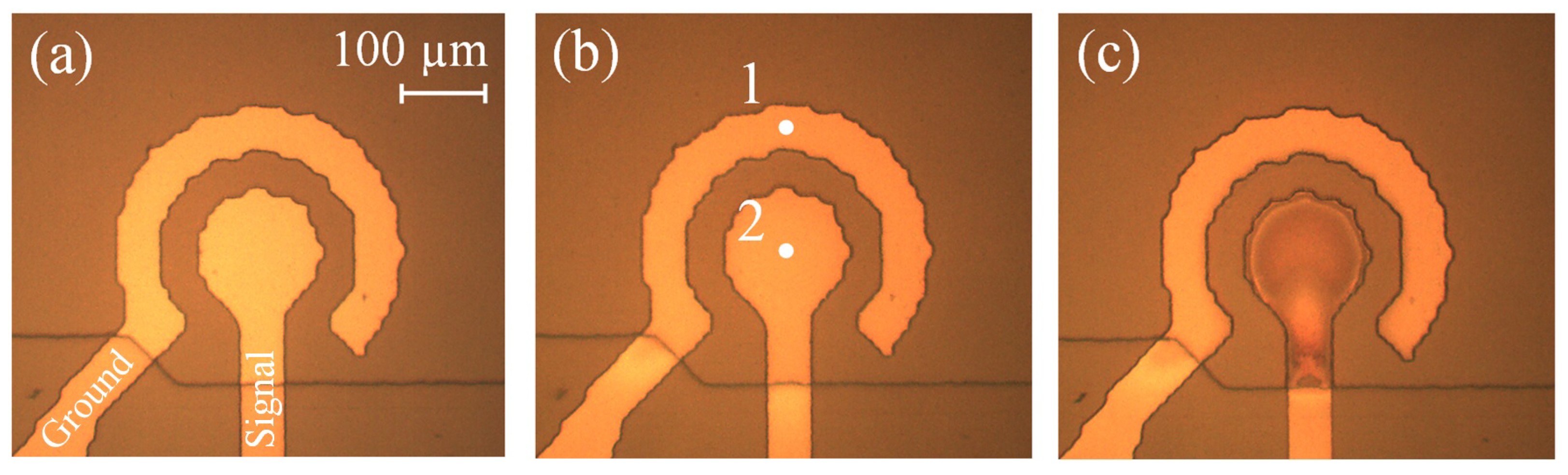
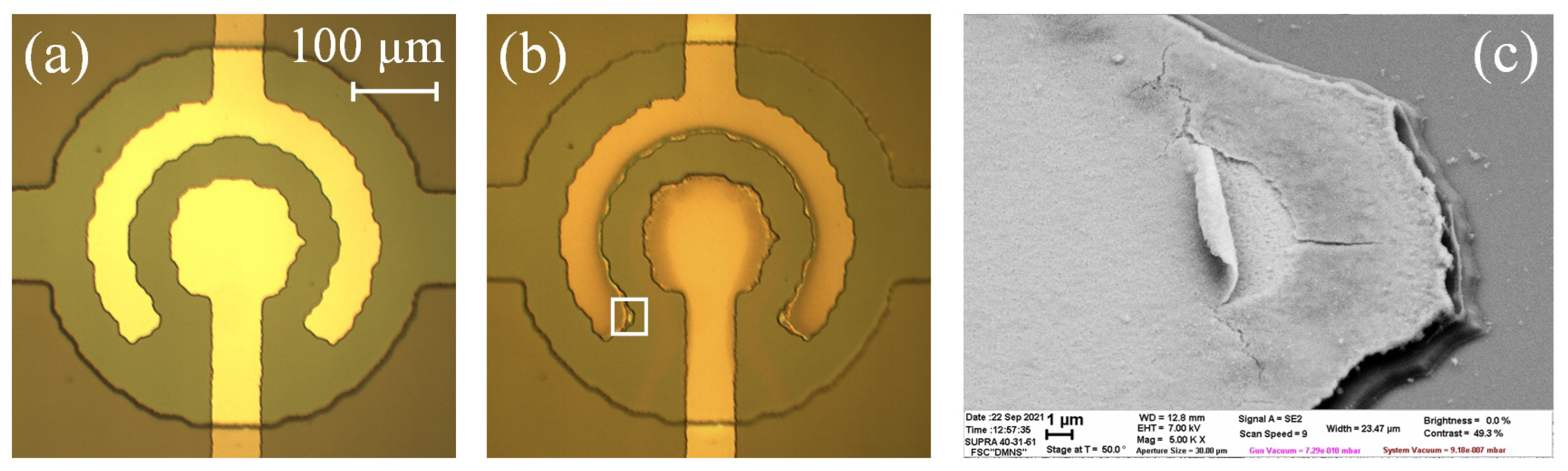
3.3. Oxidation of the Concentric Electrodes
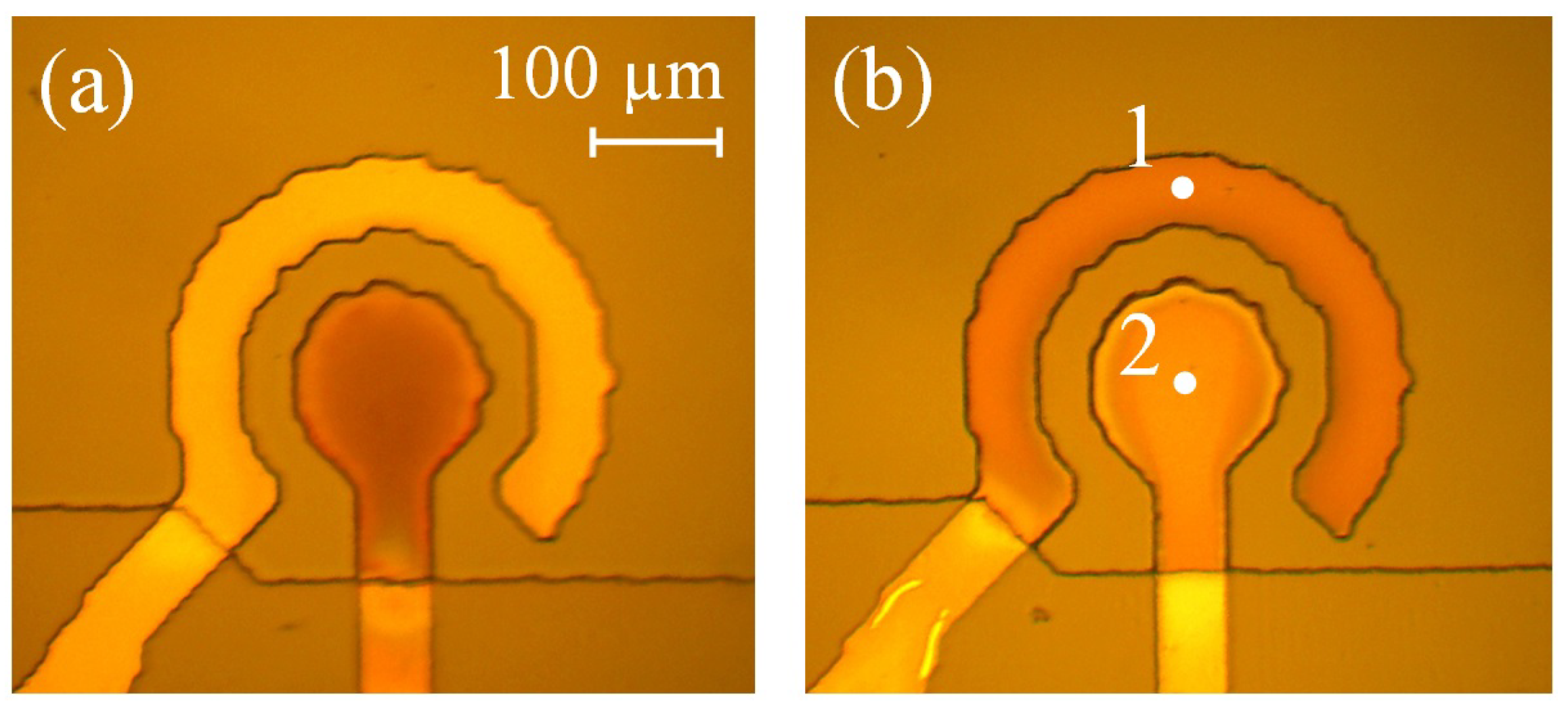
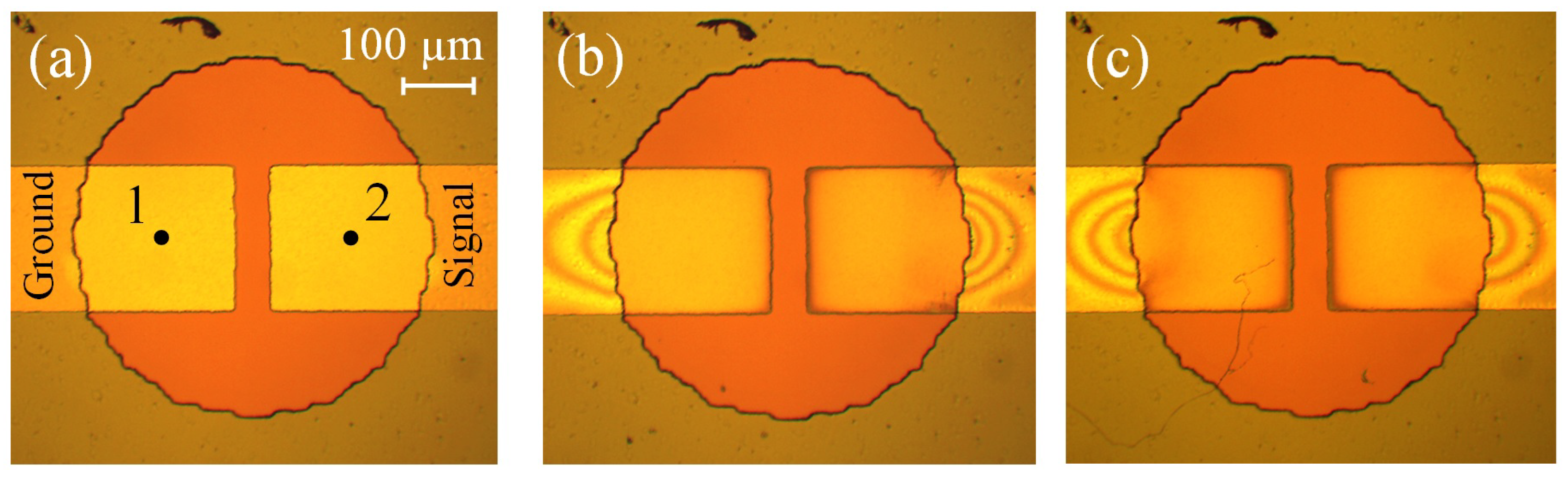
3.4. Oxidation of the Rectangular Electrodes
3.5. Damage of the Electrodes
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coluccio, M.L.; Perozziello, G.; Malara, N.; Parrotta, E.; Zhang, P.; Gentile, F.; Limongi, T.; Raj, P.M.; Cuda, G.; Candeloro, P.; et al. Microfluidic platforms for cell cultures and investigations. Microelectron. Eng. 2019, 208, 14–28. [Google Scholar] [CrossRef]
- Luo, T.; Fan, L.; Zhu, R.; Sun, D. Microfluidic Single-Cell Manipulation and Analysis: Methods and Applications. Micromachines 2019, 10, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, J.; Wang, P.; DeMello, A.; Feng, L.; Zhu, X.; Wen, W.; Kodzius, R.; Gong, X. Synthesis of Biomaterials Utilizing Microfluidic Technology. Genes 2018, 9, 283. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wang, Y.; Liu, J. Biomaterials Meet Microfluidics: From Synthesis Technologies to Biological Applications. Micromachines 2017, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- Basiri, A.; Heidari, A.; Nadi, M.F.; Fallahy, M.T.P.; Nezamabadi, S.S.; Sedighi, M.; Saghazadeh, A.; Rezaei, N. Microfluidic devices for detection of RNA viruses. Rev. Med. Virol. 2021, 31, e2154. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef]
- Villarruel Mendoza, L.A.; Scilletta, N.A.; Bellino, M.G.; Desimone, M.F.; Catalano, P.N. Recent Advances in Micro-Electro-Mechanical Devices for Controlled Drug Release Applications. Front. Bioeng. Biotechnol. 2020, 8, 827. [Google Scholar] [CrossRef]
- Pons-Faudoa, F.P.; Ballerini, A.; Sakamoto, J.; Grattoni, A. Advanced implantable drug delivery technologies: Transforming the clinical landscape of therapeutics for chronic diseases. Biomed. Microdevices 2019, 21, 47. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [Green Version]
- Sanjay, S.T.; Zhou, W.; Dou, M.; Tavakoli, H.; Ma, L.; Xu, F.; Li, X. Recent advances of controlled drug delivery using microfluidic platforms. Adv. Drug Deliv. Rev. 2018, 128, 3–28. [Google Scholar] [CrossRef]
- Asadi Dereshgi, H.; Dal, H.; Yildiz, M.Z. Piezoelectric micropumps: State of the art review. Microsyst. Technol. 2021, 27, 4127–4155. [Google Scholar] [CrossRef]
- Mohith, S.; Karanth, P.N.; Kulkarni, S.M. Experimental investigation on performance of disposable micropump with retrofit piezo stack actuator for biomedical application. Microsyst. Technol. 2019, 25, 4741–4752. [Google Scholar] [CrossRef]
- Zhao, B.; Cui, X.; Ren, W.; Xu, F.; Liu, M.; Ye, Z.G. A Controllable and Integrated Pump-enabled Microfluidic Chip and Its Application in Droplets Generating. Sci. Rep. 2017, 7, 11319. [Google Scholar] [CrossRef]
- Dumont-Fillon, D.; Tahriou, H.; Conan, C.; Chappel, E. Insulin Micropump with Embedded Pressure Sensors for Failure Detection and Delivery of Accurate Monitoring. Micromachines 2014, 5, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y. A Peristaltic Pump Integrated on a 100% Glass Microchip Using Computer Controlled Piezoelectric Actuators. Micromachines 2014, 5, 289–299. [Google Scholar] [CrossRef]
- Wang, K.; Wang, B.; Lin, K.; Li, J.; Liu, Y. Nonlinear dynamics of electrostatically actuated micro-pumps with thermal effects and filled fluids. Int. J. Non Linear Mech. 2020, 121, 103415. [Google Scholar] [CrossRef]
- Uhlig, S.; Gaudet, M.; Langa, S.; Schimmanz, K.; Conrad, H.; Kaiser, B.; Schenk, H. Electrostatically Driven In-Plane Silicon Micropump for Modular Configuration. Micromachines 2018, 9, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.; Hong, P.; Cho, C.; Lee, B.; Chun, K.; Kim, B. Four-electrode micropump with peristaltic motion. Sens. Actuators A 2016, 245, 19–25. [Google Scholar] [CrossRef]
- Kim, H.; Astle, A.A.; Najafi, K.; Bernal, L.P.; Washabaugh, P.D. An Integrated Electrostatic Peristaltic 18-Stage Gas Micropump With Active Microvalves. J. Microelectromechanical Syst. 2015, 24, 192–206. [Google Scholar] [CrossRef]
- Machauf (Prochaska), A.; Nemirovsky, Y.; Dinnar, U. A membrane micropump electrostatically actuated across the working fluid. J. Micromech. Microeng. 2005, 15, 2309–2316. [Google Scholar] [CrossRef] [Green Version]
- Yunas, J.; Mulyanti, B.; Hamidah, I.; Mohd Said, M.; Pawinanto, R.E.; Wan Ali, W.A.F.; Subandi, A.; Hamzah, A.A.; Latif, R.; Yeop Majlis, B. Polymer-Based MEMS Electromagnetic Actuator for Biomedical Application: A Review. Polymers 2020, 12, 1184. [Google Scholar] [CrossRef]
- Nakahara, T.; Ueda, Y.; Miyagawa, H.; Kotera, H.; Suzuki, T. Self-aligned fabrication process for active membrane in magnetically driven micropump using photosensitive composite. J. Micromech. Microeng. 2020, 30, 025006. [Google Scholar] [CrossRef]
- Gidde, R.R.; Pawar, P.M.; Ronge, B.P.; Dhamgaye, V.P. Design optimization of an electromagnetic actuation based valveless micropump for drug delivery application. Microsyst. Technol. 2019, 25, 509–519. [Google Scholar] [CrossRef]
- Wang, C.; Seo, S.J.; Kim, J.S.; Lee, S.H.; Jeon, J.K.; Kim, J.W.; Kim, K.H.; Kim, J.K.; Park, J. Intravitreal implantable magnetic micropump for on-demand VEGFR-targeted drug delivery. J. Control. Release 2018, 283, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; McDonald, S.J.; Kai, E.M.; Ziaie, B. A magnetically driven PDMS micropump with ball check-valves. J. Micromech. Microeng. 2005, 15, 1021–1026. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, J.; Wu, W. Theoretical and Experimental Research on Bubble Actuated Micro-Pumps. Micromachines 2018, 9, 225. [Google Scholar] [CrossRef] [Green Version]
- Hamid, N.A.; Majlis, B.Y.; Yunas, J.; Syafeeza, A.R.; Wong, Y.C.; Ibrahim, M. A stack bonded thermo-pneumatic micro-pump utilizing polyimide based actuator membrane for biomedical applications. Microsyst. Technol. 2017, 23, 4037–4043. [Google Scholar] [CrossRef]
- Chee, P.S.; Minjal, M.N.; Leow, P.L.; Ali, M.S.M. Wireless powered thermo-pneumatic micropump using frequency-controlled heater. Sens. Actuators A 2015, 233, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Spieth, S.; Schumacher, A.; Holtzman, T.; Rich, P.D.; Theobald, D.E.; Dalley, J.W.; Nouna, R.; Messner, S.; Zengerle, R. An intra-cerebral drug delivery system for freely moving animals. Biomed. Microdevices 2012, 14, 799–809. [Google Scholar] [CrossRef]
- Elman, N.M.; Ho Duc, H.L.; Cima, M.J. An implantable MEMS drug delivery device for rapid delivery in ambulatory emergency care. Biomed. Microdevices 2009, 11, 625–631. [Google Scholar] [CrossRef]
- Dong, C.W.; Tran, L.G.; Park, W.T. A polymer membrane electrolysis micropump powered by a compact wireless power transmission system. J. Mech. Sci. Technol. 2021, 35, 697–706. [Google Scholar] [CrossRef]
- Moussi, K.; AlDajani, M.; Kosel, J. Miniaturized Drug Delivery System for Biomedical Applications. In Proceedings of the 2019 IEEE 14th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Bangkok, Thailand, 11–14 April 2019; pp. 97–100. [Google Scholar] [CrossRef]
- Kim, H.; Hwang, H.; Baek, S.; Kim, D. Design, fabrication and performance evaluation of a printed-circuit-board microfluidic electrolytic pump for lab-on-a-chip devices. Sens. Actuators A 2018, 277, 73–84. [Google Scholar] [CrossRef]
- Cobo, A.; Sheybani, R.; Tu, H.; Meng, E. A wireless implantable micropump for chronic drug infusion against cancer. Sens. Actuators A 2016, 239, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, D.; Liu, X.; Chang, H. Ultra-monodisperse droplet formation using PMMA microchannels integrated with low-pulsation electrolysis micropumps. Sens. Actuators B 2016, 229, 466–475. [Google Scholar] [CrossRef]
- Sheybani, R.; Meng, E. Acceleration techniques for recombination of gases in electrolysis microactuators with Nafion-coated electrocatalyst. Sens. Actuators B 2015, 221, 914–922. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.; Buttner, U.; Carreno, A.A.A.; Conchouso, D.; Foulds, I.G. A pulsed mode electrolytic drug delivery device. J. Micromech. Microeng. 2015, 25, 105011. [Google Scholar] [CrossRef]
- Svetovoy, V.B.; Sanders, R.G.P.; Ma, K.; Elwenspoek, M.C. New type of microengine using internal combustion of hydrogen and oxygen. Sci. Rep. 2014, 4, 4296. [Google Scholar] [CrossRef] [Green Version]
- Uvarov, I.V.; Lokhanin, M.V.; Postnikov, A.V.; Melenev, A.E.; Svetovoy, V.B. Electrochemical membrane microactuator with a millisecond response time. Sens. Actuators B 2018, 260, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Postnikov, A.V.; Uvarov, I.V.; Penkov, N.V.; Svetovoy, V.B. Collective behavior of bulk nanobubbles produced by alternating polarity electrolysis. Nanoscale 2018, 10, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Svetovoy, V.B. Spontaneous chemical reactions between hydrogen and oxygen in nanobubbles. Curr. Opin. Colloid Interface Sci. 2021, 52, 101423. [Google Scholar] [CrossRef]
- Uvarov, I.V.; Shlepakov, P.S.; Postnikov, A.V.; Svetovoy, V.B. Highly energetic impact of H2 and O2 nanobubbles on Pt surface. J. Colloid Interface Sci. 2021, 582, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Shlepakov, P.S.; Uvarov, I.V.; Naumov, V.V.; Svetovoy, V.B. Degradation of Titanium Electrodes in the Alternating Polarity Electrolysis. Int. J. Electrochem. Sci. 2019, 14, 5211–5225. [Google Scholar] [CrossRef]
- Uvarov, I.V.; Melenev, A.E.; Selukov, R.V.; Svetovoy, V.B. Improving the performance of the fast electrochemical actuator. Sens. Actuators A 2020, 315, 112346. [Google Scholar] [CrossRef]
- Van Gils, S.; Mast, P.; Stijns, E.; Terryn, H. Colour properties of barrier anodic oxide films on aluminium and titanium studied with total reflectance and spectroscopic ellipsometry. Surf. Coat. Technol. 2004, 185, 303–310. [Google Scholar] [CrossRef]
- Archibald, L. Internal stresses formed during the anodic oxidation of titanium. Electrochim. Acta 1977, 22, 657–659. [Google Scholar] [CrossRef]
- Sahu, S.N.; Scarminio, J.; Decker, F. A Laser Beam Deflection System for Measuring Stress Variations in Thin Film Electrodes. J. Electrochem. Soc. 1990, 137, 1150–1154. [Google Scholar] [CrossRef]
- Nelson, J.; Oriani, R. Stress generation during anodic oxidation of titanium and aluminum. Corros. Sci. 1993, 34, 307–326. [Google Scholar] [CrossRef]
- Shibata, T.; Zhu, Y.C. The effect of film formation conditions on the structure and composition of anodic oxide films on titanium. Corros. Sci. 1995, 37, 253–270. [Google Scholar] [CrossRef]
- Ueno, K.; Pyun, S.I.; Seo, M. Stresses of a Titanium Thin-Film Electrode Generated during Anodic Oxidation by a Beam-Bending Method. J. Electrochem. Soc. 2000, 147, 4519. [Google Scholar] [CrossRef]
- Soares, P.; Mikowski, A.; Lepienski, C.M.; Santos, E., Jr.; Soares, G.A.; Filho, V.S.; Kuromoto, N.K. Hardness and elastic modulus of TiO2 anodic films measured by instrumented indentation. J. Biomed. Mater. Res. B 2008, 84B, 524–530. [Google Scholar] [CrossRef]
- Yetim, A. Investigation of wear behavior of titanium oxide films, produced by anodic oxidation, on commercially pure titanium in vacuum conditions. Surf. Coat. Technol. 2010, 205, 1757–1763. [Google Scholar] [CrossRef]

| Chemical Element | Before the Test | After the Test, Normal Regime | After the Test, New Regime | |||
|---|---|---|---|---|---|---|
| Region 1 | Region 2 | Region 1 | Region 2 | Region 1 | Region 2 | |
| C | 1.7 | 1.9 | 1.0 | 0.8 | - | 0.8 |
| O | - | - | 14.2 | 16.3 | 13.5 | 29.3 |
| Al | 0.8 | 0.8 | 0.8 | 0.7 | 0.8 | 0.7 |
| Si | 0.4 | 0.3 | 0.6 | 0.5 | 0.4 | 0.4 |
| Ti | 97.0 | 97.0 | 83.4 | 81.7 | 86.0 | 68.9 |
| Chemical Element | Positive Polarity | Negative Polarity | ||
|---|---|---|---|---|
| Region 1 | Region 2 | Region 1 | Region 2 | |
| C | 0.6 | 2.0 | - | 0.5 |
| O | 12.4 | 37.2 | 25.6 | 18.8 |
| Al | 0.7 | 0.6 | 0.7 | 0.7 |
| Si | 0.4 | 0.3 | 0.3 | 0.3 |
| Ti | 85.9 | 59.9 | 73.4 | 79.7 |
| Chemical Element | Before the Test | After the Test, Positive Polarity | After the Test, Negative Polarity | |||
|---|---|---|---|---|---|---|
| Region 1 | Region 2 | Region 1 | Region 2 | Region 1 | Region 2 | |
| C | - | - | - | - | - | - |
| O | - | - | 7.7 | 13.5 | 13.8 | 12.2 |
| Al | 0.8 | 0.7 | 0.7 | 0.7 | 0.9 | 0.8 |
| Si | 0.4 | 0.2 | 0.1 | 0.3 | 0.4 | 0.3 |
| Ti | 98.8 | 99.1 | 91.5 | 85.4 | 85.0 | 86.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uvarov, I.V.; Melenev, A.E.; Svetovoy, V.B. Fast Electrochemical Actuator with Ti Electrodes in the Current Stabilization Regime. Micromachines 2022, 13, 283. https://doi.org/10.3390/mi13020283
Uvarov IV, Melenev AE, Svetovoy VB. Fast Electrochemical Actuator with Ti Electrodes in the Current Stabilization Regime. Micromachines. 2022; 13(2):283. https://doi.org/10.3390/mi13020283
Chicago/Turabian StyleUvarov, Ilia V., Artem E. Melenev, and Vitaly B. Svetovoy. 2022. "Fast Electrochemical Actuator with Ti Electrodes in the Current Stabilization Regime" Micromachines 13, no. 2: 283. https://doi.org/10.3390/mi13020283
APA StyleUvarov, I. V., Melenev, A. E., & Svetovoy, V. B. (2022). Fast Electrochemical Actuator with Ti Electrodes in the Current Stabilization Regime. Micromachines, 13(2), 283. https://doi.org/10.3390/mi13020283








