Kinetics Analysis and ADRC-Based Controller for a String-Driven Vascular Intervention Surgical Robotic System
Abstract
:1. Introduction
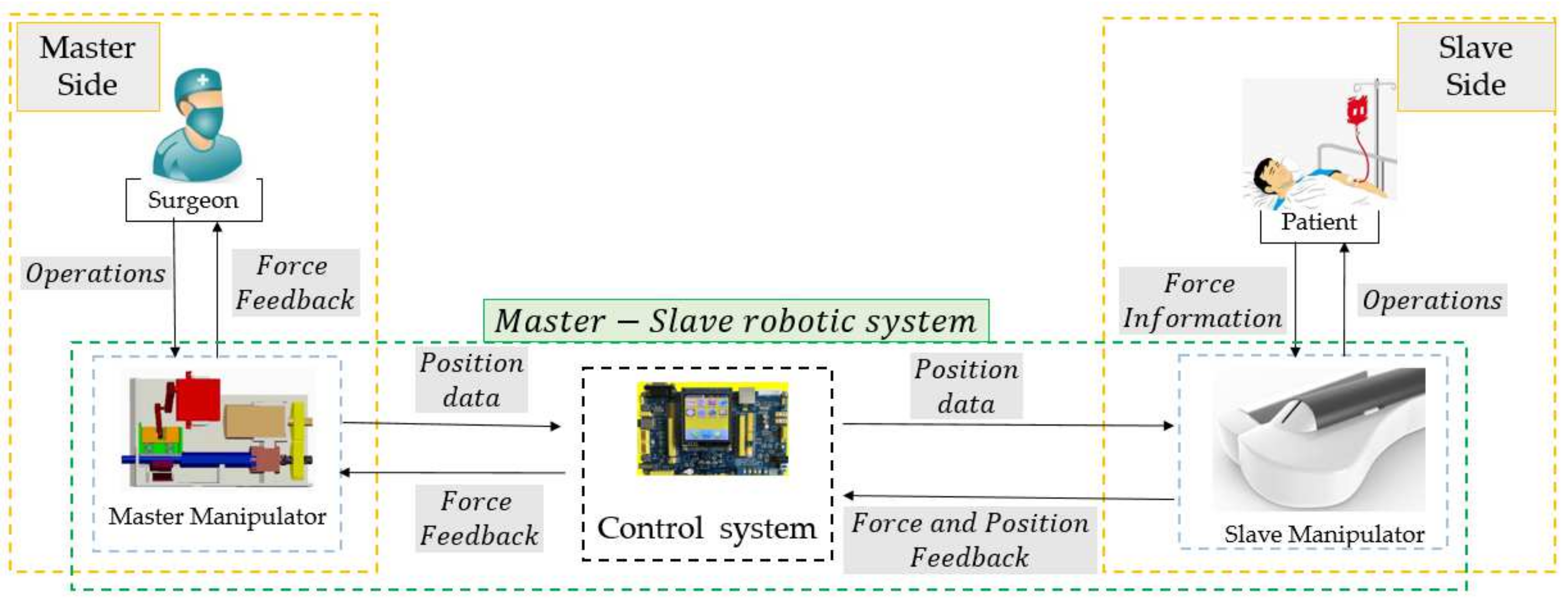
2. Overview of the Master–Slave Robotic System
2.1. Surgeons’ Habits-Based Master Manipulator
2.2. Slave Manipulator with a Multi-Slider Structure
3. Kinetics & ADRC-Based Controller to Improve the Displacement Accuracy
3.1. Kinetics Analysis for the Internal Factors of the Slave Manipulator
3.2. ADRC-Based Closed-Loop Control Method
4. Experiments and Results
4.1. Comparative Experiments
4.2. Evaluation Experiments
- (1)
- Experimental Method: The experimental setup, which includes the slave manipulator, master manipulator, catheter, camera, and two-dimensional vascular model, for the evaluation experiments is presented in Figure 8. Furthermore, the vascular model is manufactured using paraffin wax, as shown in Figure 9, in which the initial and target positions in the experiments are marked. This vascular model was used in [32] to estimate the operational skills of a surgeon, which demonstrates the efficiency of the vascular model for research.
- (2)
5. Discussions

6. Conclusions and Future Work
Author Contributions
Funding
Conflicts of Interest
References
- Rafii-Tari, H.; Payne, C.J.; Yang, G.-Z. Current and Emerging Robot-Assisted Endovascular Catheterization Technologies: A Review. Ann. Biomed. Eng. 2014, 42, 697–715. [Google Scholar] [CrossRef]
- Klein, L.W.; Miller, D.L.; Balter, S.; Laskey, W.; Naito, N.; Haines, D.; Ross, A.; Mauro, M.A.; Goldstein, J.A. Occupational health hazards in the interventional laboratory: Time for a safer environment. Catheter. Cardiovasc. Interv. 2009, 73, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Song, Y.; Yin, X.; Zhang, L.; Tamiya, T.; Hirata, H.; Ishihara, H. A Novel Robot-Assisted Endovascular Catheterization System With Haptic Force Feedback. IEEE Trans. Robot. 2019, 35, 685–696. [Google Scholar] [CrossRef]
- Patel, T.M.; Shah, S.C.; Soni, Y.Y.; Radadiya, R.C.; Patel, G.A.; Tiwari, P.O.; Pancholy, S.B. Comparison of robotic percutaneous coronary intervention with traditional percutaneous coronary intervention: A pstringnsity score–matched analysis of a large cohort. Circ. Cardiovasc. Interv. 2020, 13, e008888. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.M.; Frumkin, W.; Ng, G.A.; Neelagaru, S.; Abi-Samra, F.M.; Lee, J.; Giudici, M.; Gohn, D.; Winkle, R.A.; Sussman, J.; et al. First experience with a novel robotic remote catheter system: Amigo™ mapping trial. J. Interv. Card. Electrophysiol. 2013, 37, 121–129. [Google Scholar] [CrossRef]
- Riga, C.V.; Bicknell, C.D.; Rolls, A.; Cheshire, N.J.; Hamady, M.S. Robot-assisted Fenestrated Endovascular Aneurysm Repair (FEVAR) Using the Magellan System. J. Vasc. Interv. Radiol. 2013, 24, 191–196. [Google Scholar] [CrossRef]
- Stereotaxis Inc. Stereotaxis Epoch. 2016. Available online: www.stereotaxis.com (accessed on 11 June 2016).
- Millan, B.; Nagpal, S.; Ding, M.; Lee, J.Y.; Kapoor, A. A Scoping Review of Emerging and Established Surgical Robotic Platforms with Applications in Urologic Surgery. Société Int. d’Urologie J. 2021, 2, 300–310. [Google Scholar] [CrossRef]
- Shi, P.; Guo, S.; Zhang, L.; Jin, X.; Hirata, H.; Tamiya, T.; Kawanishi, M. Design and Evaluation of a Haptic Robot-Assisted Catheter Operating System With Collision Protection Function. IEEE Sens. J. 2021, 21, 20807–20816. [Google Scholar] [CrossRef]
- Yin, X.; Guo, S.; Xiao, N.; Tamiya, T.; Hirata, H.; Ishihara, H. Safety Operation Consciousness Realization of a MR Fluids-Based Novel Haptic Interface for Teleoperated Catheter Minimally Invasive Neurosurgery. IEEE/ASME Trans. Mechatron. 2016, 21, 1043–1054. [Google Scholar] [CrossRef]
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Yang, C.; Jiang, Y. A cooperation of catheters and guidewires-based novel remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 20. [Google Scholar] [CrossRef]
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Yang, C.; Shen, R.; Cui, J.; Jiang, Y.; Liu, X.; Liu, K. Operation evaluation in-human of a novel remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wang, C.; Xie, L.; Zhou, S.; Gu, L.; Xie, H. A novel remote-controlled robotic system for cerebrovascular intervention. Int. J. Med. Robot. Comput. Assist. Surg. 2018, 14, e1943. [Google Scholar] [CrossRef] [PubMed]
- Omisore, O.M.; Han, S.P.; Ren, L.X.; Wang, G.S.; Ou, F.L.; Li, H.; Wang, L. Towards Characterization and Adaptive Compensation of Backlash in a Novel Robotic Catheter System for Cardiovascular Interventions. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 824–838. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Song, H.-S.; Cha, H.-J.; Yi, B.-J. Advantage of Steerable Catheter and Haptic Feedback for a 5-DOF Vascular Intervention Robot System. Appl. Sci. 2019, 9, 4305. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Lee, D.Y. Hydraulically Steerable Micro Guidewire Capable of Distal Sharp Steering. IEEE Trans. Biomed. Eng. 2021, 68, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, J.; Xie, L.; Cui, G. A generalized predictive control for remote cardiovascular surgical systems. ISA Trans. 2020, 104, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, S.; Guo, S.; Meng, C.; Qi, L. Study on robust control for the vascular interventional surgical robot. In Proceedings of the 2019 IEEE International Conference on Mechatronics and Automation (ICMA), Tianjin, China, 4–7 August 2019; pp. 1361–1366. [Google Scholar]
- Sankaran, N.K.; Chembrammel, P.; Siddiqui, A.; Snyder, K.; Kesavadas, T. Design and Development of Surgeon Augmented Endovascular Robotic System. IEEE Trans. Biomed. Eng. 2018, 65, 2483–2493. [Google Scholar] [CrossRef]
- Yang, C.; Guo, S.; Bao, X.; Xiao, N.; Shi, L.; Li, Y.; Jiang, Y. A vascular interventional surgical robot based on surgeon’s operating skills. Med. Biol. Eng. Comput. 2019, 57, 1999–2010. [Google Scholar] [CrossRef]
- Yang, C.; Guo, S.; Guo, Y.; Bao, X. Cloud Communication-Based Sensing Performance Evaluation of a Vascular Interventional Robot System. IEEE Sens. J. 2022, 22, 9005–9017. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, H.; Yu, H.; Wang, F.; Fang, J.; Niu, J.; Guo, S. Machine Learning-Based Surgical State Perception and Collaborative Control for a Vascular Interventional Robot. IEEE Sens. J. 2022, 22, 7106–7118. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, S.; Yu, H.; Song, Y.; Tamiya, T.; Hirata, H.; Ishihara, H. Design and performance evaluation of collision protection-based safety operation for a haptic robot-assisted catheter operating system. Biomed. Microdevices 2018, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Haidegger, T.; Benyó, B.; Kovacs, L.; Benyó, Z. Force Sensing and Force Control for Surgical Robots. IFAC Proc. Vol. 2009, 42, 401–406. [Google Scholar] [CrossRef]
- Haidegger, T. Probabilistic Method to Improve the Accuracy of Computer-Integrated Surgical Systems. Acta Polytech. Hung. 2019, 16, 119–141. [Google Scholar] [CrossRef]
- Haidegger, T.; Kovács, L.; Preitl, S.; Precup, R.-E.; Benyó, B.; Benyó, Z. Controller design solutions for long distance telesurgical applications. Int. J. Artif. Intell. 2011, 6, 48–71. [Google Scholar]
- Zhou, X.; Bian, G.; Xie, X.; Hou, Z.; Qu, X.; Guan, S. Analysis of Interventionalists’ Natural Behaviors for Recognizing Motion Patte ems of Endovascular Tools during Percutaneous Coronary Interventions. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Guo, S.; Guo, J.; Meng, F.; Chen, Z.; Lyu, C. A Surgeon’s Habits-based Novel Master Manipulator for the Vascular Interventional Surgical Master-Slave Robotic System. IEEE Sens. J. 2022. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, S.; Guo, J.; Meng, F.; Chen, Z. ADRC-Based Control Method for the Vascular Intervention Master–Slave Surgical Robotic System. Micromachines 2021, 12, 1439. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, S.; Bao, X.; Guo, Y. Evaluation method of linear displacement precision for a string-driven vascular intervention surgery robot. In Proceedings of the 2019 IEEE International Conference on Mechatronics and Automation (ICMA), Tianjin, China, 4–7 August 2019; pp. 1344–1349. [Google Scholar]
- Han, J. From PID to Active Disturbance Rejection Control. IEEE Trans. Ind. Electron. 2009, 56, 900–906. [Google Scholar] [CrossRef]
- Guo, S.; Cui, J.; Zhao, Y.; Wang, Y.; Ma, Y.; Gao, W.; Mao, G.; Hong, S. Machine learning-based operation skills assessment with vascular difficulty index for vascular intervention surgery. Med. Biol. Eng. Comput. 2020, 58, 1707–1721. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Guo, S.; Guo, J.; Chen, Z.; Meng, F. Kinetics Analysis and ADRC-Based Controller for a String-Driven Vascular Intervention Surgical Robotic System. Micromachines 2022, 13, 770. https://doi.org/10.3390/mi13050770
Zhou W, Guo S, Guo J, Chen Z, Meng F. Kinetics Analysis and ADRC-Based Controller for a String-Driven Vascular Intervention Surgical Robotic System. Micromachines. 2022; 13(5):770. https://doi.org/10.3390/mi13050770
Chicago/Turabian StyleZhou, Wei, Shuxiang Guo, Jin Guo, Zhengyang Chen, and Fanxu Meng. 2022. "Kinetics Analysis and ADRC-Based Controller for a String-Driven Vascular Intervention Surgical Robotic System" Micromachines 13, no. 5: 770. https://doi.org/10.3390/mi13050770





