Electrochemical Glue for Binding Chitosan–Alginate Hydrogel Fibers for Cell Culture
Abstract
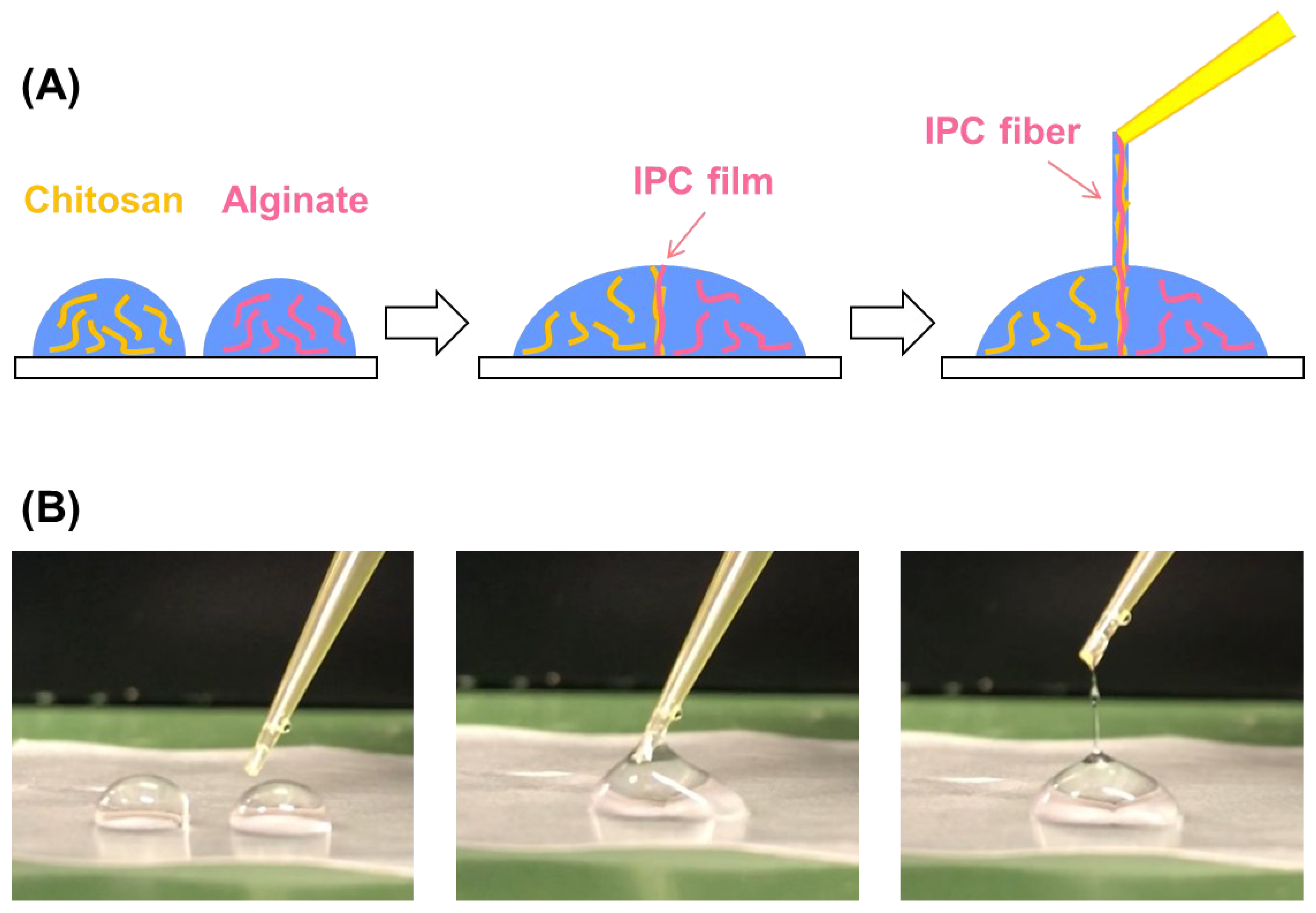
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Hydrogel Fibers
2.2. Electrochemical Gluing Induced by HClO
2.3. Electrochemical Gluing Induced by Ca2+
2.4. Cell Culture
2.5. Electrochemical Gluing Induced by Ca2+ for Binding of Hydrogel Fibers Containing HUVECs
3. Results and Discussion
3.1. Fabrication of Hydrogel Fibers
3.2. Electrochemical Gluing Induced by HClO
3.3. Electrochemical Gluing Induced by Ca2+
3.4. Electrochemical Gluing Induced by Ca2+ of Hydrogel Fibers Containing Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dababneh, A.B.; Ozbolat, I.T. Bioprinting technology: A current state-of-the-art review. J. Manuf. Sci. Eng.-Trans. ASME 2014, 136, 061016. [Google Scholar] [CrossRef]
- Chung, B.G.; Lee, K.-H.; Khademhosseini, A.; Lee, S.-H. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab Chip 2012, 12, 45–59. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhang, H.Y.; Zhan, Z.; Nan, H.C.; Huang, N.; Xu, T.; Gong, X.H.; Hu, C.Z. Mild formation of core-shell hydrogel microcapsules for cell encapsulation. Biofabrication 2021, 13, 025002. [Google Scholar] [CrossRef]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef] [Green Version]
- Yanagawa, F.; Sugiura, S.; Kanamori, T. Hydrogel microfabrication technology toward three dimensional tissue engineering. Regen. Ther. 2016, 3, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 2013, 12, 584–590. [Google Scholar] [CrossRef]
- Ino, K.; Fukuda, M.T.; Hiramoto, K.; Taira, N.; Nashimoto, Y.; Shiku, H. Fabrication of three-dimensional calcium alginate hydrogels using sacrificial templates of sugar. J. Biosci. Bioeng. 2020, 130, 539–544. [Google Scholar] [CrossRef]
- Wan, A.C.A.; Liao, I.C.; Yim, E.K.F.; Leong, K.W. Mechanism of fiber formation by interfacial polyelectrolyte complexation. Macromolecules 2004, 37, 7019–7025. [Google Scholar] [CrossRef]
- Wan, A.C.A.; Yim, E.K.F.; Liao, I.C.; Le Visage, C.; Leong, K.W. Encapsulation of biologics in self-assembled fibers as biostructural units for tissue engineering. J. Biomed. Mater. Res. Part A 2004, 71A, 586–595. [Google Scholar] [CrossRef]
- Wang, F.Y.; Liu, Z.; Wang, B.; Feng, L.H.; Liu, L.B.; Lv, F.T.; Wang, Y.L.; Wang, S. Multi-colored fibers by self-assembly of DNA, histone proteins, and cationic conjugated polymers. Angew. Chem. Int. Ed. 2014, 53, 424–428. [Google Scholar] [CrossRef]
- Leong, M.F.; Toh, J.K.C.; Du, C.; Narayanan, K.; Lu, H.F.; Lim, T.C.; Wan, A.C.A.; Ying, J.Y. Patterned prevascularised tissue constructs by assembly of polyelectrolyte hydrogel fibres. Nat. Commun. 2013, 4, 2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, A.C.A.; Cutiongco, M.F.A.; Tai, B.C.U.; Leong, M.F.; Lu, H.F.; Yim, E.K.F. Fibers by interfacial polyelectrolyte complexation—Processes, materials and applications. Mater. Today 2016, 19, 437–450. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kobayashi, Y.; Kobayashi, R.; Takashima, Y.; Hashidzume, A.; Harada, A. Photoswitchable gel assembly based on molecular recognition. Nat. Commun. 2012, 3, 603. [Google Scholar] [CrossRef]
- Asoh, T.A.; Kawai, W.; Kikuchi, A. Electrophoretic adhesion of biodegradable hydrogels through the intermediary of oppositely charged polyelectrolytes. Soft Matter 2012, 8, 1923–1927. [Google Scholar] [CrossRef]
- Phadke, A.; Zhang, C.; Arman, B.; Hsu, C.C.; Mashelkar, R.A.; Lele, A.K.; Tauber, M.J.; Arya, G.; Varghese, S. Rapid self-healing hydrogels. Proc. Natl. Acad. Sci. USA 2012, 109, 4383–4388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.Y.; Antoniadou, E.; Lee, M.; Jeong, J.H.; Ahmed, W.W.; Saif, T.A.; Boppart, S.A.; Kong, H. Tailoring hydrogel adhesion to polydimethylsiloxane substrates using polysaccharide glue. Angew. Chem. Int. Ed. 2013, 52, 6949–6952. [Google Scholar] [CrossRef]
- Saito, J.; Furukawa, H.; Kurokawa, T.; Kuwabara, R.; Kuroda, S.; Hu, J.; Tanaka, Y.; Gong, J.P.; Kitamura, N.; Yasuda, K. Robust bonding and one-step facile synthesis of tough hydrogels with desirable shape by virtue of the double network structure. Polym. Chem. 2011, 2, 575–580. [Google Scholar] [CrossRef]
- Nagamine, K.; Okamoto, K.; Kaji, H.; Nishizawa, M. Bonding of synthetic hydrogels with fibrin as the glue to engineer hydrogel-based biodevices. J. Biosci. Bioeng. 2014, 118, 94–97. [Google Scholar] [CrossRef]
- Hong, S.H.; Shin, M.; Park, E.; Ryu, J.H.; Burdick, J.A.; Lee, H. Alginate-boronic acid: pH-triggered bioinspired glue for hydrogel assembly. Adv. Funct. Mater. 2020, 30, 1908497. [Google Scholar] [CrossRef]
- Ino, K.; Matsumoto, T.; Taira, N.; Kumagai, T.; Nashimoto, Y.; Shiku, H. Hydrogel electrodeposition based on bipolar electrochemistry. Lab Chip 2018, 18, 2425–2432. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Ozawa, F.; Dang, N.; Hiramoto, K.; Hino, S.; Akasaka, R.; Nashimoto, Y.; Shiku, H. Biofabrication using electrochemical devices and systems. Adv. Biosyst. 2020, 4, 1900234. [Google Scholar] [CrossRef] [PubMed]
- Ino, K.; Terauchi, M.; Gakumasawa, M.; Taira, N.; Suda, A.; Kunikata, R.; Matsue, T.; Shiku, H. Local hydrogel fabrication based on electrodeposition with a large-scale integration (LSI)-based amperometric device. Sens. Actuator B Chem. 2018, 277, 95–101. [Google Scholar] [CrossRef]
- Ozawa, F.; Ino, K.; Arai, T.; Ramon-Azcon, J.; Takahashi, Y.; Shiku, H.; Matsue, T. Alginate gel microwell arrays using electrodeposition for three-dimensional cell culture. Lab Chip 2013, 13, 3128–3135. [Google Scholar] [CrossRef]
- Ozawa, F.; Ino, K.; Shiku, H.; Matsue, T. Electrochemical hydrogel lithography of calcium-alginate hydrogels for cell culture. Materials 2016, 9, 744. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, F.; Ino, K.; Shiku, H.; Matsue, T. Cell sheet fabrication using RGD peptide-coupled alginate hydrogels fabricated by an electrodeposition method. Chem. Lett. 2017, 46, 605–608. [Google Scholar] [CrossRef]
- Ozawa, F.; Ino, K.; Takahashi, Y.; Shiku, H.; Matsue, T. Electrodeposition of alginate gels for construction of vascular-like structures. J. Biosci. Bioeng. 2013, 115, 459–461. [Google Scholar] [CrossRef]
- Taira, N.; Ino, K.; Ida, H.; Nashimoto, Y.; Shiku, H. Electrodeposition-based rapid bioprinting of 3D-designed hydrogels with a pin art device. Biofabrication 2019, 11, 035018. [Google Scholar] [CrossRef]
- Taira, N.; Ino, K.; Kumagai, T.; Nashimoto, Y.; Shiku, H. Electrochemical fabrication of fibrin gels via cascade reaction for cell culture. Chem. Commun. 2019, 55, 5335–5338. [Google Scholar] [CrossRef]
- Taira, N.; Ino, K.; Robert, J.; Shiku, H. Electrochemical printing of calcium alginate/gelatin hydrogel. Electrochim. Acta 2018, 281, 429–436. [Google Scholar] [CrossRef]
- Tamura, A.; Hiramoto, K.; Ino, K.; Taira, N.; Nashimoto, Y.; Shiku, H. Genipin crosslinking of electrodeposited chitosan/gelatin hydrogels for cell culture. Chem. Lett. 2019, 48, 1178–1180. [Google Scholar] [CrossRef]
- Dang, N.; Etienne, M.; Walcarius, A.; Liu, L. Scanning gel electrochemical microscopy (SGECM): Lateral physical resolution by current and shear force feedback. Anal. Chem. 2020, 92, 6415–6422. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, D.M.; Capuano, J.A.; Corr, D.T. On-demand radial electrodeposition of alginate tubular structures. ACS Biomater. Sci. Eng. 2019, 5, 3184–3189. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Liu, Y.; Wan, W.; Hu, C.; Liu, Z.; Wong, C.T.; Fukuda, T.; Shen, Y. Hybrid 3D printing and electrodeposition approach for controllable 3D alginate hydrogel formation. Biofabrication 2017, 9, 025032. [Google Scholar] [CrossRef]
- Yan, K.; Wan, Y.; Yang, C.; Chen, Y.; Wei, W.; Li, X.; Wang, D. Rational programming of polysaccharide-based double network hydrogel with heterogeneous architecture and multifunction via electrical signal/temperature triggered sequential self-assembly. Compos. Part B Eng. 2021, 226, 109343. [Google Scholar] [CrossRef]
- Xie, F.; Li, C.Y.; Hua, X.Q.; Ma, L. Biofabrication of controllable alginate hydrogel cell scaffolds based on bipolar electrochemistry. J. Bioact. Compat. Polym. 2021, 36, 497–509. [Google Scholar] [CrossRef]
- Xie, F.; Cao, H.; Ma, L.; Hua, X.; Li, C. Biofabrication of controllable tubular calcium alginate hydrogel for tissue engineering. J. Mater. Res. 2021, 36, 1487–1495. [Google Scholar] [CrossRef]
- Liu, Z.; Takeuchi, M.; Nakajima, M.; Hasegawa, Y.; Huang, Q.; Fukuda, T. Shape-controlled high cell-density microcapsules by electrodeposition. Acta Biomater. 2016, 37, 93–100. [Google Scholar] [CrossRef]
- Lei, M.; Qu, X.; Liu, H.; Liu, Y.; Wang, S.; Wu, S.; Bentley, W.E.; Payne, G.F.; Liu, C. Programmable electrofabrication of porous Janus films with tunable Janus balance for anisotropic cell guidance and tissue regeneration. Adv. Funct. Mater. 2019, 29, 1900065. [Google Scholar] [CrossRef]
- Cross, E.R. The electrochemical fabrication of hydrogels: A short review. SN Appl. Sci. 2020, 2, 397. [Google Scholar] [CrossRef] [Green Version]
- Gray, K.M.; Liba, B.D.; Wang, Y.F.; Cheng, Y.; Rubloff, G.W.; Bentley, W.E.; Montembault, A.; Royaud, I.; David, L.; Payne, G.F. Electrodeposition of a biopolymeric hydrogel: Potential for one-step protein electroaddressing. Biomacromolecules 2012, 13, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H.; Kanada, M.; Oyamatsu, D.; Matsue, T.; Nishizawa, M. Microelectrochemical approach to induce local cell adhesion and growth on substrates. Langmuir 2004, 20, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H.; Tsukidate, K.; Hashimoto, M.; Matsue, T.; Nishizawa, M. Patterning the surface cytophobicity of an albumin-physisorbed substrate by electrochemical means. Langmuir 2005, 21, 6966–6969. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H.; Tsukidate, K.; Matsue, T.; Nishizawa, M. In situ control of cellular growth and migration on substrates using microelectrodes. J. Am. Chem. Soc. 2004, 126, 15026–15027. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Z.; Rzasa, J.R.; Kim, E.; Li, J.; VanArsdale, E.; Bentley, W.E.; Shi, X.; Payne, G.F. Hydrogel patterning with catechol enables networked electron flow. Adv. Funct. Mater. 2021, 31, 2007709. [Google Scholar] [CrossRef]






| Applied Time (min) | Cl− Concentration | ||
|---|---|---|---|
| 0.15 M | 0.30 M | 0.50 M | |
| 1 | 0/3 | 0/3 | 2/6 |
| 2 | 0/3 | 0/3 | 2/10 |
| 3 | 0/3 | 1/4 | 3/6 |
| 5 | 1/4 | 3/3 | 3/4 |
| 10 | 0/3 | 3/3 | 3/3 |
| 15 | 0/3 | 2/3 | 3/3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utagawa, Y.; Ino, K.; Kumagai, T.; Hiramoto, K.; Takinoue, M.; Nashimoto, Y.; Shiku, H. Electrochemical Glue for Binding Chitosan–Alginate Hydrogel Fibers for Cell Culture. Micromachines 2022, 13, 420. https://doi.org/10.3390/mi13030420
Utagawa Y, Ino K, Kumagai T, Hiramoto K, Takinoue M, Nashimoto Y, Shiku H. Electrochemical Glue for Binding Chitosan–Alginate Hydrogel Fibers for Cell Culture. Micromachines. 2022; 13(3):420. https://doi.org/10.3390/mi13030420
Chicago/Turabian StyleUtagawa, Yoshinobu, Kosuke Ino, Tatsuki Kumagai, Kaoru Hiramoto, Masahiro Takinoue, Yuji Nashimoto, and Hitoshi Shiku. 2022. "Electrochemical Glue for Binding Chitosan–Alginate Hydrogel Fibers for Cell Culture" Micromachines 13, no. 3: 420. https://doi.org/10.3390/mi13030420
APA StyleUtagawa, Y., Ino, K., Kumagai, T., Hiramoto, K., Takinoue, M., Nashimoto, Y., & Shiku, H. (2022). Electrochemical Glue for Binding Chitosan–Alginate Hydrogel Fibers for Cell Culture. Micromachines, 13(3), 420. https://doi.org/10.3390/mi13030420








