In-Plane Si Microneedles: Fabrication, Characterization, Modeling and Applications
Abstract
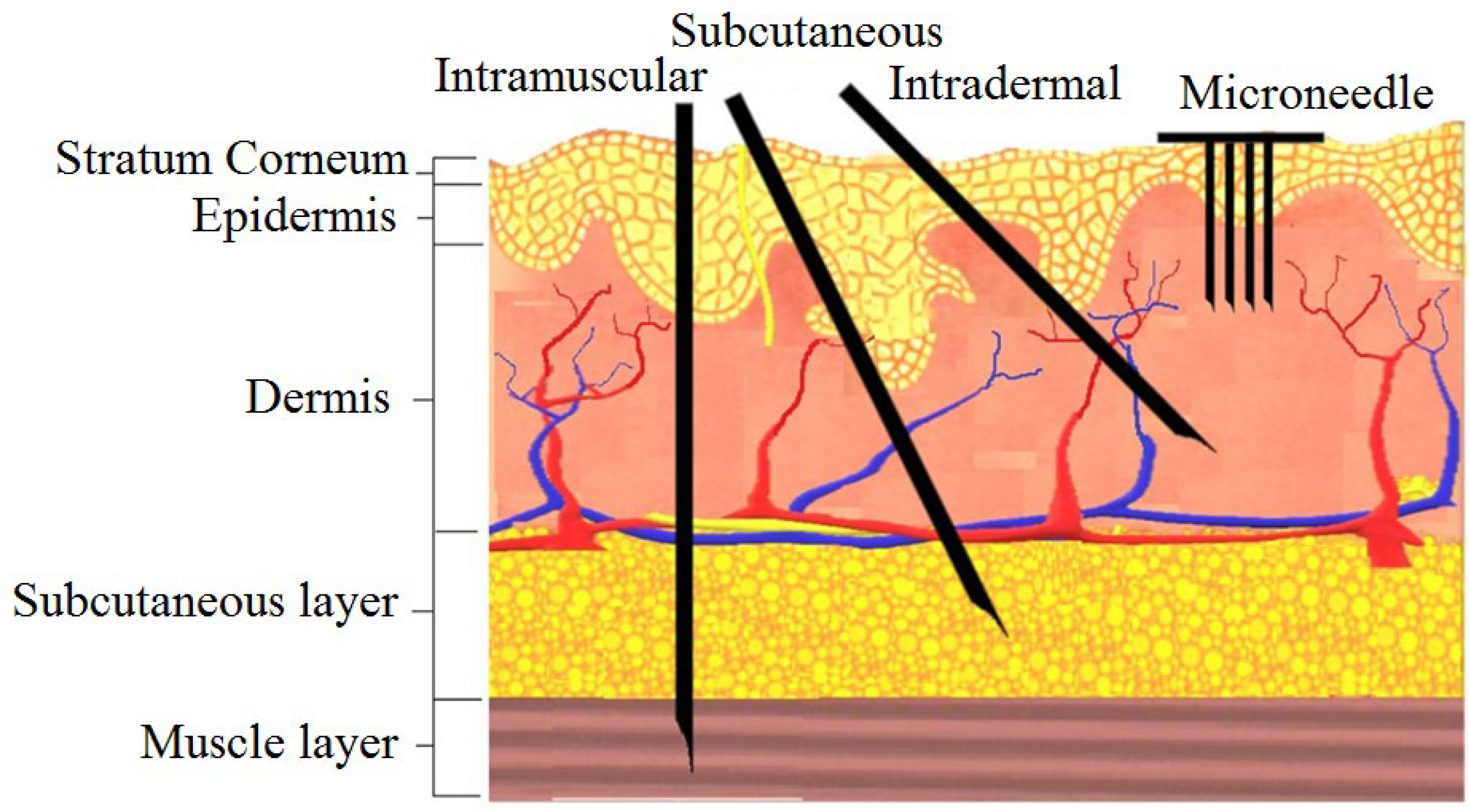
1. Introduction
2. Design
3. Fabrication
3.1. Etching Mask Materials
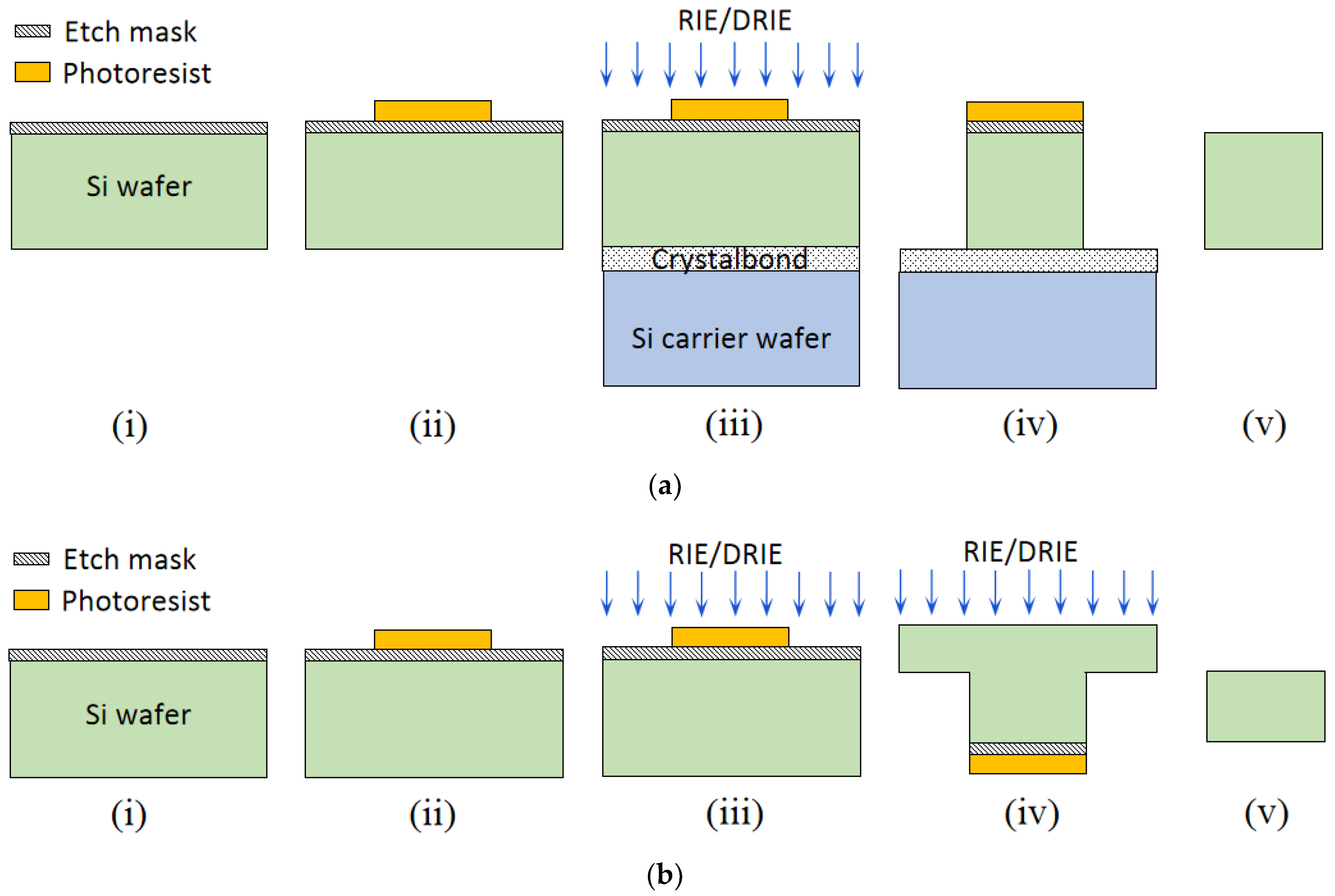
3.2. Pattern Transfer
3.3. Etching
3.3.1. TMAH Etching
3.3.2. EDP Etching
3.3.3. KOH Etching
3.3.4. HF and HNO3 Etching
3.3.5. RIE and DRIE Etching
3.4. Coating
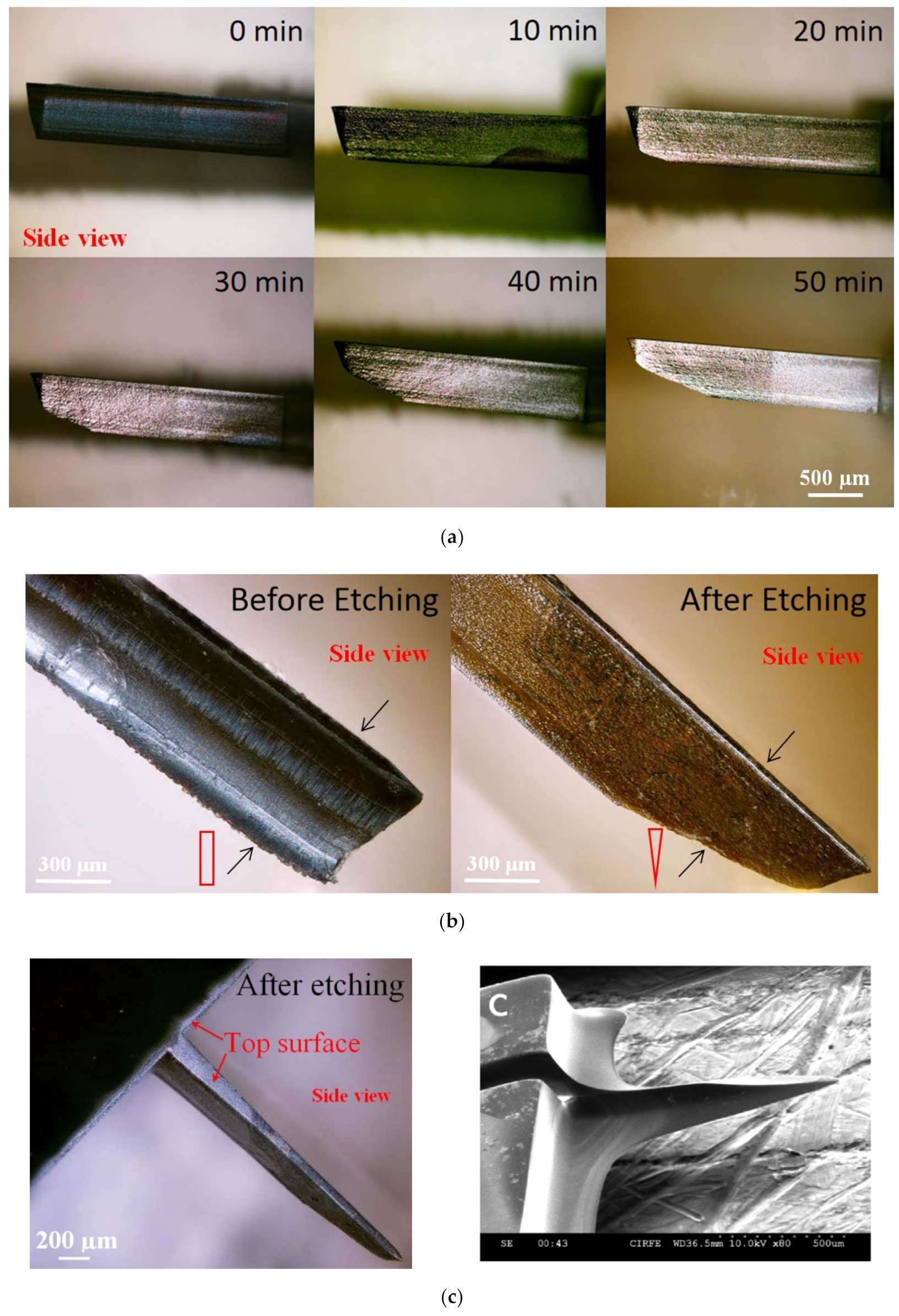
3.5. Tip Sharpening
4. Analysis
4.1. Theoretical Analysis
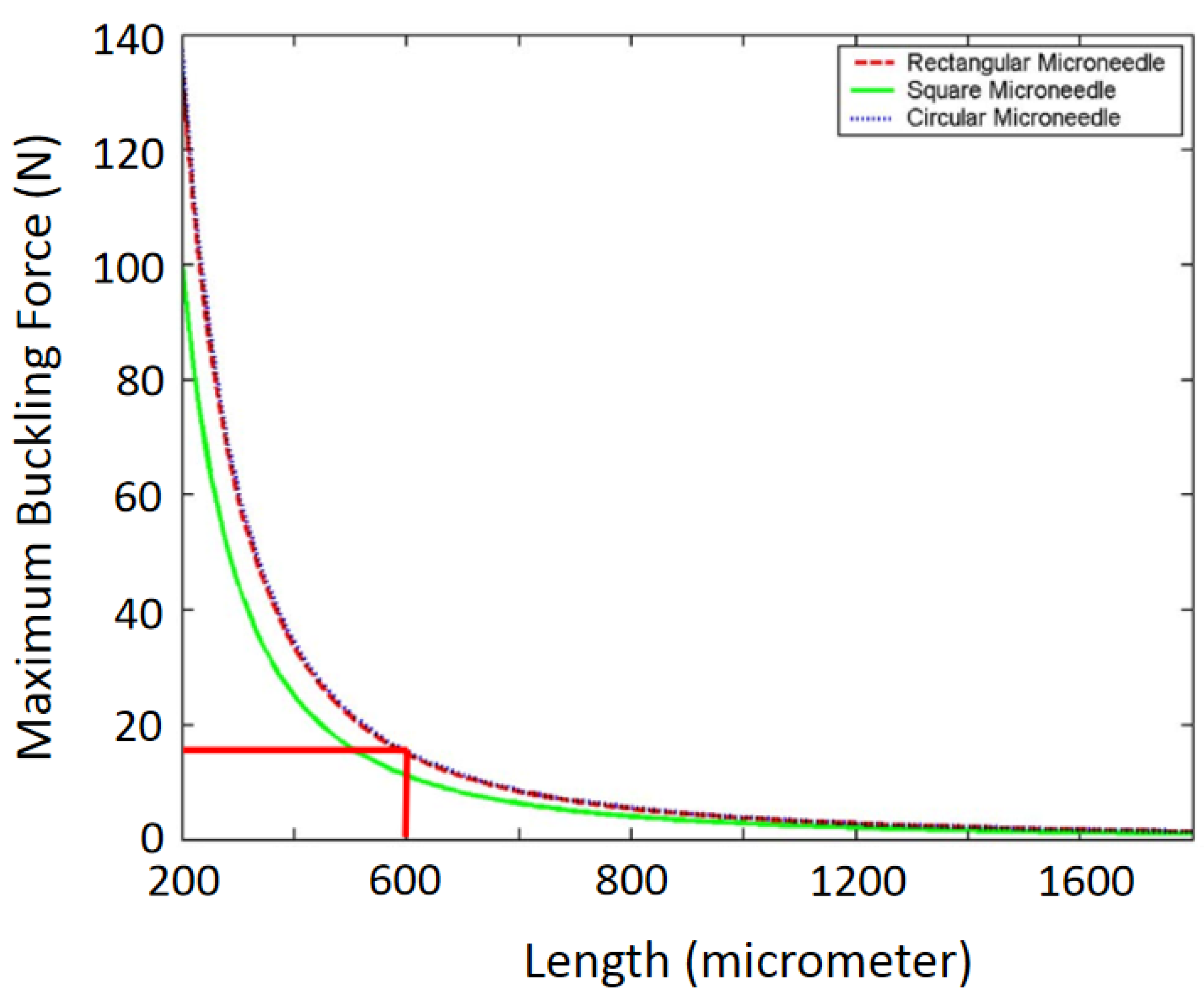
4.1.1. Mechanical Strength Analysis
- (a)
- Buckling Force
- (b)
- Free Bending Force
- (c)
- Constrained Bending Force
- (d)
- Compressive Force
- (e)
- Shear Force
- (f)
- Penetration Force
4.1.2. Microfluidic Analysis
4.2. Computational Analysis
4.2.1. Structural Analysis
4.2.2. Computational Fluid Dynamics
- The flow rate increases with inlet pressure;
- The flow rate is slightly less in numerical analysis than in theoretical analysis. It is because frictional losses were not considered during numerical analysis;
- Pressure drop increases with flow rate and inlet pressure.
4.3. Experimental Analysis
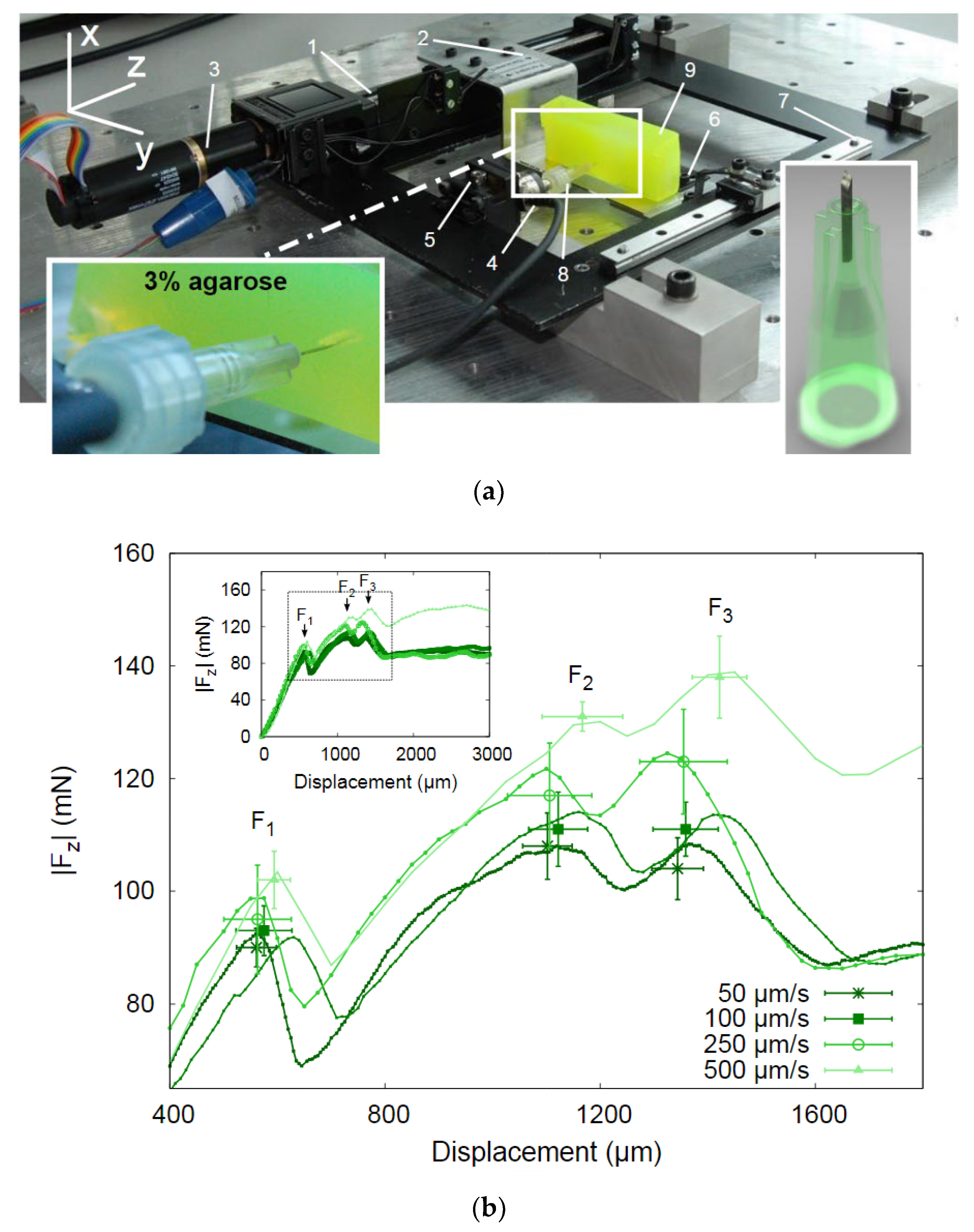
4.3.1. Mechanical Strength
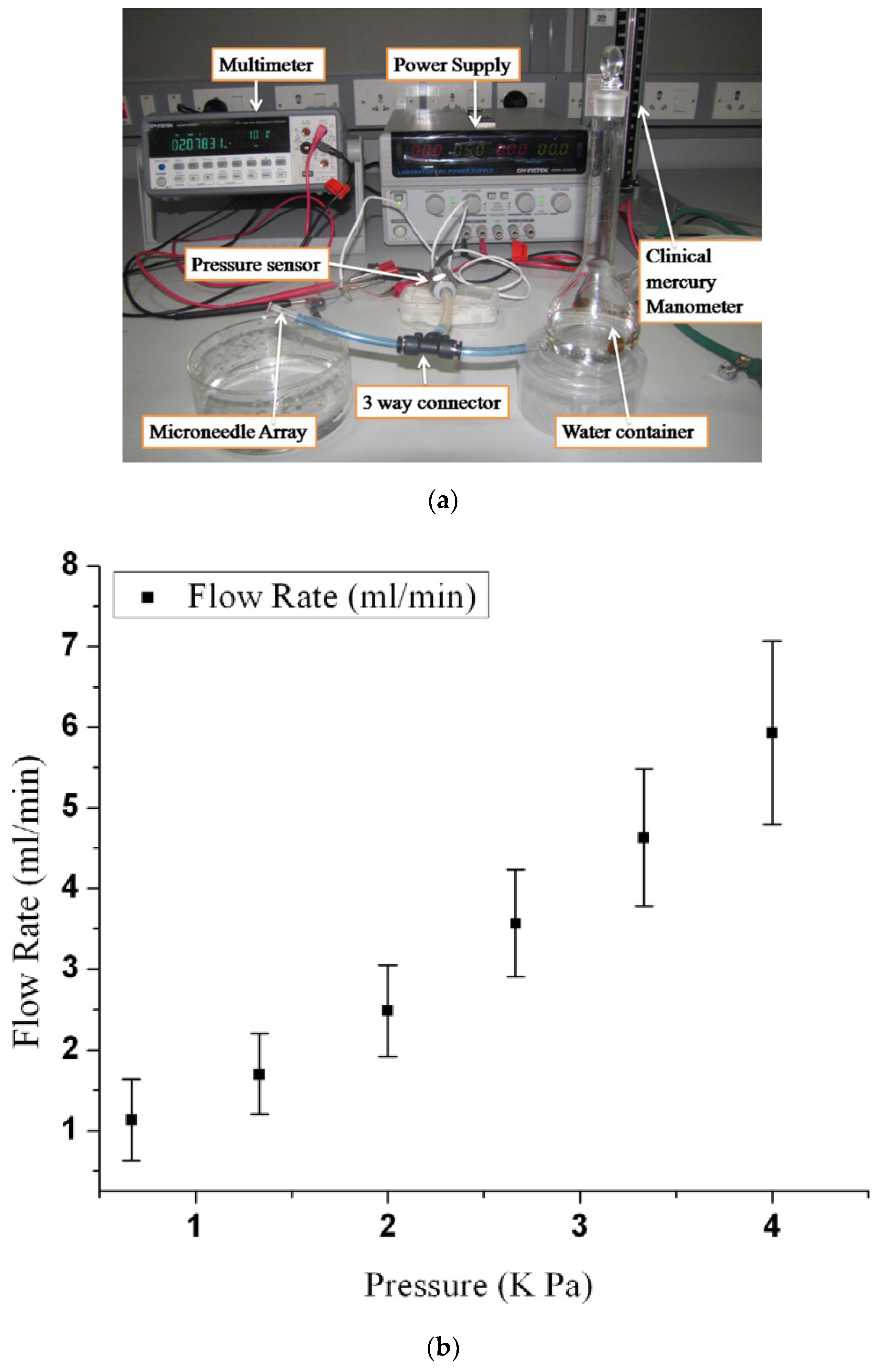
4.3.2. Experiment on Fluid Flow
5. Applications
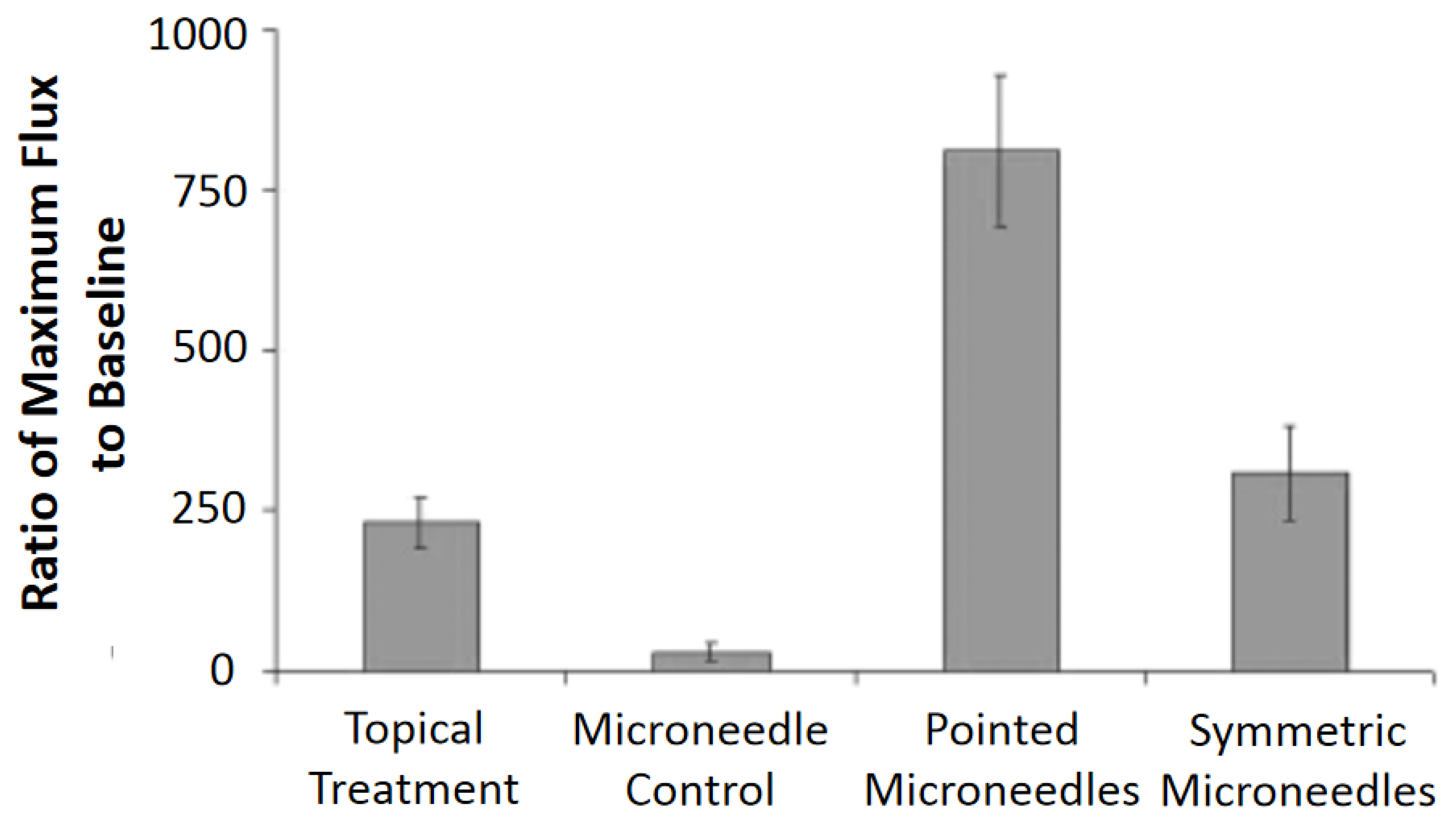
5.1. Drug Delivery
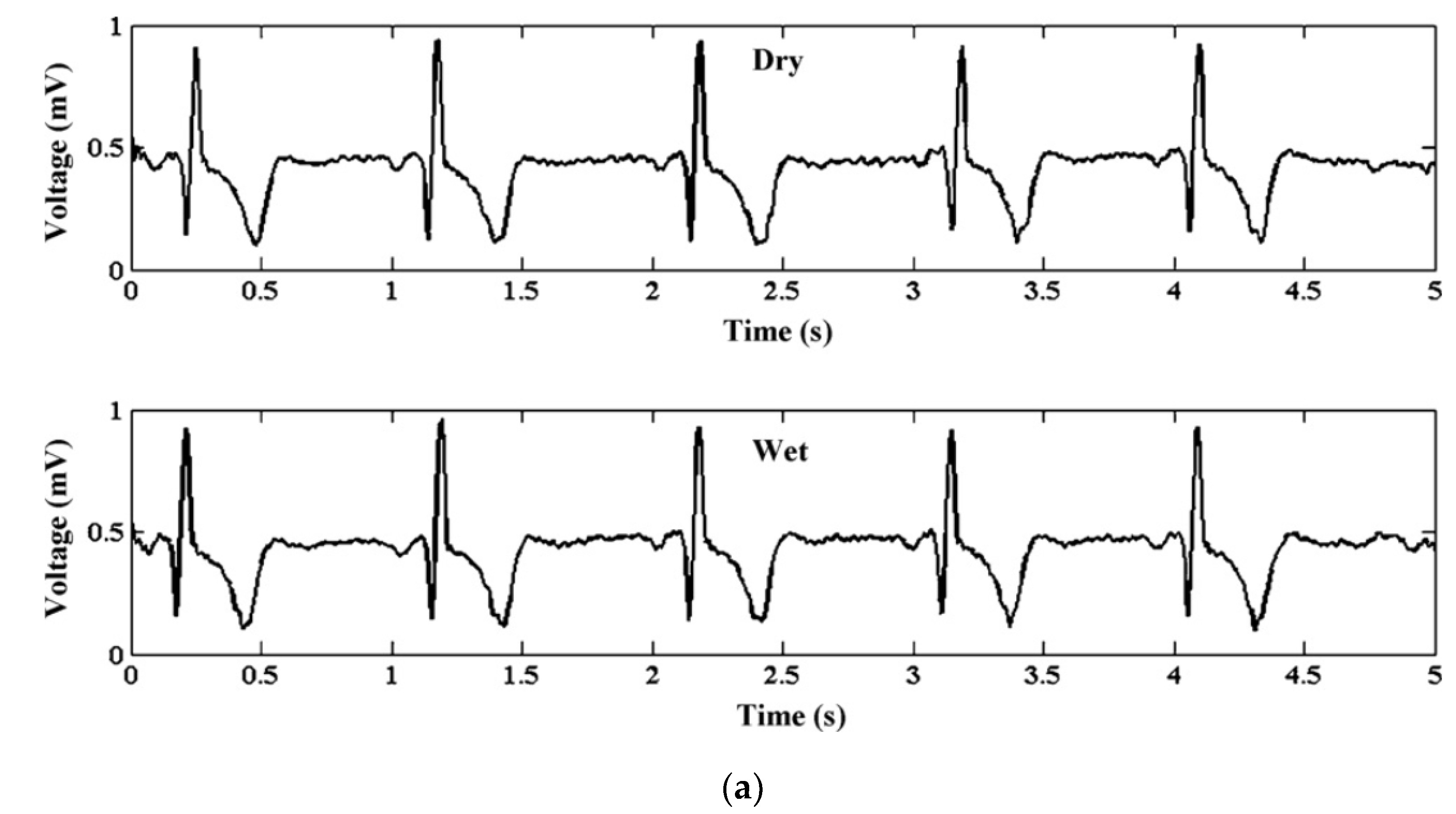
5.2. Bio-Signal Monitoring
5.3. Bio-Markers and Drug Monitoring
5.4. Pediatrics
5.5. Delivery of Peptides
5.6. Neural Implant
6. Future Prospects and Challenges
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zahn, J.D.; Talbot, N.H.; Liepmann, D.; Pisano, A.P.; Liepmann, D.; Pisano, A.P. Microfabricated polysilicon microneedles for minimally invasive devices. Biomed. Microdevices 2000, 2, 295–303. [Google Scholar] [CrossRef]
- Aggarwal, P.; Johnston, C.R. Geometrical effects in mechanical characterizing of microneedle for biomedical applications. Sens. Actuators B Chem. 2004, 102, 226–234. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Yang, R.; Tazrin, F.; Zhu, C.; Kaddoura, M.; Blondeel, E.J.M.M.; Cui, B. In-plane silicon microneedles with open capillary microfluidic networks by deep reactive ion etching and sacrificial layer based sharpening. Sens. Actuators A Phys. 2019, 292, 149–157. [Google Scholar] [CrossRef]
- Zahn, J.D.; Deshmukh, A.; Pisano, A.P.; Liepmann, D. Continuous on-chip micropumping for microneedle enhanced drug delivery. Biomed. Microdevices 2004, 6, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef]
- Doraiswamy, A.; Ovsianikov, A.; Gittard, S.D.; Monteiro-Riviere, N.A.; Crombez, R.; Montalvo, E.; Shen, W.; Chichkov, B.N.; Narayan, R.J. Fabrication of microneedles using two photon polymerization for transdermal delivery of nanomaterials. J. Nanosci. Nanotechnol. 2010, 10, 6305–6312. [Google Scholar] [CrossRef]
- Jiang, J.; Gill, H.S.; Ghate, D.; McCarey, B.E.; Patel, S.R.; Edelhauser, H.F.; Prausnitz, M.R. Coated microneedles for drug delivery to the eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4038–4043. [Google Scholar] [CrossRef]
- Gerstel, M.S.; Virgil, A.P.; Place, V.A. Drug delivery device. US Patent No: 3964482, 22 June 1976. [Google Scholar]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef]
- Yun, S.S.; An, J.Y.; Moon, S.H.; Lee, J.H. In-plane microneedle chip fabricated by crystalline wet etching of (110) silicon wafer. Transducers. In Proceedings of the 15th International Conference on Solid-State Sensors, Actuators Microsystems, Denver, CO, USA, 21–25 June 2009; IEEE: Manhattan, NY, USA, 2009; pp. 204–207. [Google Scholar] [CrossRef]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef]
- Mukerjee, E.V.; Collins, S.D.; Isseroff, R.R.; Smith, R.L. Microneedle array for transdermal biological fluid extraction and in situ analysis. Sens. Actuators A Phys. 2004, 114, 267–275. [Google Scholar] [CrossRef]
- Zhang, S.; Song, Y.; Wang, M.; Xiao, G.; Gao, F.; Li, Z.; Tao, G.; Zhuang, P.; Yue, F.; Chan, P.; et al. Real-time simultaneous recording of electrophysiological activities and dopamine overflow in the deep brain nuclei of a non-human primate with parkinson’s disease using nano-based microelectrode arrays. Microsyst. Nanoeng. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Ren, L.; Liu, B.; Zhou, W.; Jiang, L. A mini review of microneedle array electrode for bio-signal recording: A review. IEEE Sens. J. 2020, 20, 577–590. [Google Scholar] [CrossRef]
- Paik, S.J.; Byun, S.; Lim, J.M.; Park, Y.; Lee, A.; Chung, S.; Chang, J.; Chun, K.; Cho, D.; Dan, D. In-plane single-crystal-silicon microneedles for minimally invasive microfluid systems. Sens. Actuators A Phys. 2004, 114, 276–284. [Google Scholar] [CrossRef]
- Jung, M.; Jeong, D.; Yun, S.S.; Lee, J.H.; Sik, S.; Jong, Y.; Lee, H.; Yun, S.S.; Lee, J.H. Fabrication of a 2-D in-plane microneedle array integrated with microfluidic components using crystalline wet etching of (110) silicon. Microsyst. Technol. 2016, 22, 2287–2294. [Google Scholar] [CrossRef]
- Jin, C.Y.; Han, M.H.; Lee, S.S.; Choi, Y.H. Mass producible and biocompatible microneedle patch and functional verification of its usefulness for transdermal drug delivery. Biomed. Microdevices 2009, 11, 1195–1203. [Google Scholar] [CrossRef]
- Bodhale, D.W.; Nisar, A.; Afzulpurkar, N.; Nisar, Æ.A.; Nisar, A.; Afzulpurkar, N. Structural and microfluidic analysis of hollow side-open polymeric microneedles for transdermal drug delivery applications. Microfluid. Nanofluidics 2010, 8, 373–392. [Google Scholar] [CrossRef]
- Uenishi, Y.; Istigai, M.; Mehregany, M. Micro-opto-mechanical devices fabricated by anisotropic etching of (110) silicon. J. Micromech. Microeng. 1995, 5, 305–312. [Google Scholar] [CrossRef]
- Park, J.H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66. [Google Scholar] [CrossRef]
- Sparks, D.; Hubbard, T. Micromachined needles and lancets with design adjustable bevel angles. J. Micromech. Microeng. 2004, 14, 1230–1233. [Google Scholar] [CrossRef]
- Koelmans, W.W.; Krishnamoorthy, G.; Heskamp, A.; Wissink, J.; Misra, S.; Tas, N. Microneedle characterization using a double-layer skin simulant. Mech. Eng. Res. 2013, 3, 51. [Google Scholar] [CrossRef][Green Version]
- Bhuyan, M.K.; Rodriguez-Devora, J.I.; Fraser, K.; Tseng, T.L.B. Silicon substrate as a novel cell culture device for myoblast cells. J. Biomed. Sci. 2014, 21, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Pisano, A.P. Silicon-processed microneedles. J. Microelectromech. Syst. 1999, 8, 78–84. [Google Scholar] [CrossRef]
- Bodhale, D.W.; Nisar, A.; Afzulpurkar, N. Design, fabrication and analysis of silicon microneedles for transdermal drug delivery applications. IFMBE Proc. 2010, 27, 84–89. [Google Scholar] [CrossRef]
- Le-Thanh, H.; Tran-Minh, N.; The, H.L.; Karlsen, F.; Le The, H.; Karlsen, F. A novel design of hollow microneedle for blood sample collection. In Proceedings of the 9th IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Waikiki Beach, HI, USA, 13–16 April 2014; IEEE-NEMS: Manhattan, NY, USA, 2014; pp. 430–435. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Aggarwal, P.; Kaler, K.V.I.S.; Badawy, W. Design and implementation of mems based micro-needles for biomedical applications. Can. Conf. Electr. Comput. Eng. 2003, 3, 1505–1508. [Google Scholar] [CrossRef]
- Ganesan, A.V.; Kumar, H.; Swaminathan, S.; Singh, K.K.; Joy, R.A.; Sood, N.; Gokhale, T.; Mittal, R.K. Analysis of MEMS-based microneedles for blood monitoring. Bionanoscience 2014, 4, 128–135. [Google Scholar] [CrossRef]
- Khumpuang, S.; Maeda, R.; Sugiyama, S. Design and Fabrication of a coupled microneedle array and insertion guide array for safe penetration through skin. In Proceedings of the 2003 International Symposium on Micromechatronics and Human Science, Nagoya, Japan, 19–22 October 2003; IEEE: Manhattan, NY, USA, 2003; Volume 1, pp. 233–237. [Google Scholar] [CrossRef]
- Seidl, K.; Spieth, S.; Herwik, S.; Steigert, J.; Zengerle, R.; Paul, O.; Ruther, P. In-plane silicon probes for simultaneous neural recording and drug delivery. J. Micromech. Microeng. 2010, 20, 105006. [Google Scholar] [CrossRef]
- Spence, A.; Lence, R. Basic Human Anatomy; Benjamin-Cumming Publishing Company: San Fransico, CA, USA, 1982. [Google Scholar]
- Wang, L.F.; Liu, J.Q.; Yan, X.X.; Yang, B.; Yang, C.S. A MEMS-based pyramid micro-needle electrode for long-term EEG measurement. Microsyst. Technol. 2013, 19, 269–276. [Google Scholar] [CrossRef]
- Barrett, K.; Heddwen, B.; Boitano, S.; Barman, S. Ganong’s Review of Medical Physiology; McGraw Hill: New York, NY, USA, 2010. [Google Scholar]
- Brown, S.; Zambrana, P.N.; Ge, X.; Bagdure, D.; Stinchcomb, A.L.; Rao, G.; Tolosa, L. Minimally Invasive technique for measuring transdermal glucose with a fluorescent biosensor. Anal. Bioanal. Chem. 2018, 410, 7249–7260. [Google Scholar] [CrossRef]
- Quinn, H.L.; Kearney, M.-C.; Courtenay, A.J.; McCrudden, M.T.C.; Donnelly, R.F. The role of microneedles for drug and vaccine delivery. Expert Opin. Drug Deliv. 2014, 11, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, R.; Singh, M.; Nguyen, H.X.; Jonnalagadda, S. A review of recent advances in microneedle technology for transdermal drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101923. [Google Scholar] [CrossRef]
- Won Ban, J.; Koo, K.; Park, S.; Kim, G.; Jeon, D.; Cho, D. Il Microneedle system for localized drug injection using embedded microfluid source. Sens. Mater. 2007, 19, 453–464. [Google Scholar]
- Jeong, D.-H.H.; Myung Kim, J.; Noh, D.Y.; Hyun Kim, K.; Lee, J.-H.H. Micromachined anti-scatter grid fabricated using crystalline wet etching of (1 1 0) silicon and metal electroplating for X-ray imaging. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 652, 846–849. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Yang, R.; Laffitte, Y.; Schmill, U.; Hu, W.; Kaddoura, M.; Blondeel, E.J.M.; Cui, B. Fabrication of sharp silicon hollow microneedles by deep-reactive ion etching towards minimally invasive diagnostics. Microsyst. Nanoeng. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Al Mamun, A.; Sueoka, B.; Allison, N.; Huang, Y.; Zhao, F. Design and evaluation of in-plane silicon microneedles fabricated with post-CMOS compatible processes. Sens. Actuators A Phys. 2022, 336, 113407. [Google Scholar] [CrossRef]
- Izumi, H.; Aoyagi, S. Novel fabrication method for long silicon microneedles with three-dimensional sharp tips and complicated shank shapes by isotropic dry etching. IEEJ Trans. Electr. Electron. Eng. 2007, 2, 328–334. [Google Scholar] [CrossRef]
- Teo, E.J.; Liu, M.; Breese, M.B.H.; Tavernier, E.P.; Bettiol, A.A.; Blackwood, D.J.; Watt, F. Fabrication of Silicon Microstructures Using a High-Energy Ion Beam. Micromachining Technology for Micro-Optics and Nano-Optics; Johnson, E.G., II, Nordin, G.P., Eds.; SPIE International Society for Optics and Photonics: San Jose, CA, USA, 2003; Volume 5347, pp. 264–270. [Google Scholar]
- Cheung, K.; West, G.; Das, D.B. Delivery of large molecular protein using flat and short microneedles prepared using focused ion beam (FIB) as a skin ablation tool. Drug Deliv. Transl. Res. 2015, 5, 462–467. [Google Scholar] [CrossRef]
- Rouhi, N.; Jung-Kubiak, C.; White, V.; Wilson, D.; Anderson, J.; Marrese-Reading, C.; Forouhar, S. Fabrication of 3-D silicon microneedles using a single-step DRIE process. J. Microelectromech. Syst. 2015, 24, 1409–1414. [Google Scholar] [CrossRef]
- Gittard, S.D.; Ovsianikov, A.; Chichkov, B.N.; Doraiswamy, A.; Narayan, R.J. Two-photon polymerization of microneedles for transdermal drug delivery. Expert Opin. Drug Deliv. 2010, 7, 513–533. [Google Scholar] [CrossRef]
- You, J.S.; Kim, D.; Huh, J.Y.; Park, H.J.; Pak, J.J.; Kang, C.S. Experiments on anisotropic etching of Si in TMAH. Sol. Energy Mater. Sol. Cells 2001, 66, 37–44. [Google Scholar] [CrossRef]
- Thong, J.T.L.; Choi, W.K.; Chong, C.W. TMAH etching of silicon and the interaction of etching parameters. Sens. Actuators A Phys. 1997, 63, 243–249. [Google Scholar] [CrossRef]
- Dutta, S.; Imran, M.; Kumar, P.; Pal, R.; Datta, P.; Chatterjee, R. Comparison of etch characteristics of KOH, TMAH and EDP for bulk micromachining of silicon (110). Microsyst. Technol. 2011, 17, 1621–1628. [Google Scholar] [CrossRef]
- Iosub, R.; Moldovan, C.; Modreanu, M. Silicon membranes fabrication by wet anisotropic etching. Sens. Actuators A Phys. 2002, 99, 104–111. [Google Scholar] [CrossRef]
- Burham, N.; Hamzah, A.A.; Majlis, B.Y. Effect of isopropyl alcohol (IPA) on etching rate and surface roughness of silicon etched in KOH solution. In Proceedings of the 2015 IEEE Regional Symposium on Micro and Nanoelectronics (RSM), Kuala Terengganu, Malaysia, 19–21 August 2015; IEEE: Manhattan, NY, USA, 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Bhandari, R.; Negi, S.; Rieth, L.; Solzbacher, F. A wafer-scale etching technique for high aspect ratio implantable MEMS structures. Sens. Actuators A Phys. 2010, 162, 130–136. [Google Scholar] [CrossRef]
- Tilli, M.; Motooka, T.; Airaksinen, V.M.; Franssila, S.; Paulasto-Kröckel, M.; Lindroos, V. Handbook of Silicon Based MEMS Materials and Technologies, 2nd ed.; William Andrew Publications, Elsevier: Norwich, NY, USA, 2015; ISBN 9780323312233. [Google Scholar]
- Roberts, M.; Johns, P.; Owen, J.; Brandell, D.; Edstrom, K.; El Enany, G.; Guery, C.; Golodnitsky, D.; Lacey, M.; Lecoeur, C.; et al. 3D lithium ion batteries-From fundamentals to fabrication. J. Mater. Chem. 2011, 21, 9876–9890. [Google Scholar] [CrossRef]
- James, M.G. Mechanics of Materials, 6th ed.; Bill Stenquist: Belmont, CA, USA, 2004. [Google Scholar]
- Najafi, K.; Mochizuki, T.; Wise, K.D.; Mochizuki, T. A high-yield IC-compatible multichannel array recording. IEEE Trans. Electron. Devices 1985, 32, 1206–1211. [Google Scholar] [CrossRef]
- Park, J.-H.; Prausnitz, M.R. Analysis of mechanical failure of polymer microneedles by axial force. J. Korean Phys. Soc. 2010, 56, 1223–1227. [Google Scholar] [CrossRef]
- Choi, S.-O.; Kim, Y.-C.; Lee, J.W.; Park, J.-H.; Prausnitz, M.R.; Allen, M.G. Intracellular protein delivery and gene transfection by electroporation using a microneedle electrode array. Small 2012, 8, 1081–1091. [Google Scholar] [CrossRef]
- Wilson, C.J.; Beck, P.A. Fracture testing of bulk silicn microcantilever beams subjected to a side load. Microelectromech. Syst. 1996, 5, 142–150. [Google Scholar] [CrossRef]
- Brett, P.N.; Parker, T.J.; Harrison, A.J.; Thomas, T.A.; Carr, A. Simulation of resistance forces acting on surgical needles. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1997, 211, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Brett, P.N.; Fraser, C.A.; Hennigan, M.; Griffiths, M.V.; Kamel, Y.; Griffithsq, M.V.; Kome, Y. Automatic surgical tools for penetrating flexible tissues. IEEE Eng. Med. Biol. Mag. 1995, 14, 264–270. [Google Scholar] [CrossRef]
- Frick, T.B.; Marucci, D.D.; Cartmill, J.A.; Martin, C.J.; Walsh, W.R. Resistance forces acting on suture needles. J. Biomech. 2001, 34, 1335–1340. [Google Scholar] [CrossRef]
- Aggarwal, P.; Johnston, C.R. Human blood rheology in MEMS-based microneedles. Biomed. Appl. Micro Nanoeng. II 2005, 5651, 185. [Google Scholar] [CrossRef]
- Davis, S.P.; Chandrasekaran, S.; Frazier, A.B. Characterization of surface micromachined metallic microneedles. J. Microelectromech. Syst. 2003, 12, 289–295. [Google Scholar] [CrossRef]
- Wilke, N.; Hibert, C.; O’Brien, J.; Morrissey, A. Silicon Microneedle electrode array with temperature monitoring for electroporation. Sens. Actuators A Phys. 2005, 123, 319–325. [Google Scholar] [CrossRef]
- Herwik, S.; Kisban, S.; Aarts, A.A.A.; Seidl, K.; Girardeau, G.; Benchenane, K.; Zugaro, M.B.; Wiener, S.I.; Paul, O.; Neves, H.P.; et al. Fabrication technology for silicon-based microprobe arrays used in acute and sub-chronic neural recording. J. Micromech. Microeng. 2009, 19, 074008. [Google Scholar] [CrossRef]
- Wang, R.; Huang, X.; Liu, G.; Wang, W.; Dong, F.; Li, Z. Fabrication and characterization of a parylene-based three-dimensional microelectrode array for use in retinal prosthesis. J. Microelectromech. Syst. 2010, 19, 367–374. [Google Scholar] [CrossRef]
- Vinayakumar, K.B.; Hegde, G.M.; Nayak, M.M.; Dinesh, N.S.; Rajanna, K. Fabrication and characterization of gold coated hollow silicon microneedle array for drug delivery. Microelectron. Eng. 2014, 128, 12–18. [Google Scholar] [CrossRef]
- Resnik, D.; Možek, M.; Pečar, B.; Dolžan, T.; Janež, A.; Urbančič, V.; Vrtačnik, D. Characterization of skin penetration efficacy by Au-coated Si microneedle array electrode. Sens. Actuators A Phys. 2015, 232, 299–309. [Google Scholar] [CrossRef]
- Chen, K.; Ren, L.; Chen, Z.P.; Pan, C.F.; Zhou, W.; Jiang, L.L. Fabrication of micro-needle electrodes for bio-signal recording by a magnetization-induced self-assembly method. Sensors 2016, 16, 1533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Desai, T.; Ferrari, M. Proteins and cells on peg immobilized silicon surfaces. Biomaterials 1998, 19, 953–960. [Google Scholar] [CrossRef]
- Oka, K.; Aoyagi, S.; Arai, Y.; Isono, Y.; Hashiguchi, G.; Fujita, H. Fabrication of a micro needle for a trace blood test. Sens. Actuators A Phys. 2002, 97–98, 478–485. [Google Scholar] [CrossRef]
- Stoeber, B.; Liepmann, D. Fluid Injection through out-of-plane microneedles. In Proceedings of the 1st Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine and Biology, Lyon, France, 12–14 October 2000; IEEE: Manhattan, NY, USA, 2000; Volume 1, pp. 224–228. [Google Scholar]
- Sivamani, R.K.; Stoeber, B.; Wu, G.C.; Zhai, H.; Liepmann, D.; Maibach, H. Clinical microneedle injection of methyl nicotinate: Stratum corneum penetration. Ski. Res. Technol. 2005, 11, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Bystrova, S.; Luttge, R. Micromolding for ceramic microneedle arrays. Microelectron. Eng. 2011, 88, 1681–1684. [Google Scholar] [CrossRef]
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 42. [Google Scholar] [CrossRef]
- Varvel, J.R.; Shafer, S.L.; Hwang, S.S. Absorption characteristics of transdermally administered fentanyl. Am. Soc. Anesthesiol. 1989, 70, 928–934. [Google Scholar] [CrossRef]
- Yang, S.-I.; Park, H.-Y.; Lee, S.-H.; Lee, S.-J.; Han, O.-Y.; Lim, S.-S.; Jang, C.-G.; Lee, W.-S.; Shin, Y.-H.; Kim, J.-J.; et al. Transdermal eperisone elicits more potent and longer-lasting muscle relaxation than oral eperisone. Pharmacology 2004, 71, 150–156. [Google Scholar] [CrossRef]
- Coling, L.C. Common Skin Disorders and Their Topical Treatment. In Dermatological and Transdermal Formulations; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Kornick, C.A.; Santiago-Palma, J.; Moryl, N.; Payne, R.; Obbens, E.A.M.T. Benefit-risk assessment of transdermal fentanyl for the treatment of chronic pain. Drug Saf. 2003, 26, 951–973. [Google Scholar] [CrossRef]
- Cramer, M.P.; Saks, S.R. Translating safety, efficacy and compliance into economic value for controlled release dosage forms. Pharmacoeconomics 1994, 5, 482–504. [Google Scholar] [CrossRef]
- Sivamani, R.K.; Liepmann, D.; Maibach, H.I. Microneedles and transdermal applications. Expert Opin. Drug Deliv. 2007, 4, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and Transdermal drug delivery systems: Current and future prospects. Drug Deliv. J. Deliv. Target. Ther. Agents 2006, 13, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Wermeling, D.P.; Banks, S.L.; Hudson, D.A.; Gill, H.S.; Gupta, J.; Prausnitz, M.R.; Stinchcomb, A.L. Microneedles Permit transdermal delivery of a skin-impermeant medication to humans. Proc. Natl. Acad. Sci. USA 2008, 105, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Daddona, P.E.; Matriano, J.A.; Mandema, J.; Maa, Y.-F. Parathyroid hormone (1-34)-coated microneedle patch system: Clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm. Res. 2011, 28, 159–165. [Google Scholar] [CrossRef]
- Atmar, R.L.; Patel, S.M.; Keitel, W.A. Intanza®: A new intradermal vaccine for seasonal influenza. Expert Rev. Vaccines 2010, 9, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, J.; Zhuang, J.; Xu, H.; Liu, Y.; Wu, D. Microneedle system for transdermal drug and vaccine delivery: Devices, safety, and prospects. Dose-Response 2019, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, X.; Li, C.; Wu, W.; Li, Z. A novel fabrication method of hollow nanoneedles applicable for single cell operation. In Proceedings of the 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS 2010), Groningen, The Netherlands, 3–7 October 2010; Curran Associates: Red Hook, NY, USA, 2010; Volume 3, pp. 1895–1897. [Google Scholar]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Morrow, D.I.J.; McCarron, P.A.; Woolfson, A.D.; Morrissey, A.; Juzenas, P.; Juzeniene, A.; Iani, V.; McCarthy, H.O.; Moan, J. Microneedle-mediated intradermal delivery of 5-aminolevulinic acid: Potential for enhanced topical photodynamic therapy. J. Control. Release 2008, 129, 154–162. [Google Scholar] [CrossRef]
- Paleco, R.; Vučen, S.R.; Crean, A.M.; Moore, A.; Scalia, S. Enhancement of the in vitro penetration of quercetin through pig skin by combined microneedles and lipid microparticles. Int. J. Pharm. 2014, 472, 206–213. [Google Scholar] [CrossRef]
- Smart, W.H.; Subramanian, K. The use of silicon microfabrication technology in painless blood glucose monitoring. Diabetes Technol. Ther. 2000, 2, 549–559. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Brazzle, J.D.; Frazier, A.B. Surface micromachined metallic microneedles. J. Microelectromech. Syst. 2003, 12, 281–288. [Google Scholar] [CrossRef]
- Gardeniers, H.J.G.E.; Luttge, R.; Berenschot, E.J.W.; de Boer, M.J.; Yeshurun, S.Y.; Hefetz, M.; van’t Oever, R.; van den Berg, A. Silicon micromachined hollow microneedles for transdermal liquid transport. J. Microelectromech. Syst. 2003, 12, 855–862. [Google Scholar] [CrossRef]
- Griss, P.; Stemme, G. Side-opened out-of-plane microneedles for microfluidic transdermal liquid transfer. J. Microelectromech. Syst. 2003, 12, 296–301. [Google Scholar] [CrossRef]
- Chi, Y.M.; Jung, T.-P.; Cauwenberghs, G. Dry-contact and noncontact biopotential electrodes: Methodological review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Pini, F.; Blake, A.; Webster, C.; O’Brien, J.; McCarthy, K.G. Microneedle-based electrodes with integrated through-silicon via for biopotential recording. Sens. Actuators A Phys. 2012, 186, 130–136. [Google Scholar] [CrossRef]
- Peng, H.-L.; Liu, J.-Q.; Dong, Y.-Z.; Yang, B.; Chen, X.; Yang, C.-S. Parylene-Based flexible dry electrode for bioptential recording. Sens. Actuators B Chem. 2016, 231, 1–11. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, D.; Song, R.; Zhang, C.; Xu, W.; Pan, X. Characterization of alternating current impedance properties of biomedical electrodes. J. Cent. South Univ. 2013, 20, 1254–1258. [Google Scholar] [CrossRef]
- Kato, T.; Ueno, A.; Kataoka, S.; Hoshino, H.; Ishiyama, Y. An application of capacitive electrode for detecting electrocardiogram of neonates and infants. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; IEEE: Manhattan, NY, USA, 2006; pp. 916–919. [Google Scholar]
- Griss, P.; Enoksson, P.; Tolvanen-Laakso, H.; Merilainen, P.; Ollmar, S.; Stemme, G. Spiked biopotential electrodes. In Proceedings of the IEEE Thirteenth Annual International Conference on Micro Electro Mechanical Systems, Miyazaki, Japan, 23–27 January 2000; IEEE: Manhattan, NY, USA, 2002; pp. 323–328. [Google Scholar]
- Srivastava, A.K.; Bhartia, B.; Mukhopadhyay, K.; Sharma, A. Long term biopotential recording by body conformable photolithography fabricated low cost polymeric microneedle arrays. Sens. Actuators A Phys. 2015, 236, 164–172. [Google Scholar] [CrossRef]
- Yu, L.M.; Tay, F.E.H.; Guo, D.G.; Xu, L.; Yap, K.L. A microfabricated electrode with hollow microneedles for ECG measurement. Sens. Actuators A Phys. 2009, 151, 17–22. [Google Scholar] [CrossRef]
- Forvi, E.; Bedoni, M.; Carabalona, R.; Soncini, M.; Mazzoleni, P.; Rizzo, F.; O’Mahony, C.; Morasso, C.; Cassarà, D.G.; Gramatica, F. Preliminary technological assessment of microneedles-based dry electrodes for biopotential monitoring in clinical examinations. Sens. Actuators A Phys. 2012, 180, 177–186. [Google Scholar] [CrossRef]
- Hsu, L.S.; Tung, S.W.; Kuo, C.H.; Yang, Y.J. Developing barbed microtip-based electrode arrays for biopotential measurement. Sensors 2014, 14, 12370–12386. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Murphy, R.K.J.; Hwang, S.W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S.; et al. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Salvo, P.; Raedt, R.; Carrette, E.; Schaubroeck, D.; Vanfleteren, J.; Cardon, L. A 3D printed dry electrode for ECG/EEG recording. Sens. Actuators A Phys. 2012, 174, 96–102. [Google Scholar] [CrossRef]
- Arai, M.; Nishinaka, Y.; Miki, N. Electroencephalogram measurement using polymer-based dry microneedle electrode. JPN J. Appl. Phys. 2015, 54, 06FP14. [Google Scholar] [CrossRef]
- Kim, M.; Kim, T.; Kim, D.S.; Chung, W.K. Curved microneedle array-based sEMG electrode for robust long-term measurements and high selectivity. Sensors 2015, 15, 16265–16280. [Google Scholar] [CrossRef] [PubMed]
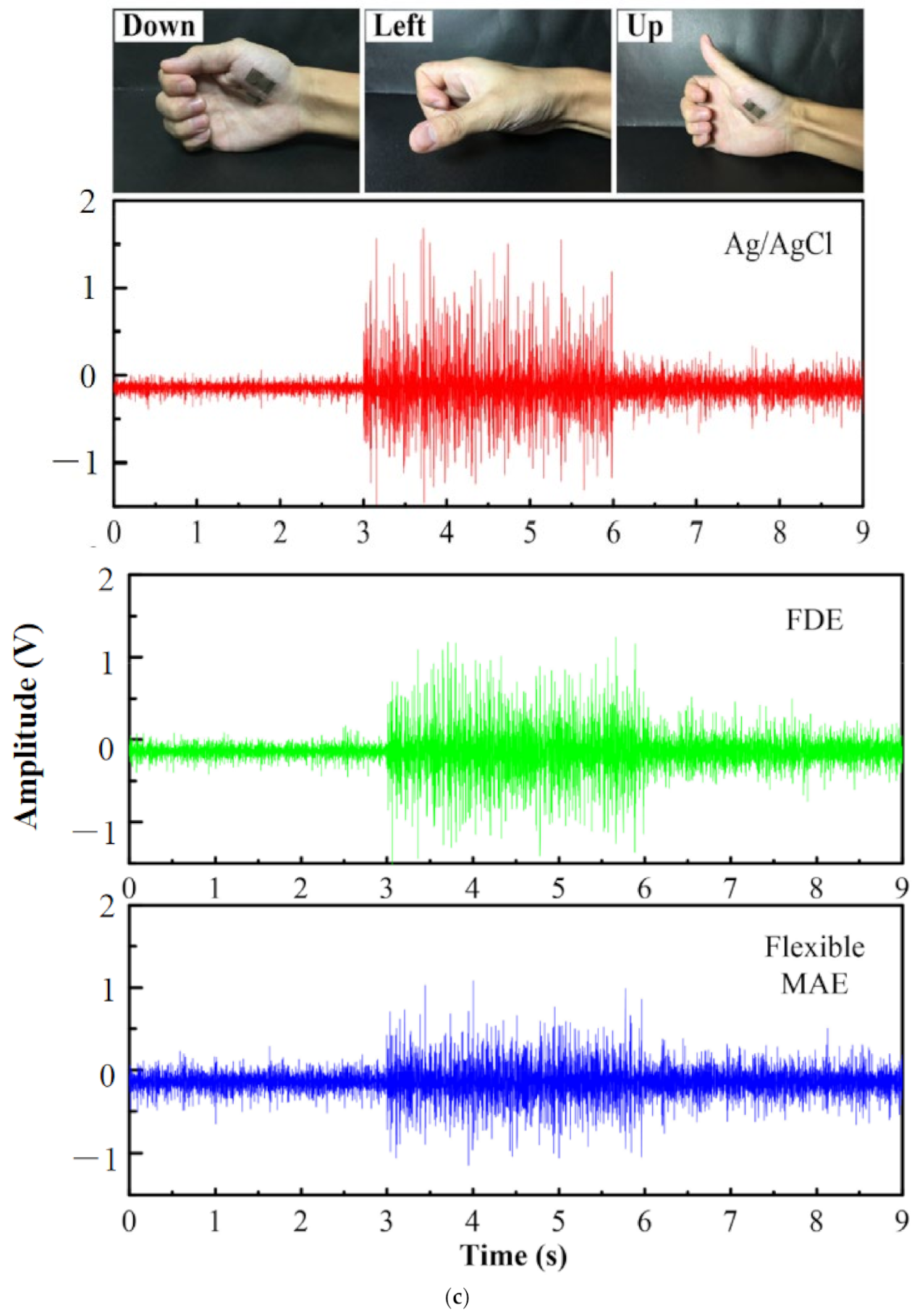
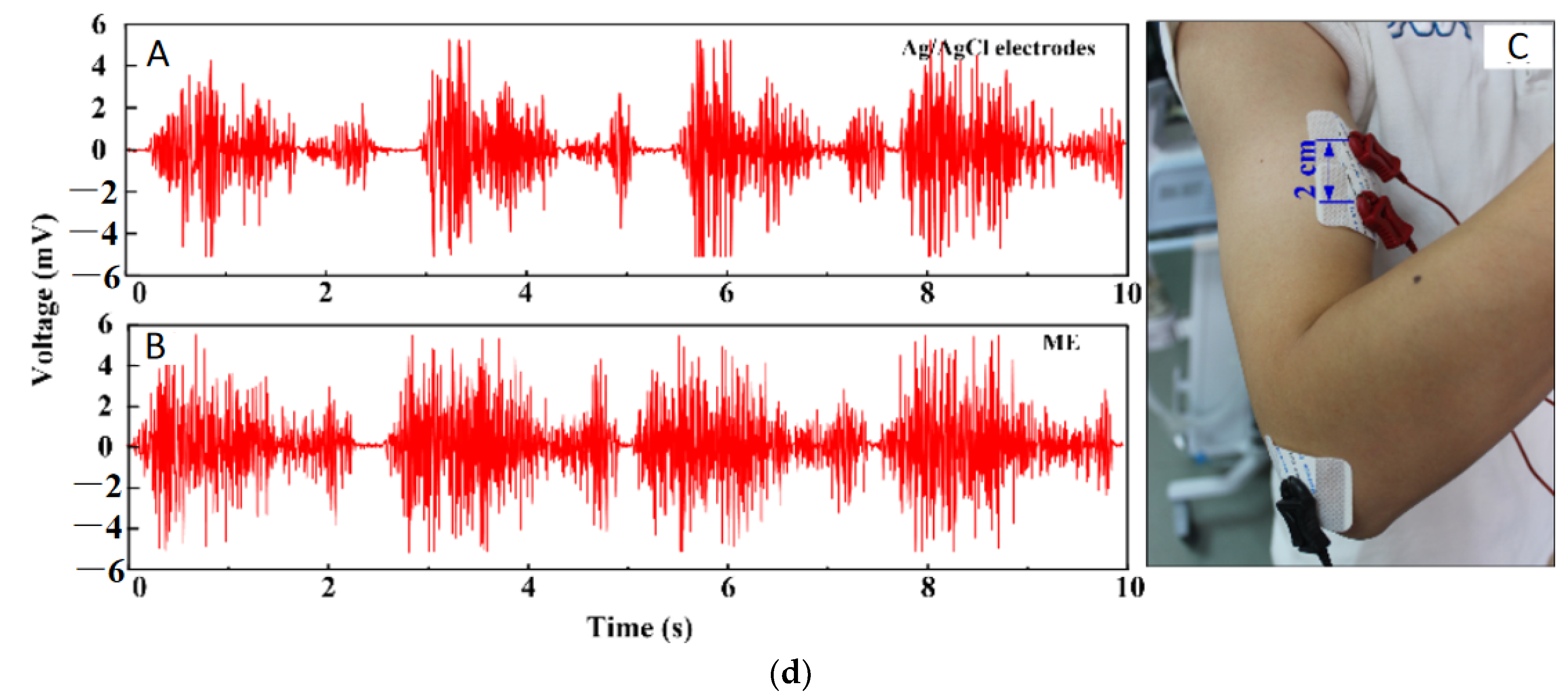
- Ren, L.; Xu, S.; Gao, J.; Lin, Z.; Chen, Z.; Liu, B.; Liang, L.; Jiang, L. Fabrication of flexible microneedle array electrodes for wearable bio-signal recording. Sensors 2018, 18, 1191. [Google Scholar] [CrossRef]
- O’Mahony, C.; Pini, F.; McCarthy, K.G. Microneedle-based electrodes with integrated through-silicon via for biopotential recording. Procedia Eng. 2011, 25, 992–995. [Google Scholar] [CrossRef]
- Ren, L.; Jiang, Q.; Chen, K.; Chen, Z.; Pan, C.; Jiang, L. Fabrication of a micro-needle array electrode by thermal drawing for bio-signals monitoring. Sensors 2016, 16, 908. [Google Scholar] [CrossRef]
- Jina, A.; Tierney, M.J.; Tamada, J.A.; McGill, S.; Desai, S.; Chua, B.; Chang, A.; Christiansen, M. Design, Development, and Evaluation of a novel microneedle array-based continuous glucose monitor. J. Diabetes Sci. Technol. 2014, 8, 483–487. [Google Scholar] [CrossRef]
- Sharma, S.; El-Laboudi, A.; Reddy, M.; Jugnee, N.; Sivasubramaniyam, S.; El Sharkawy, M.; Georgiou, P.; Johnston, D.; Oliver, N.; Cass, A.E.G. A pilot study in humans of microneedle sensor arrays for continuous glucose monitoring. Anal. Methods 2018, 10, 2088–2095. [Google Scholar] [CrossRef]
- Sharma, S.; Huang, Z.; Rogers, M.; Boutelle, M.; Cass, A.E.G. Evaluation of a minimally invasive glucose biosensor for continuous tissue monitoring. Anal. Bioanal. Chem. 2016, 408, 8427–8435. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.R.; Xiao, X.; Brener, I.; Burckel, D.B.; Narayan, R.; Polsky, R. Diagnostic devices: Microneedle-based transdermal sensor for on-chip potentiometric determination of K+ (Adv. Healthcare Mater. 6/2014). Adv. Healthc. Mater. 2014, 3, 948. [Google Scholar] [CrossRef]
- Parrilla, M.; Cuartero, M.; Padrell Sánchez, S.; Rajabi, M.; Roxhed, N.; Niklaus, F.; Crespo, G.A. Wearable all-solid-state potentiometric microneedle patch for intradermal potassium detection. Anal. Chem. 2019, 91, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.M.V.; Windmiller, J.R.; Mishra, R.K.; Wang, J. Continuous minimally-invasive alcohol monitoring using microneedle sensor arrays. Biosens. Bioelectron. 2017, 91, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ren, L.; Jiang, L.; Tang, Y.; Liu, B. Fabrication of composite microneedle array electrode for temperature and bio-signal monitoring. Sensors 2018, 18, 1193. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Vinu Mohan, A.M.; Soto, F.; Chrostowski, R.; Wang, J. A microneedle biosensor for minimally-invasive transdermal detection of nerve agents. Analyst 2017, 142, 918–924. [Google Scholar] [CrossRef]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; Narayan, R.; Litvan, I.; Wang, J. Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: Toward parkinson management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef]
- Duarah, S.; Sharma, M.; Wen, J. Recent advances in microneedle-based drug delivery: Special emphasis on its use in paediatric population. Eur. J. Pharm. Biopharm. 2019, 136, 48–69. [Google Scholar] [CrossRef]
- Birchall, J.C. Microneedle array technology: The time is right but is the science ready? Expert Rev. Med. Devices 2006, 3, 1–4. [Google Scholar] [CrossRef]
- Giudice, E.L.; Campbell, J.D. Needle-free vaccine delivery. Adv. Drug Deliv. Rev. 2006, 58, 68–89. [Google Scholar] [CrossRef]
- Merletti, R. The electrode-skin interface and optimal detection of bioelectric signals. Physiol. Meas. 2010, 31, 10. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207. [Google Scholar] [CrossRef] [PubMed]
- Mooney, K.; McElnay, J.C.; Donnelly, R.F. Children’s Views on microneedle use as an alternative to blood sampling for patient monitoring. Int. J. Pharm. Pract. 2013, 22, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Caffarel-Salvador, E.; Tuan-Mahmood, T.-M.; McElnay, J.C.; McCarthy, H.O.; Mooney, K.; Woolfson, A.D.; Donnelly, R.F. Potential of hydrogel-forming and dissolving microneedles for use in paediatric populations. Int. J. Pharm. 2015, 489, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Cormier, M.; Johnson, B.; Ameri, M.; Nyam, K.; Libiran, L.; Zhang, D.D.; Daddona, P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J. Control. Release 2004, 97, 503–511. [Google Scholar] [CrossRef]
- Gupta, J.; Felner, E.I.; Prausnitz, M.R. Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol. Ther. 2011, 13, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Brown, M.R.; Raviele, N.A.; Prausnitz, M.R.; Felner, E.I. Faster pharmacokinetics and increased patient acceptance of intradermal insulin delivery using a single hollow microneedle in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2013, 14, 459–465. [Google Scholar] [CrossRef]
- Birchall, J.C.; Clemo, R.; Anstey, A.; John, D.N. Microneedles in clinical practice–an exploratory study into the opinions of healthcare professionals and the public. Pharm. Res. 2011, 28, 95–106. [Google Scholar] [CrossRef]
- Adrienne Stinson BCAAs: Benefits of Branched-Chain Amino Acids. Available online: https://www.medicalnewstoday.com/articles/324605#exercise-performance (accessed on 14 April 2022).
- GhavamiNejad, A.; Lu, B.; Samarikhalaj, M.; Liu, J.F.; Mirzaie, S.; Pereira, S.; Zhou, L.; Giacca, A.; Wu, X.Y. Transdermal delivery of a somatostatin receptor type 2 antagonist using microneedle patch technology for hypoglycemia prevention. Drug Deliv. Transl. Res. 2022, 12, 792–804. [Google Scholar] [CrossRef]
- Kim, H.; Seong, K.-Y.; Lee, J.H.; Park, W.; Yang, S.Y.; Hahn, S.K. Biodegradable microneedle patch delivering antigenic peptide–hyaluronate conjugate for cancer immunotherapy. ACS Biomater. Sci. Eng. 2019, 5, 5150–5158. [Google Scholar] [CrossRef]
- Mohammed, Y.H.; Yamada, M.; Lin, L.L.; Grice, J.E.; Roberts, M.S.; Raphael, A.P.; Benson, H.A.E.; Prow, T.W. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS ONE 2014, 9, e101956. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L. Mineral Bioavailability. Nutr. Food Sci. 2001, 31, 179–182. [Google Scholar] [CrossRef]
- Li, H.; Low, Y.S.J.; Chong, H.P.; Zin, M.T.; Lee, C.-Y.; Li, B.; Leolukman, M.; Kang, L. Microneedle-mediated delivery of copper peptide through skin. Pharm. Res. 2015, 32, 2678–2689. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, M.; Du, X.; Xu, J.; Zhai, Y.; Ji, J.; He, S.; Zhai, G. Recent progress of micro-needle formulations: Fabrication strategies and delivery applications. J. Drug Deliv. Sci. Technol. 2019, 50, 18–26. [Google Scholar] [CrossRef]
- Chen, W.; Wainer, J.; Ryoo, S.W.; Qi, X.; Chang, R.; Li, J.; Lee, S.H.; Min, S.; Wentworth, A.; Collins, J.E.; et al. Dynamic omnidirectional adhesive microneedle system for oral macromolecular drug delivery. Sci. Adv. 2022, 8, eabk1792. [Google Scholar] [CrossRef]
- Driscoll, M.S.; Kwon, E.-K.M.; Skupsky, H.; Kwon, S.-Y.; Grant-Kels, J.M. Nutrition and the deleterious side effects of nutritional supplements. Clin. Dermatol. 2010, 28, 371–379. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Prausnitz, M. Individually coated microneedles for co-delivery of multiple compounds with different properties. Drug Deliv. Transl. Res. 2018, 8, 1043–1052. [Google Scholar] [CrossRef]
- Van der Maaden, K.; Heuts, J.; Camps, M.; Pontier, M.; Terwisscha van Scheltinga, A.; Jiskoot, W.; Ossendorp, F.; Bouwstra, J. Hollow microneedle-mediated micro-injections of a liposomal HPV E743–63 synthetic long peptide vaccine for efficient induction of cytotoxic and T-helper responses. J. Control. Release 2018, 269, 347–354. [Google Scholar] [CrossRef]
- Thukral, A.; Ershad, F.; Enan, N.; Rao, Z.; Yu, C. Soft ultrathin silicon electronics for soft neural interfaces: A review of recent advances of soft neural interfaces based on ultrathin silicon. IEEE Nanotechnol. Mag. 2018, 12, 21–34. [Google Scholar] [CrossRef]
- Zhang, S.; Yen, S.-C.; Xiang, Z.; Liao, L.-D.; Kwong, D.-L.; Lee, C. Development of Silicon probe with acute study on in vivo neural recording and implantation behavior monitored by integrated Si-nanowire strain sensors. J. Microelectromech. Syst. 2015, 24, 1303–1313. [Google Scholar] [CrossRef]
- Kita, Y.; Tsuruhara, S.; Kubo, H.; Yamashita, K.; Seikoba, Y.; Idogawa, S.; Sawahata, H.; Yamagiwa, S.; Leong, X.L.A.; Numano, R.; et al. Three-micrometer-diameter needle electrode with an amplifier for extracellular in vivo recordings. Proc. Natl. Acad. Sci. USA 2021, 118, e2008233118. [Google Scholar] [CrossRef] [PubMed]
- Shikida, M.; Hasegawa, Y.; Al Farisi, M.S.; Matsushima, M.; Kawabe, T. Advancements in MEMS technology for medical applications: Microneedles and miniaturized sensors. Jpn. J. Appl. Phys. 2021, 61, SA0803. [Google Scholar] [CrossRef]
- Lee, S.H.; Thunemann, M.; Lee, K.; Cleary, D.R.; Tonsfeldt, K.J.; Oh, H.; Azzazy, F.; Tchoe, Y.; Bourhis, A.M.; Hossain, L.; et al. Scalable thousand channel penetrating microneedle arrays on flex for multimodal and large area coverage brainmachine interfaces. Adv. Funct. Mater. 2022, 2112045. [Google Scholar] [CrossRef]
- Sawahata, H.; Yamagiwa, S.; Moriya, A.; Dong, T.; Oi, H.; Ando, Y.; Numano, R.; Ishida, M.; Koida, K.; Kawano, T. Single 5 µm diameter needle electrode block modules for unit recordings in vivo. Sci. Rep. 2016, 6, 35806. [Google Scholar] [CrossRef] [PubMed]
- Shikida, M.; Odagaki, M.; Todoroki, N.; Ando, M.; Ishihara, Y.; Ando, T.; Sato, K. Non-photolithographic pattern transfer for fabricating arrayed three-dimensional microstructures by chemical anisotropic etching. Sens. Actuators A Phys. 2004, 116, 264–271. [Google Scholar] [CrossRef]
- Sakata, M.; Goryu, A.; Ikedo, A.; Harimoto, T.; Ishida, M.; Kawano, T. A vertical micro-scale light guiding silicon dioxide tube array for optical neurostimulator. In Proceedings of the 2011 IEEE 24th International Conference on Micro Electro Mechanical Systems, Cancun, MX, USA, 23–27 January 2011; Curran Associates: Red Hook, NY, USA, 2011; pp. 1015–1018. [Google Scholar]
- Son, Y.; Lee, H.J.; Kim, J.; Lee, C.J.; Yoon, E.-S.; Kim, T.G.; Cho, I.-J. A new monolithically integrated multi-functional MEMS neural probe for optical stimulation and drug delivery. In Proceedings of the 2015 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; IEEE: Manhattan, NY, USA, 2015; pp. 158–161. [Google Scholar]
- Saxena, T.; Karumbaiah, L.; Gaupp, E.A.; Patkar, R.; Patil, K.; Betancur, M.; Stanley, G.B.; Bellamkonda, R. V The impact of chronic blood–brain barrier breach on intracortical electrode function. Biomaterials 2013, 34, 4703–4713. [Google Scholar] [CrossRef]
- Yan, D.; Jiman, A.; Ratze, D.; Huang, S.; Parizi, S.; Welle, E.; Ouyang, Z.; Patel, P.; Kushner, M.J.; Chestek, C.; et al. Microneedle penetrating array with axon-sized dimensions for cuff-less peripheral nerve interfacing. In Proceedings of the 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER), San Francisco, CA, USA, 20–23 March 2019; IEEE: Manhattan, NY, USA, 2019; pp. 827–830. [Google Scholar]
- Fujishiro, A.; Kaneko, H.; Kawashima, T.; Ishida, M.; Kawano, T. In Vivo neuronal action potential recordings via three-dimensional microscale needle-electrode arrays. Sci. Rep. 2014, 4, 4868. [Google Scholar] [CrossRef]
- Gao, J.; Huang, W.; Chen, Z.; Yi, C.; Jiang, L. Simultaneous detection of glucose, uric acid and cholesterol using flexible microneedle electrode array-based biosensor and multi-channel portable electrochemical analyzer. Sens. Actuators B Chem. 2019, 287, 102–110. [Google Scholar] [CrossRef]
- Teymourian, H.; Moonla, C.; Tehrani, F.; Vargas, E.; Aghavali, R.; Barfidokht, A.; Tangkuaram, T.; Mercier, P.P.; Dassau, E.; Wang, J. Microneedle-based detection of ketone bodies along with glucose and lactate: Toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal. Chem. 2020, 92, 2291–2300. [Google Scholar] [CrossRef]
- Mishra, R.K.; Goud, K.Y.; Li, Z.; Moonla, C.; Mohamed, M.A.; Tehrani, F.; Teymourian, H.; Wang, J. Continuous opioid monitoring along with nerve agents on a wearable microneedle sensor array. J. Am. Chem. Soc. 2020, 142, 5991–5995. [Google Scholar] [CrossRef] [PubMed]
- Kastellorizios, M.; Burgess, D.J. Continuous metabolic monitoring based on multi-analyte biomarkers to predict exhaustion. Sci. Rep. 2015, 5, 10603. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Ramírez, G.; Windmiller, J.R.; Claussen, J.C.; Martinez, A.G.; Kuralay, F.; Zhou, M.; Zhou, N.; Polsky, R.; Miller, P.R.; Narayan, R.; et al. Multiplexed and switchable release of distinct fluids from microneedle platforms via conducting polymer nanoactuators for potential drug delivery. Sens. Actuators B Chem. 2012, 161, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Gowers, S.A.N.; Freeman, D.M.E.; Wilson, R.C.; Sharma, S.; Gilchrist, M.; MacGowan, A.; Lovering, A.; Bayliss, M.; Kyriakides, M.; et al. Microneedle biosensors for real-time, minimally invasive drug monitoring of phenoxymethylpenicillin: A first-in-human evaluation in healthy volunteers. Lancet Digit. Health 2019, 1, e335–e343. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Li, H.; Yu, J.; Chen, G.; Kahkoska, A.R.; Wu, V.; Zeng, Y.; Wen, D.; Miedema, J.R.; et al. Dual Self-regulated delivery of insulin and glucagon by a hybrid patch. Proc. Natl. Acad. Sci. USA 2020, 117, 29512–29517. [Google Scholar] [CrossRef]
- Teymourian, H.; Tehrani, F.; Mahato, K.; Wang, J. Lab under the skin: Microneedle based wearable devices. Adv. Healthc. Mater. 2021, 1, 2002255. [Google Scholar] [CrossRef]
- Pires, L.R.; Vinayakumar, K.B.; Turos, M.; Miguel, V.; Gaspar, J. A perspective on microneedle-based drug delivery and diagnostics in paediatrics. J. Pers. Med. 2019, 9, 49. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.; Kahkoska, A.R.; Wang, J.; Buse, J.B.; Gu, Z. Advances in transdermal insulin delivery. Adv. Drug Deliv. Rev. 2019, 139, 51–70. [Google Scholar] [CrossRef]
- Ruiz-Valdepeñas Montiel, V.; Sempionatto, J.R.; Esteban-Fernández de Ávila, B.; Whitworth, A.; Campuzano, S.; Pingarrón, J.M.; Wang, J. Delayed sensor activation based on transient coatings: Biofouling protection in complex biofluids. J. Am. Chem. Soc. 2018, 140, 14050–14053. [Google Scholar] [CrossRef]
- Saadat-Moghaddam, D.; Kim, J.-H. A Microneedle functionalized with polyethyleneimine and nanotubes for highly sensitive, label-free quantification of DNA. Sensors 2017, 17, 1883. [Google Scholar] [CrossRef]
- Arroyo-Currás, N.; Ortega, G.; Copp, D.A.; Ploense, K.L.; Plaxco, Z.A.; Kippin, T.E.; Hespanha, J.P.; Plaxco, K.W. High-precision control of plasma drug levels using feedback-controlled dosing. ACS Pharmacol. Transl. Sci. 2018, 1, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Currás, N.; Dauphin-Ducharme, P.; Scida, K.; Chávez, J.L. From the beaker to the body: Translational challenges for electrochemical, aptamer-based sensors. Anal. Methods 2020, 12, 1288–1310. [Google Scholar] [CrossRef]
- Parolo, C.; Idili, A.; Ortega, G.; Csordas, A.; Hsu, A.; Arroyo-Currás, N.; Yang, Q.; Ferguson, B.S.; Wang, J.; Plaxco, K.W. Real-time monitoring of a protein biomarker. ACS Sens. 2020, 5, 1877–1881. [Google Scholar] [CrossRef] [PubMed]

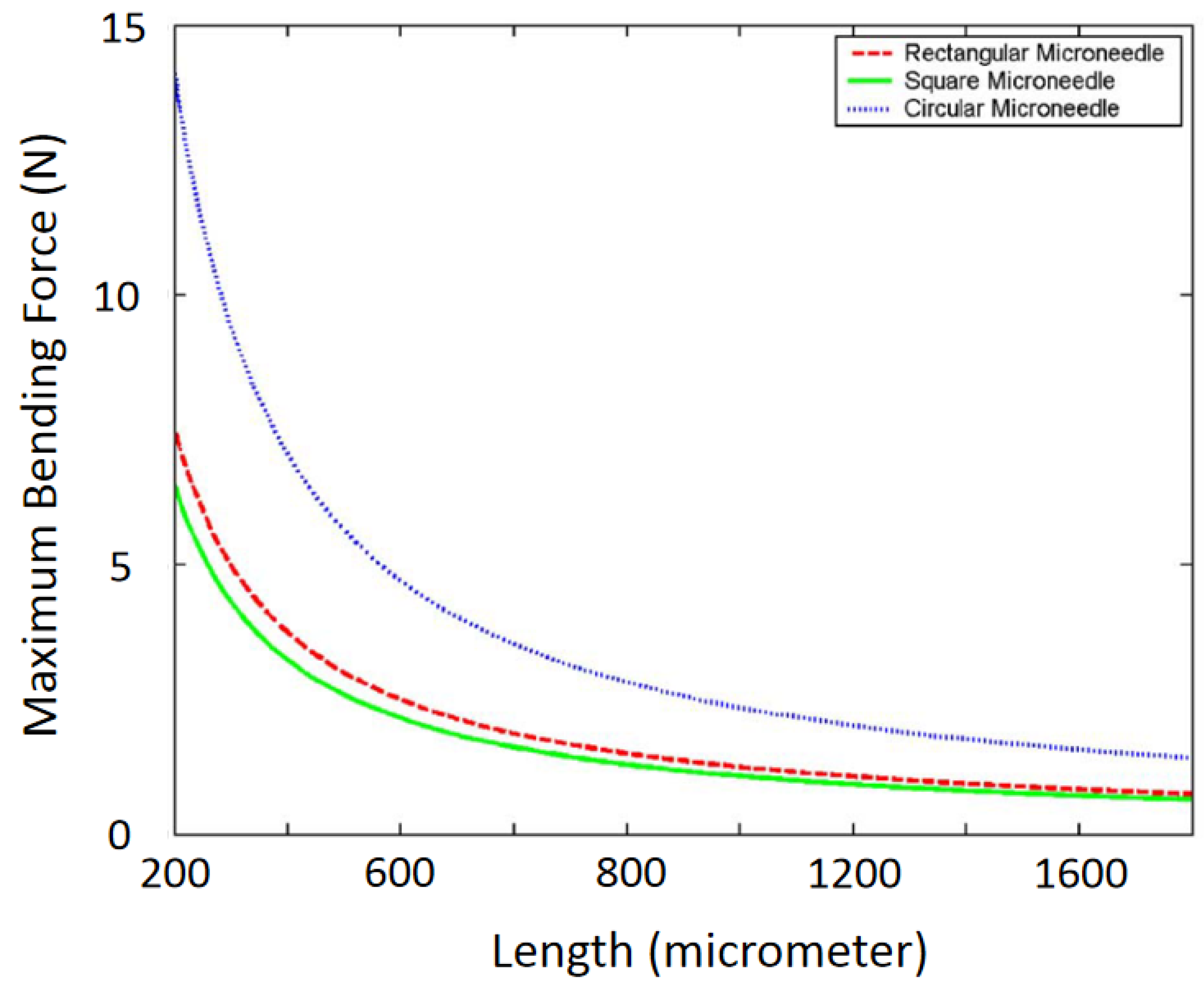
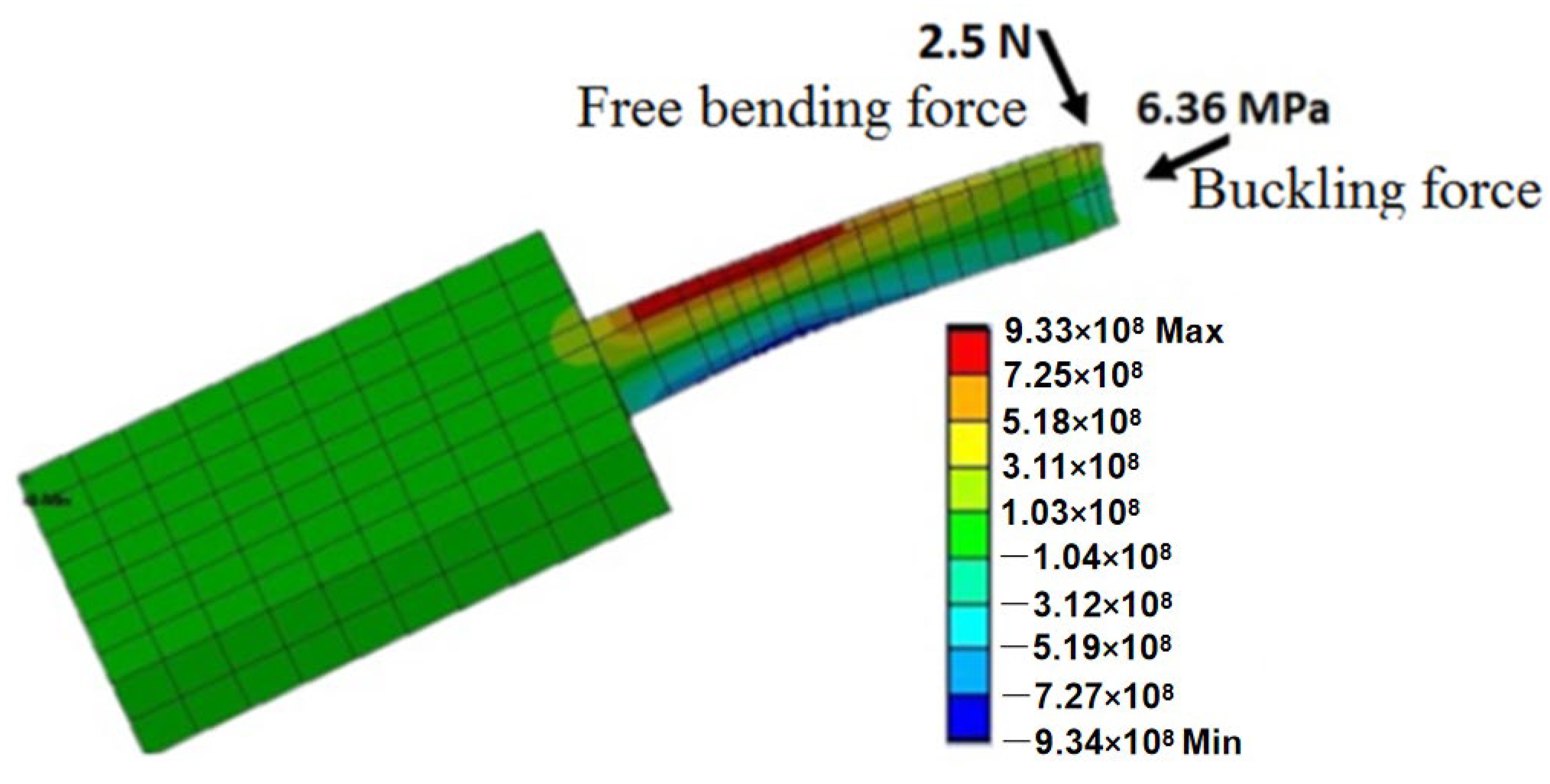
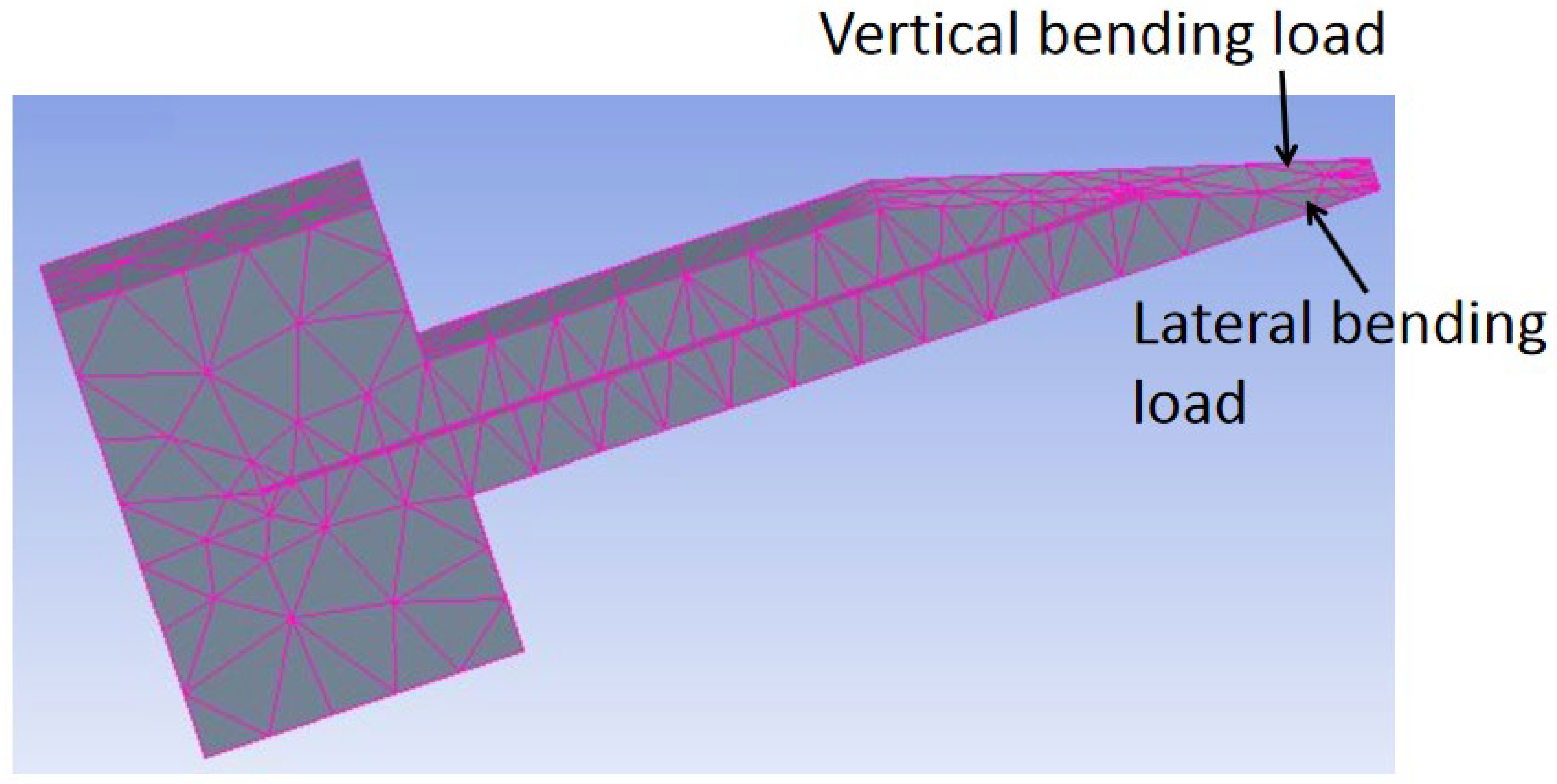



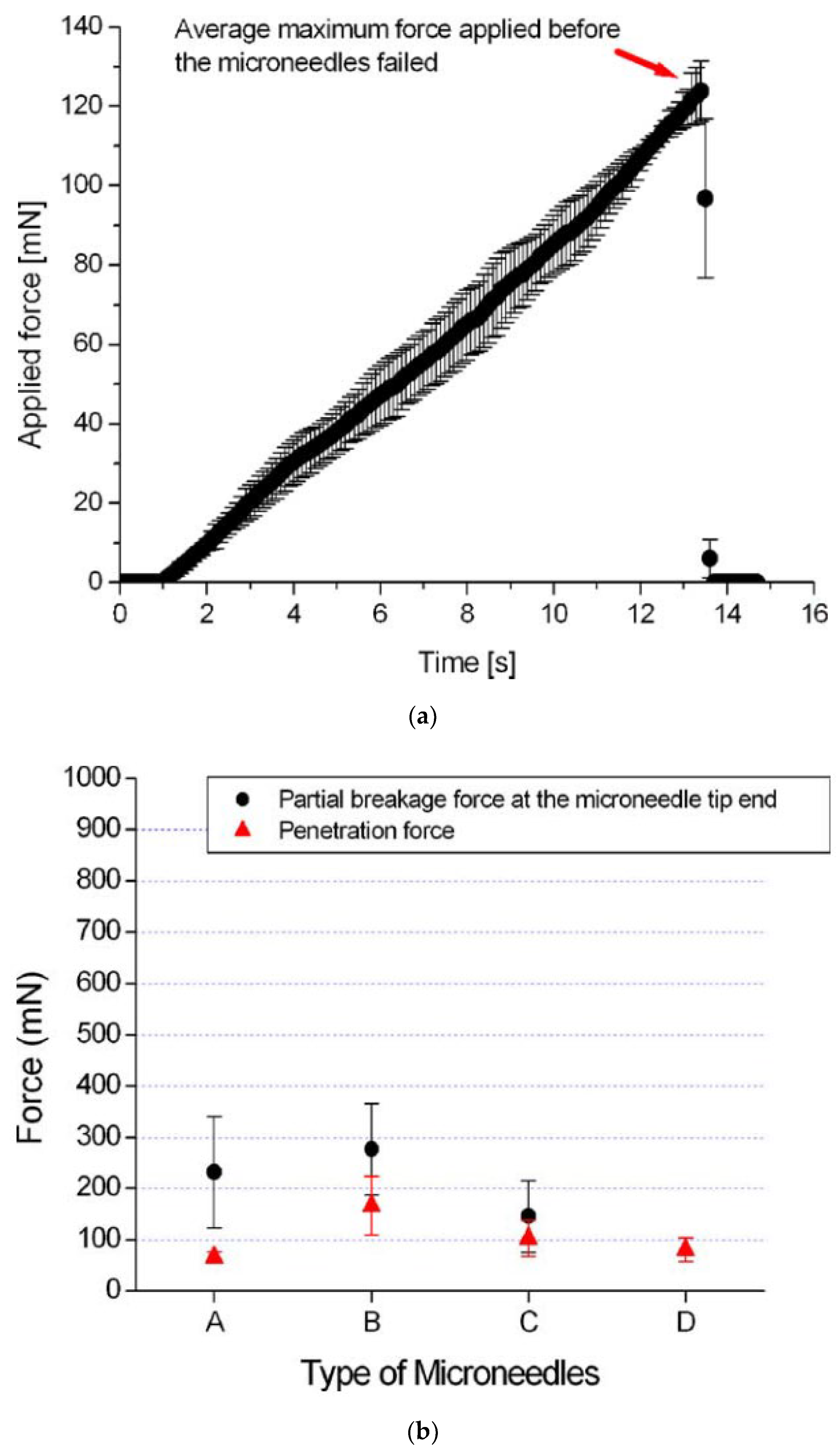

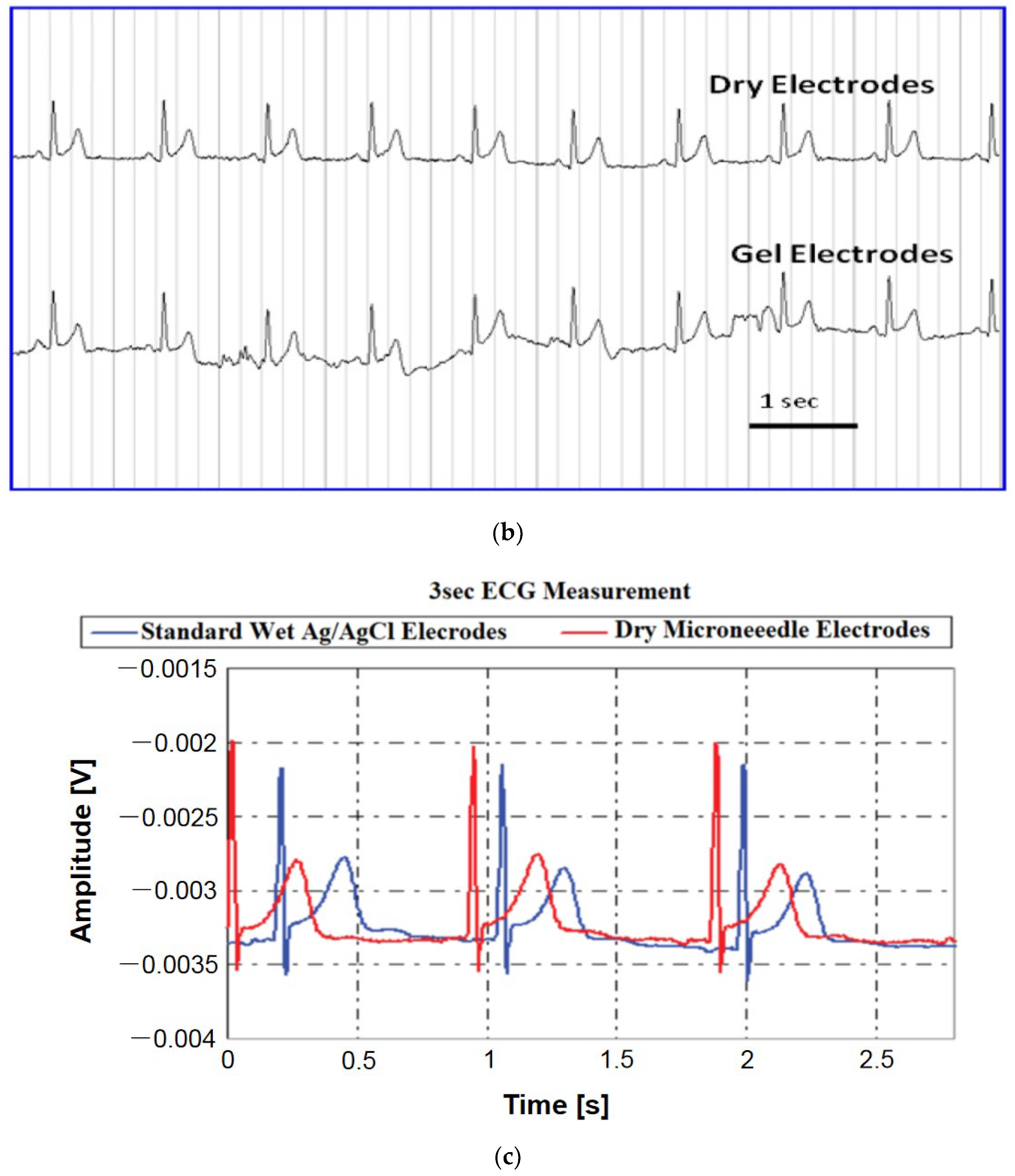
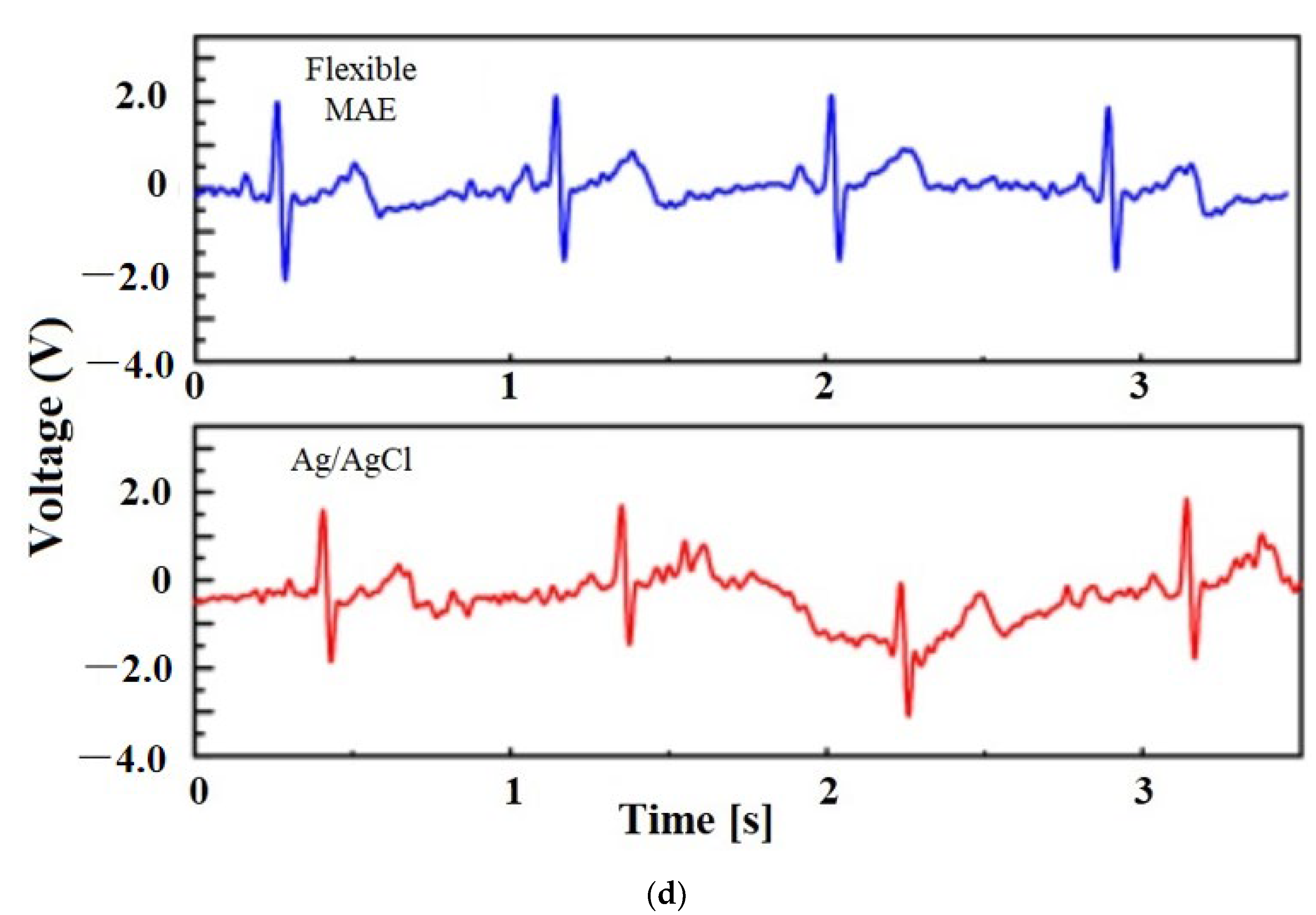
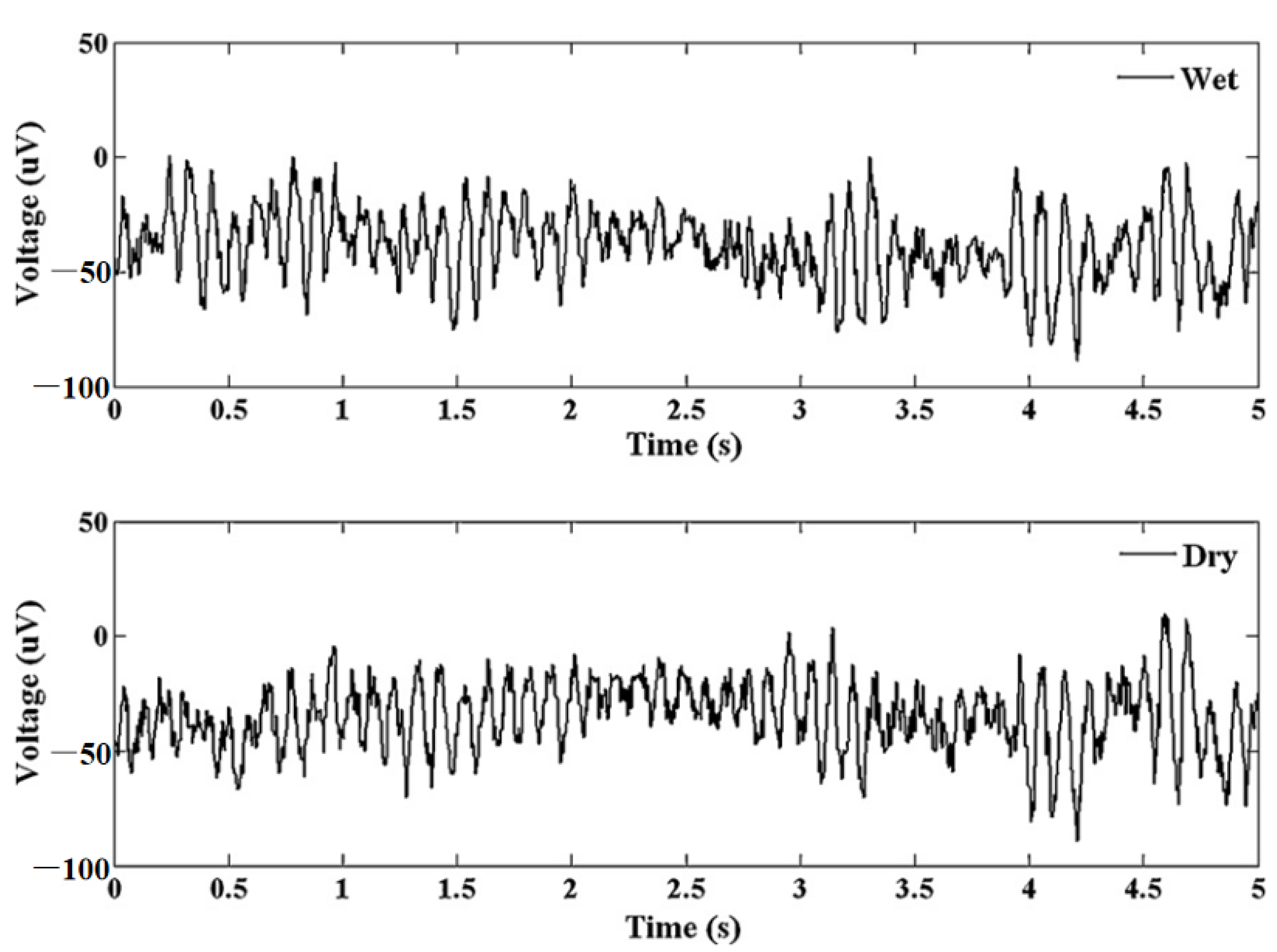
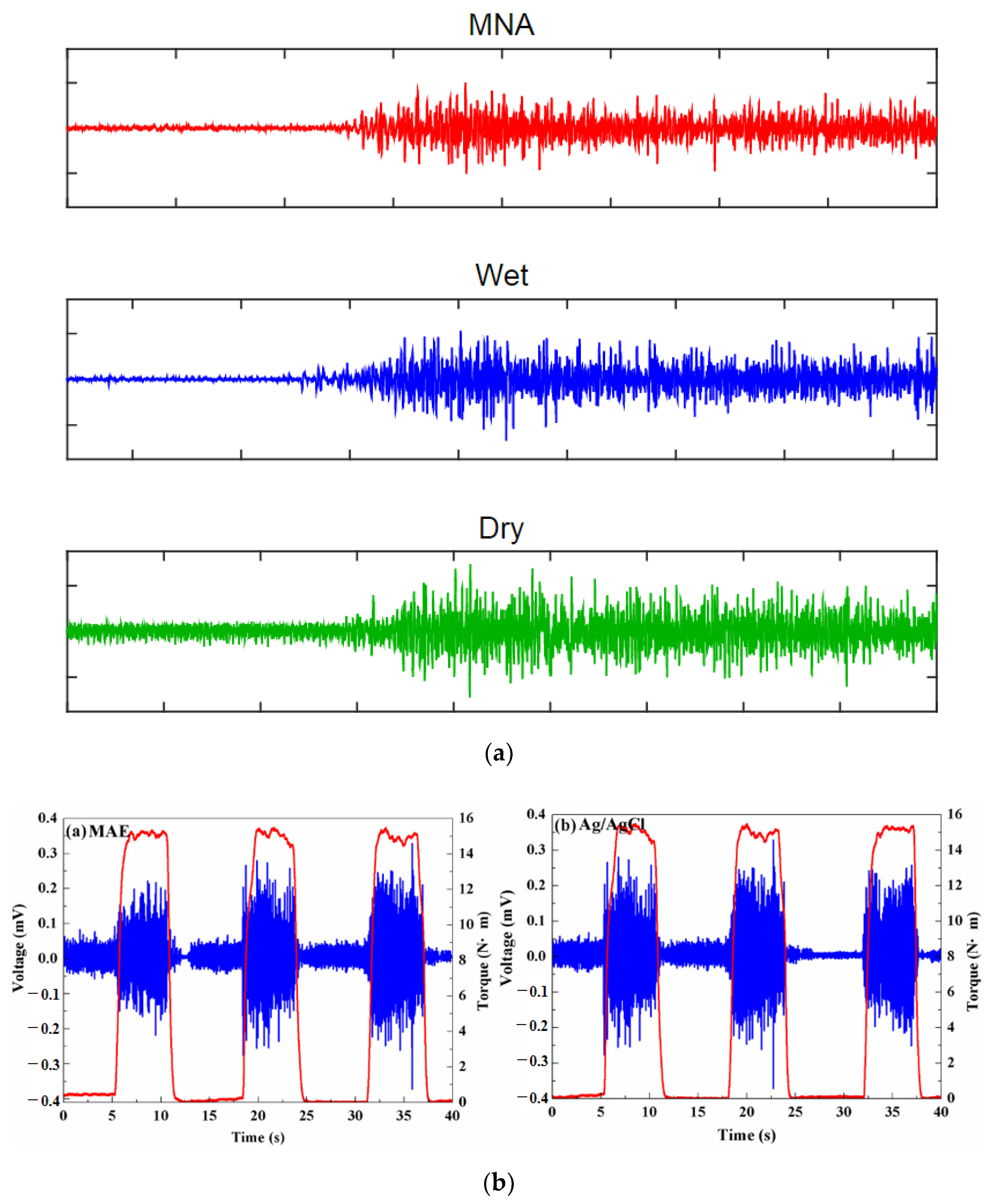
| Sl. | Reference | Schematic |
|---|---|---|
| 1. | Reprinted with permission from ref. [16] | 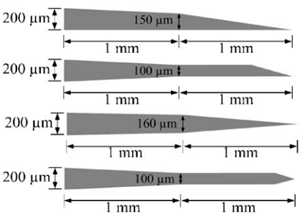 |
| 2. | Reprinted with permission from ref. [3] |  |
| 3. | Reprinted with permission from ref. [39] |  |
| Types of Microneedle | Moment of Inertia | Definition of the Term |
|---|---|---|
| Circular | D and d are the outer and inner diameters of the needle, respectively. | |
| Rectangular | B and H are outer width and thickness, and b and h are the inner width and thickness. | |
| Square | H and h are the outer and inner dimensions, respectively. | |
| Solid Conical | y is the length of the conical section, and D is the diameter. |
| SL. No | Needle Style | Number of Tests | Avg. Measured Flow Rate (cc/sec) | Computed Flow Rate (cc/sec) | Error (%) | Reynold Numbers |
|---|---|---|---|---|---|---|
| 1. | Bent, 90° | 4 | 0.082 ± 0.004 | 0.088 | 7.3 | 738 |
| 2. | Reinforced | 9 | 0.040 ± 0.004 | 0.040 | 0.0 | 503 |
| 3. | Fillet | 2 | 0.070 ± 0.01 | 0.083 | 17.9 | 688 |
| 4. | Double Channel | 1 | 0.032 | 0.o34 | 6.2 | 260 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamun, A.A.; Zhao, F. In-Plane Si Microneedles: Fabrication, Characterization, Modeling and Applications. Micromachines 2022, 13, 657. https://doi.org/10.3390/mi13050657
Mamun AA, Zhao F. In-Plane Si Microneedles: Fabrication, Characterization, Modeling and Applications. Micromachines. 2022; 13(5):657. https://doi.org/10.3390/mi13050657
Chicago/Turabian StyleMamun, Abdulla Al, and Feng Zhao. 2022. "In-Plane Si Microneedles: Fabrication, Characterization, Modeling and Applications" Micromachines 13, no. 5: 657. https://doi.org/10.3390/mi13050657
APA StyleMamun, A. A., & Zhao, F. (2022). In-Plane Si Microneedles: Fabrication, Characterization, Modeling and Applications. Micromachines, 13(5), 657. https://doi.org/10.3390/mi13050657







