Atomization Characteristics of Hydrogen Peroxide Solutions in Electrostatic Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
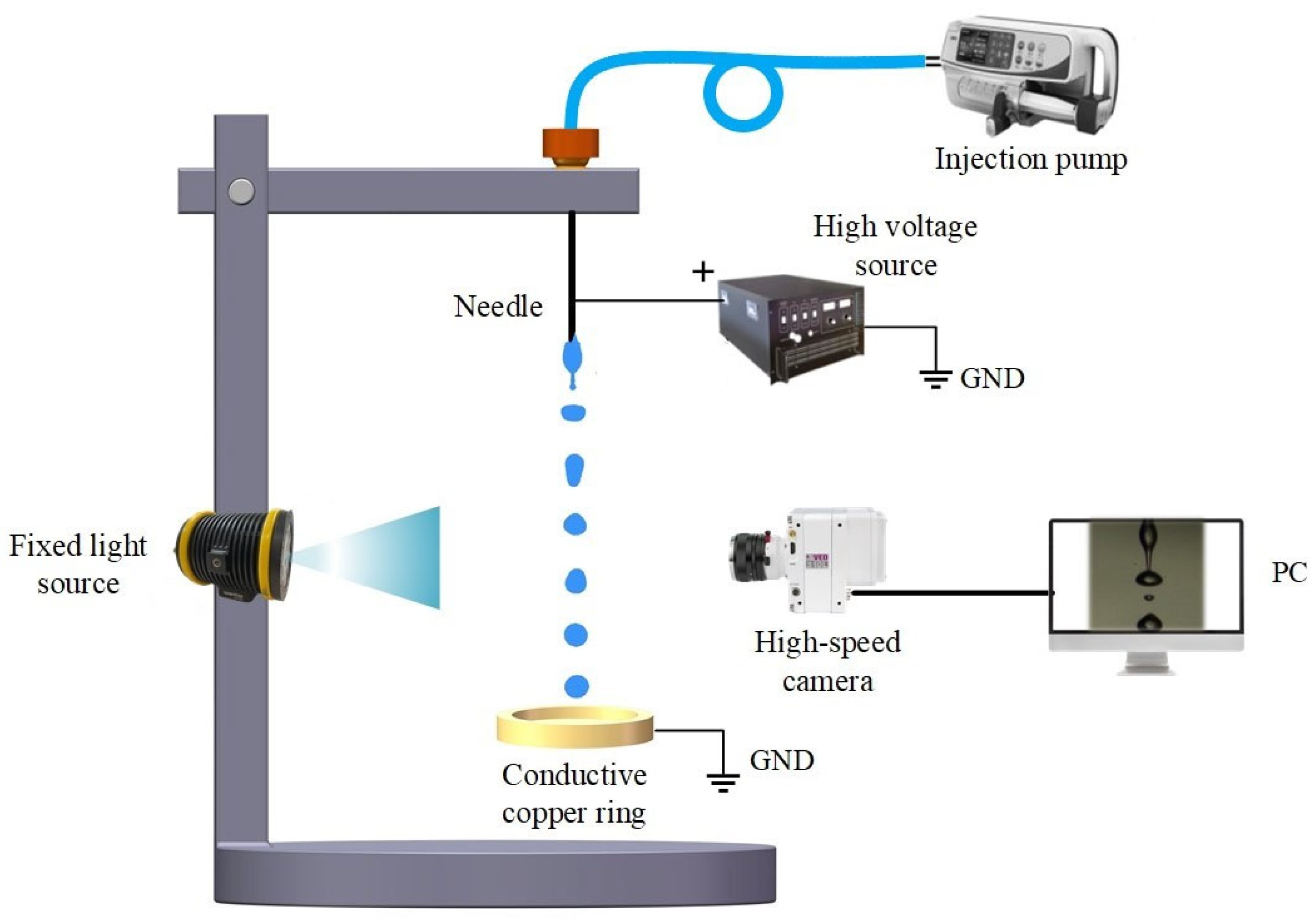
2.2. Experimental Setup
2.2.1. Liquids and Feeding System
2.2.2. Electrospray High-Voltage System
2.2.3. Illumination and Visual Imaging System
3. Results and Discussion
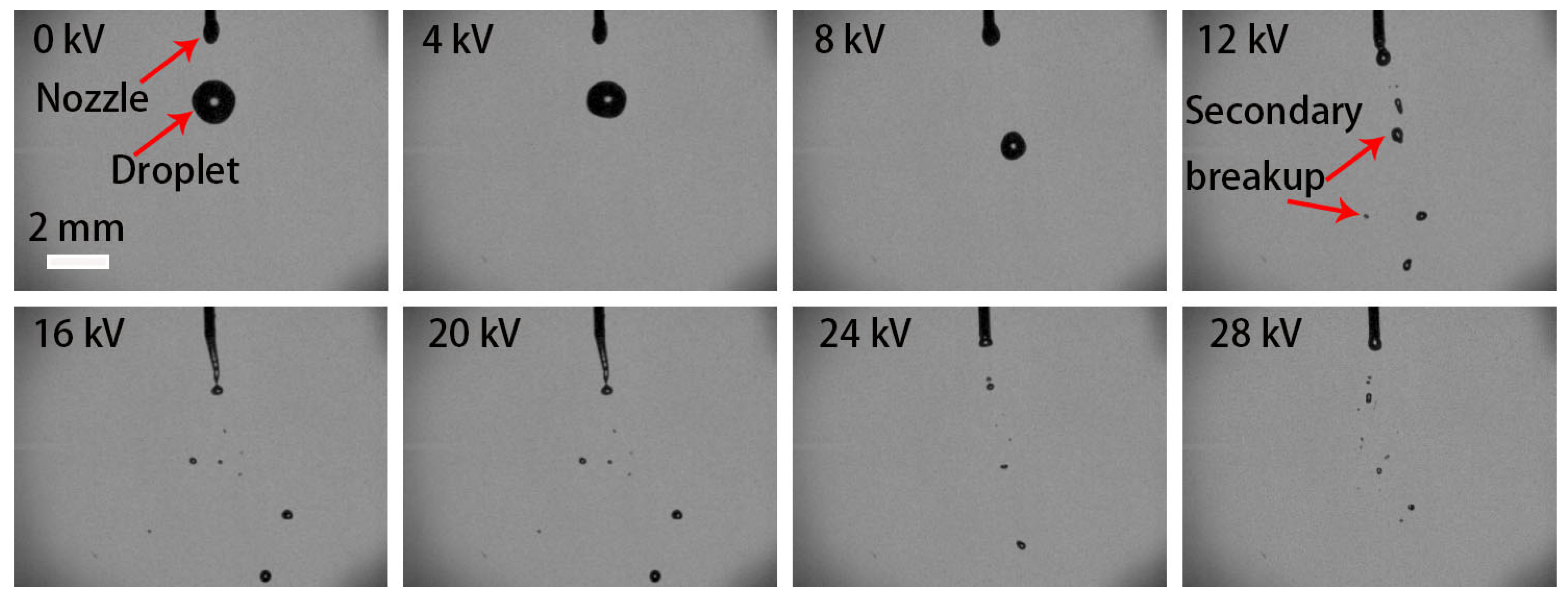
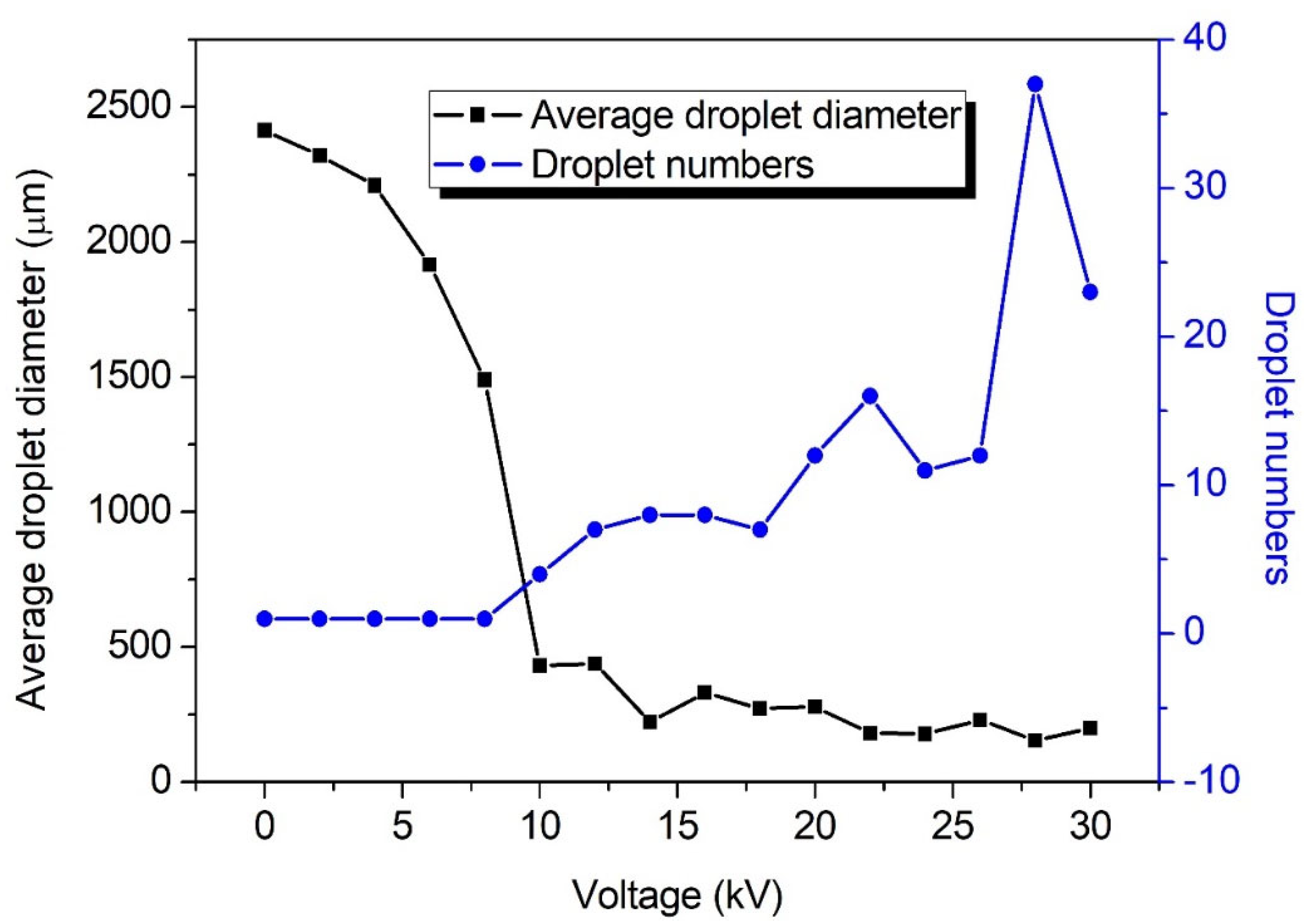
3.1. Effect of Electric-Field Voltage on the Breakup Performance
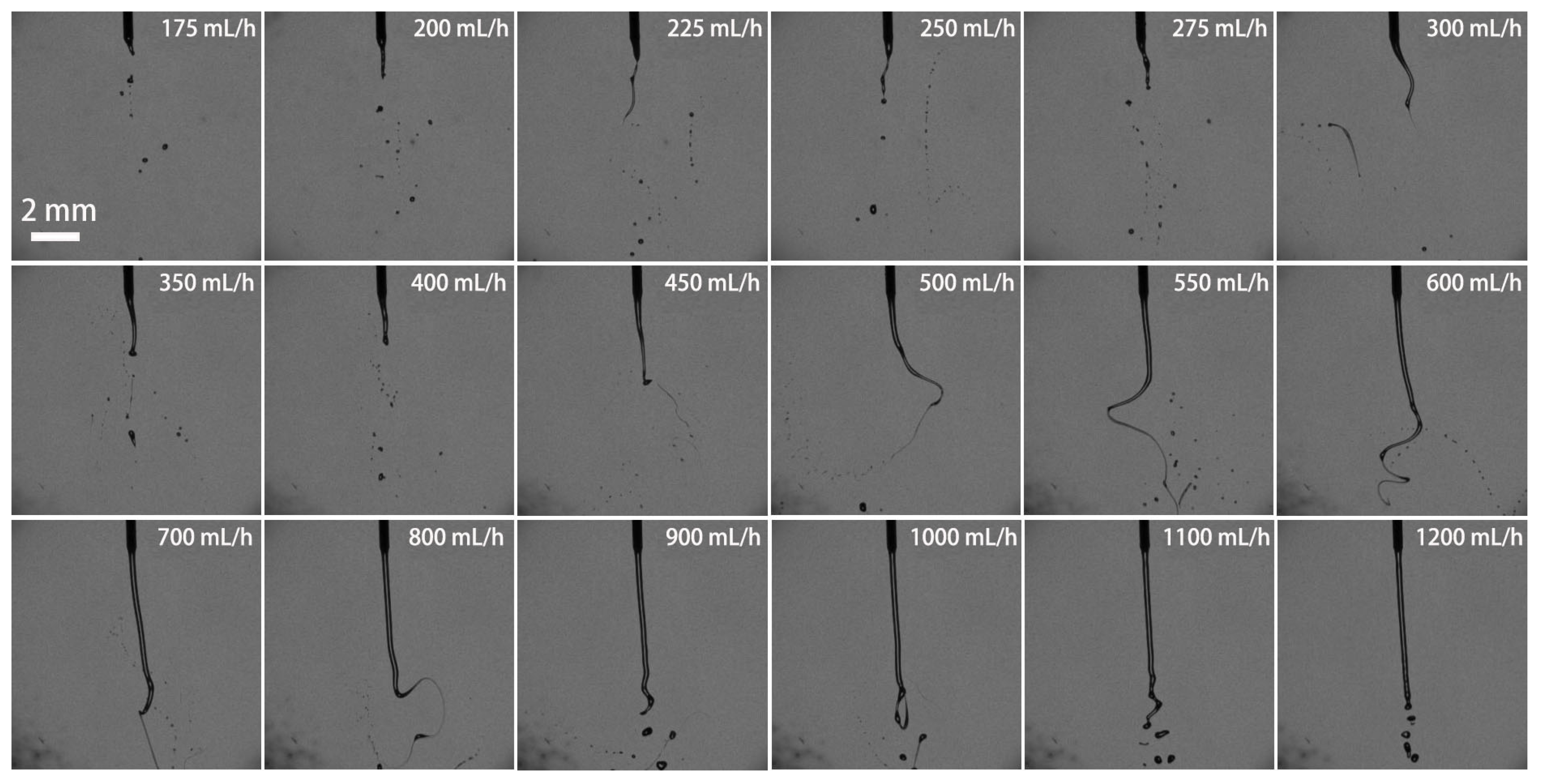
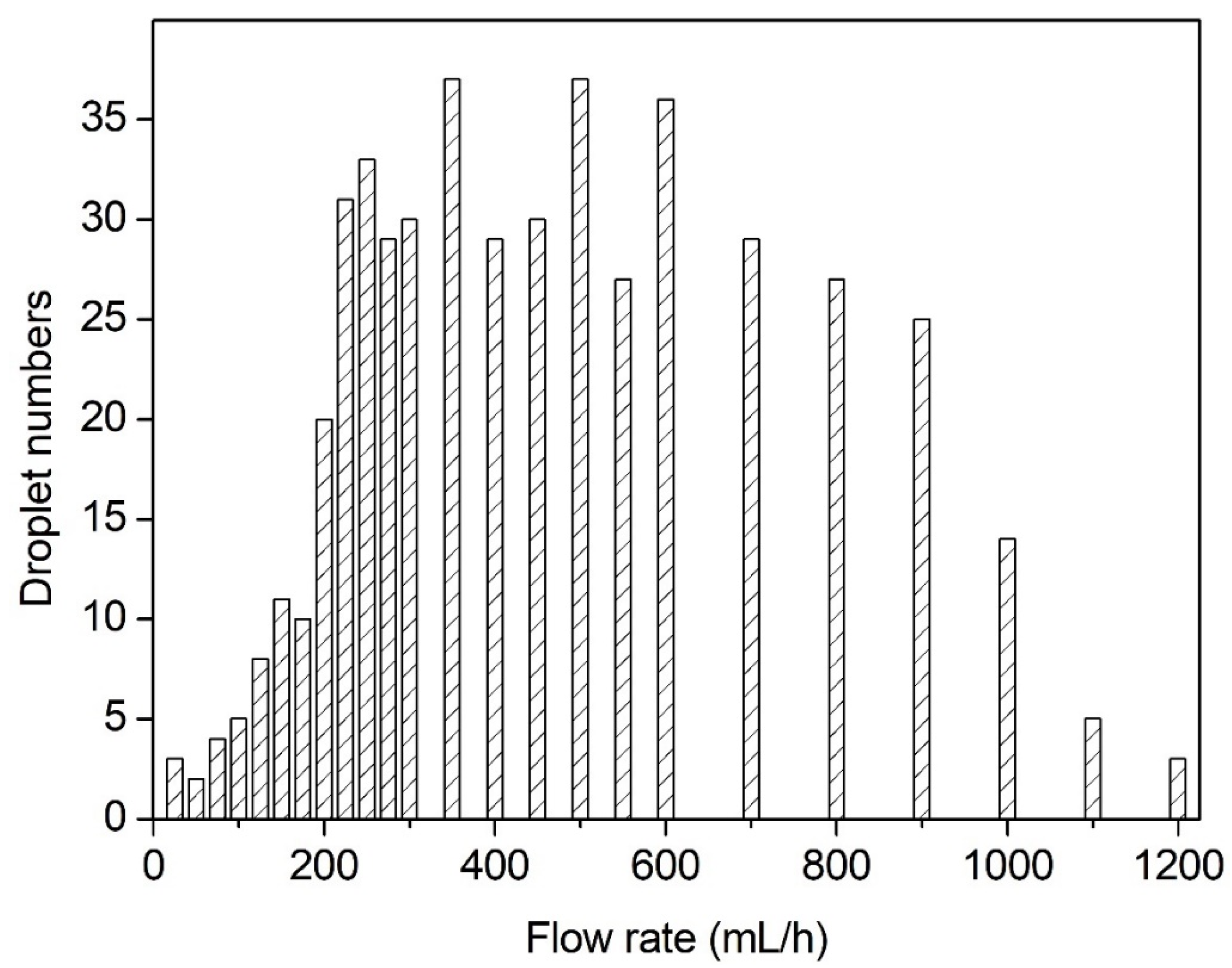
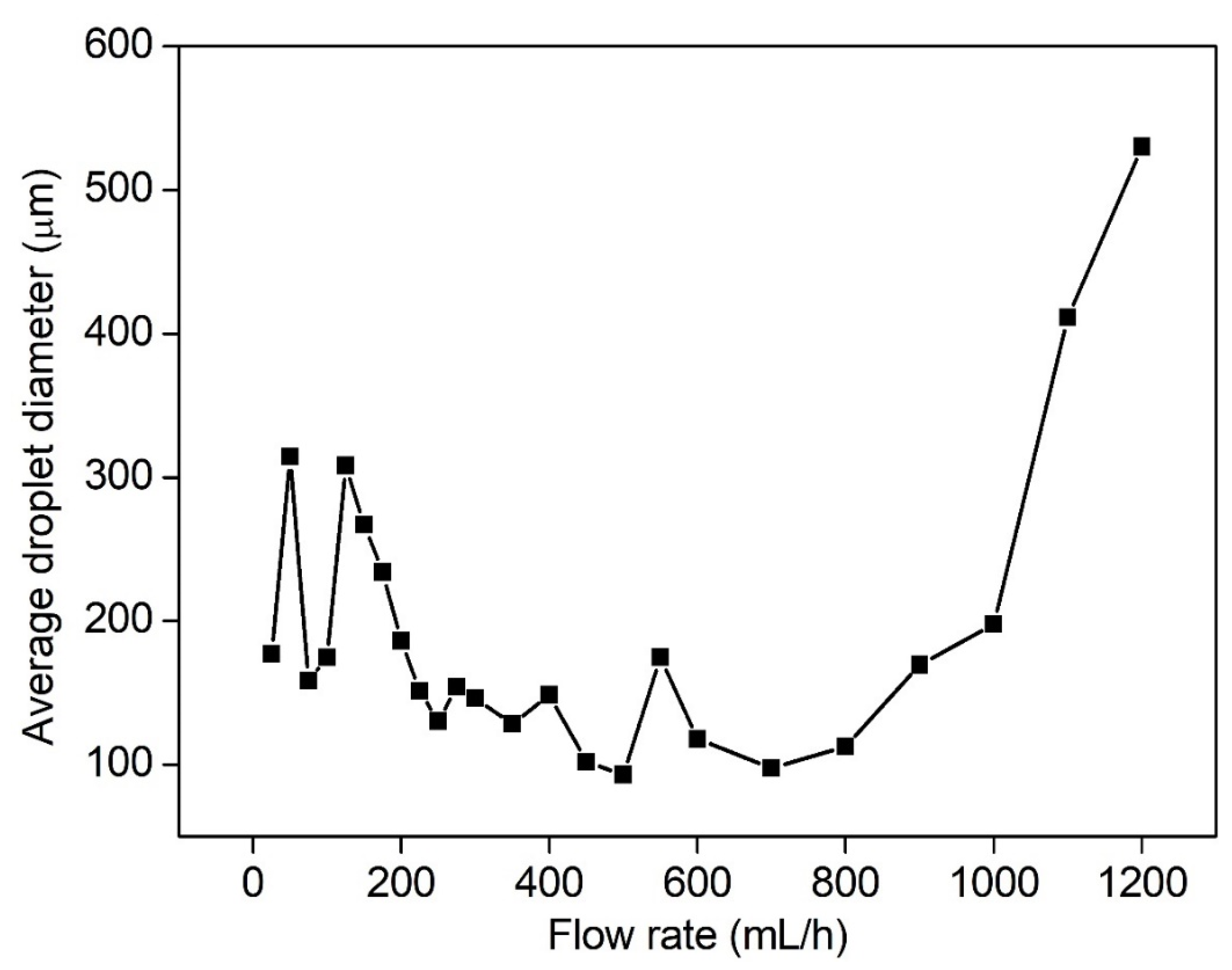
3.2. Effect of Volume Flow Rate on the Breakup Performance
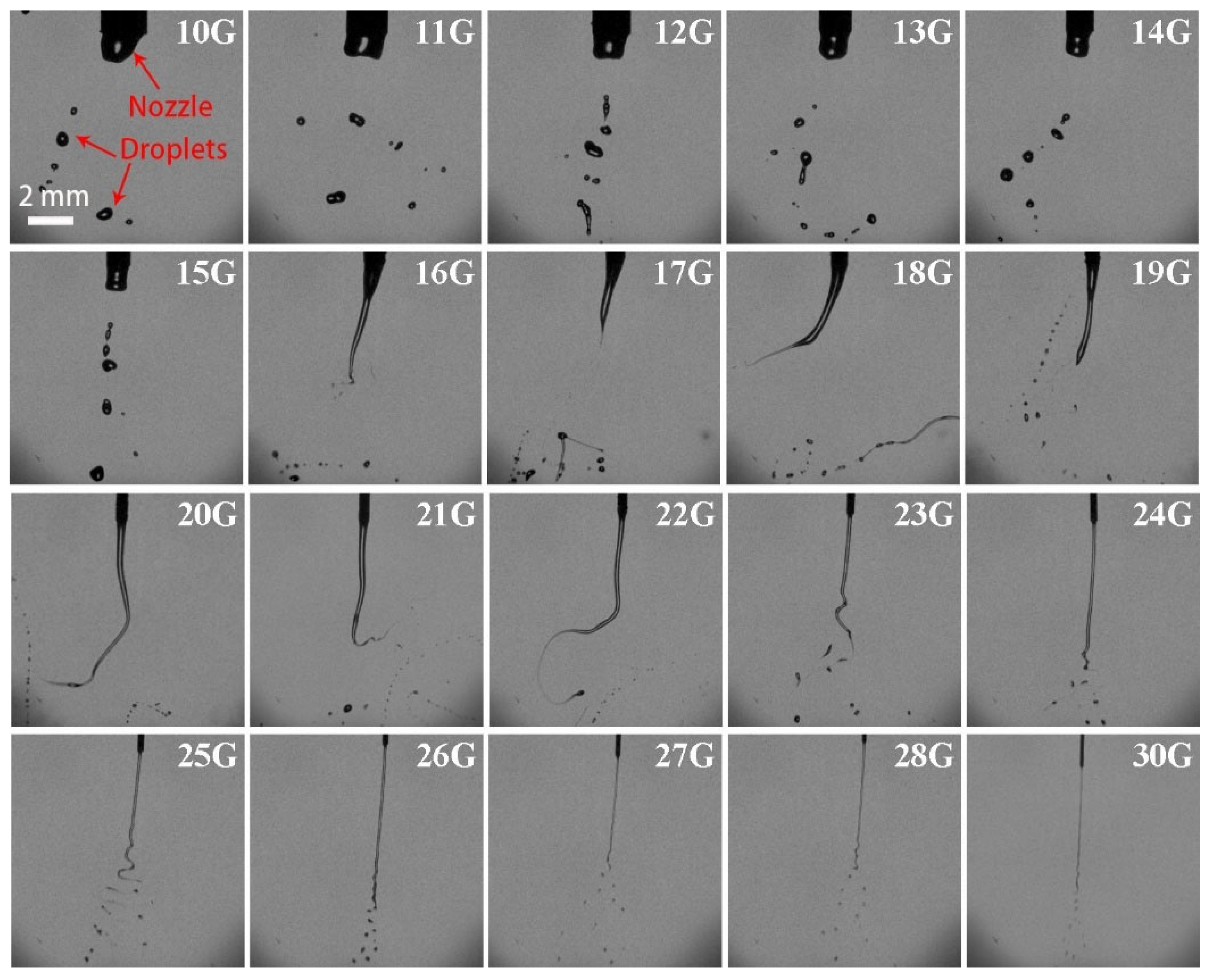
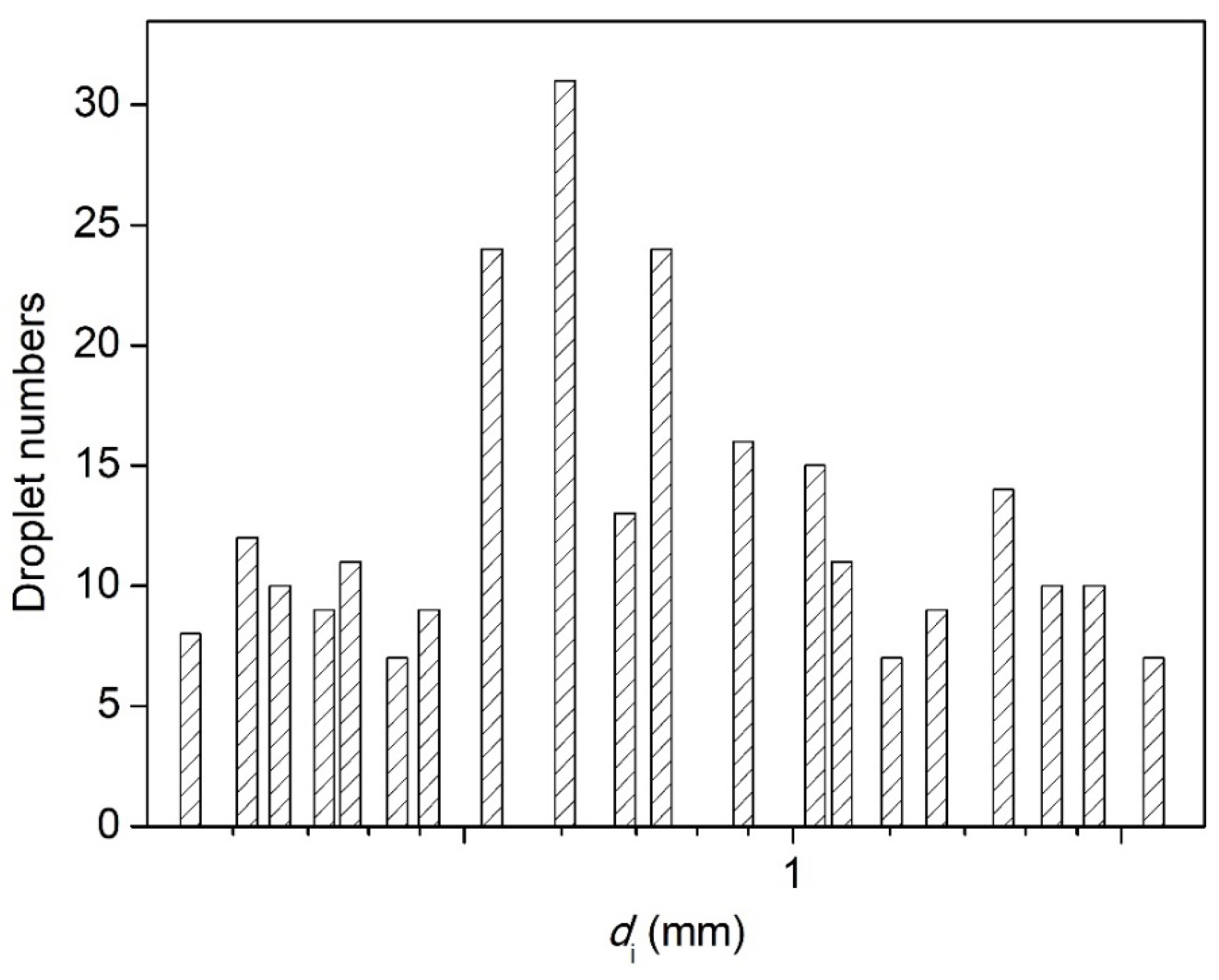
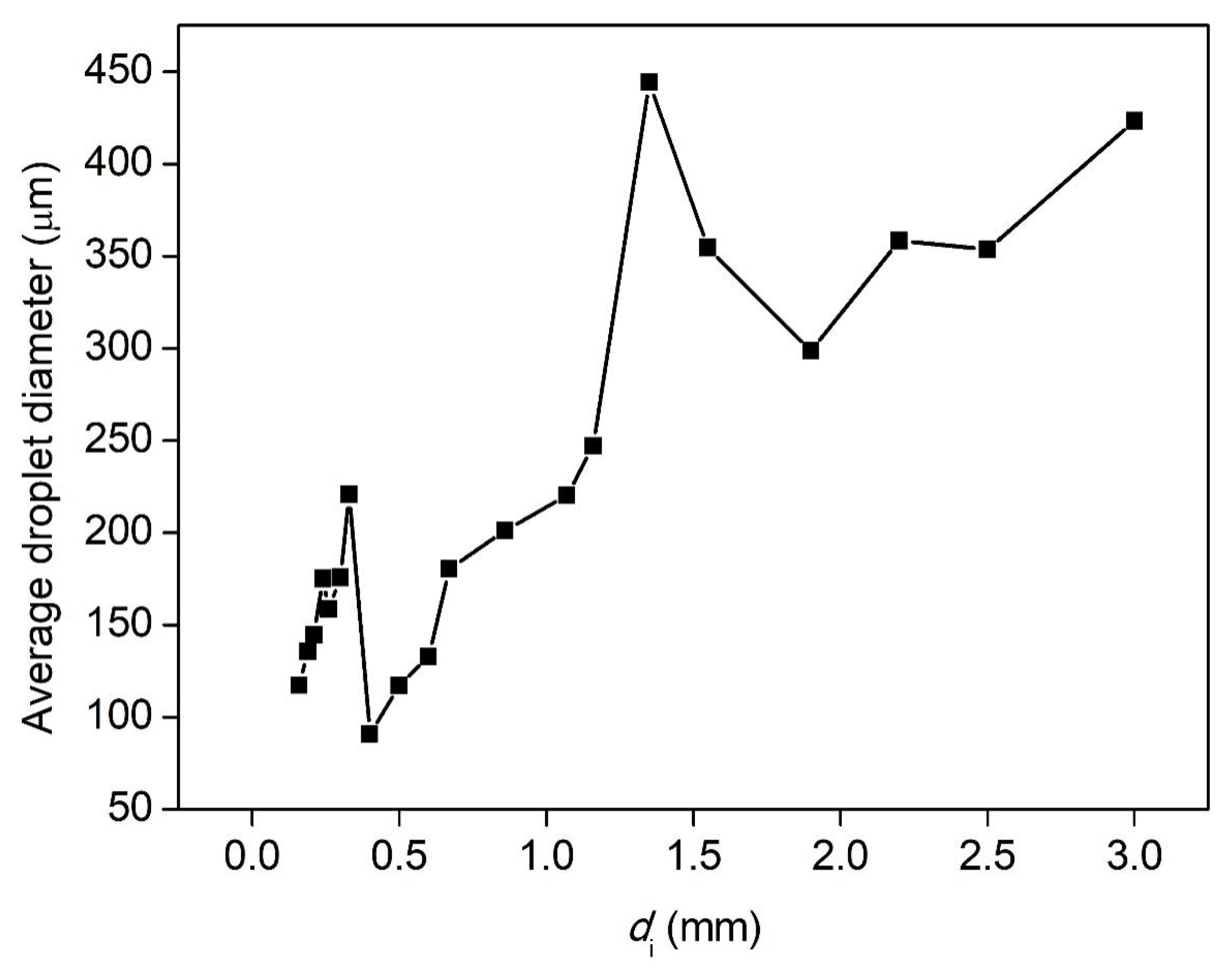
3.3. Effect of Nozzle Size on the Breakup Performance
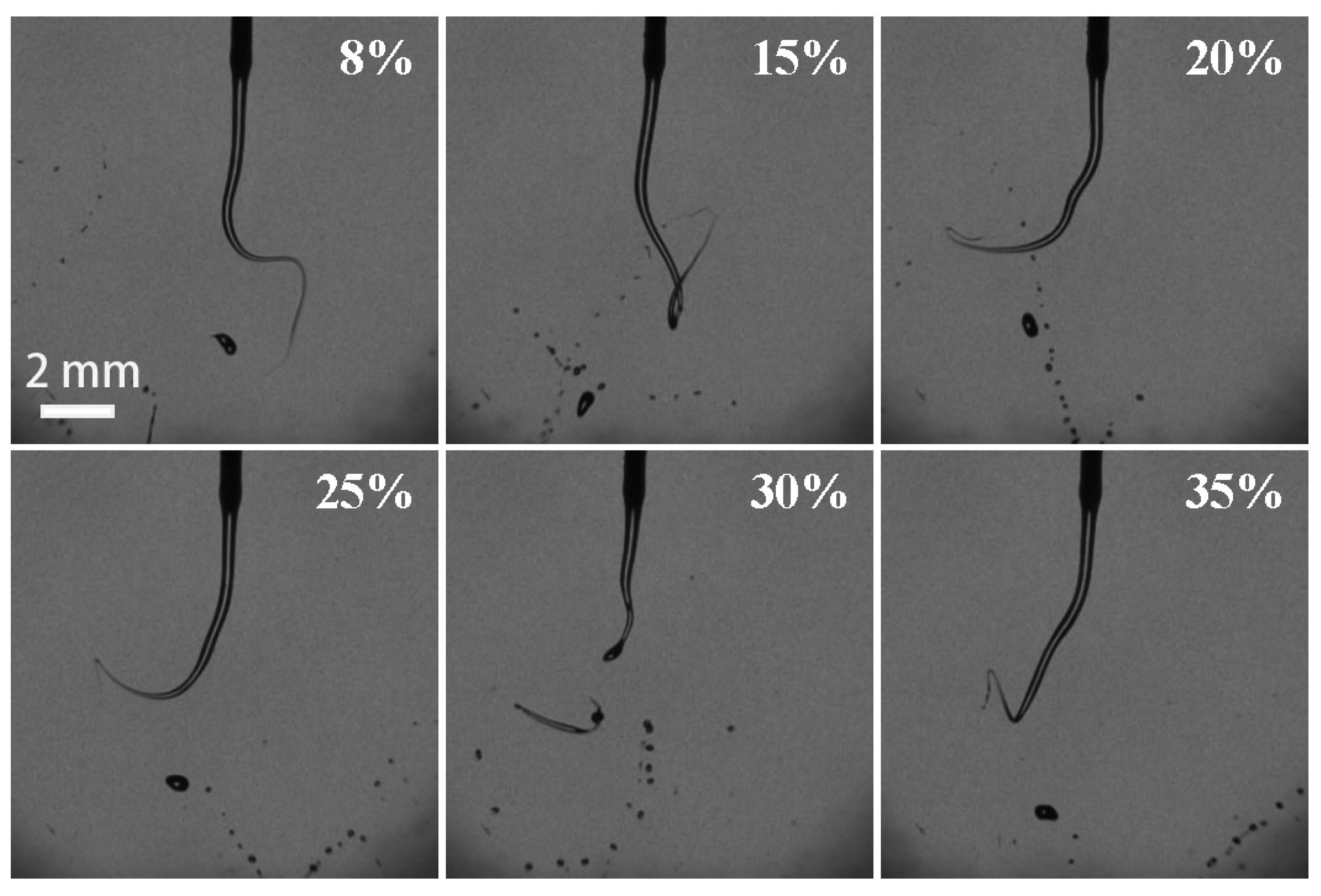
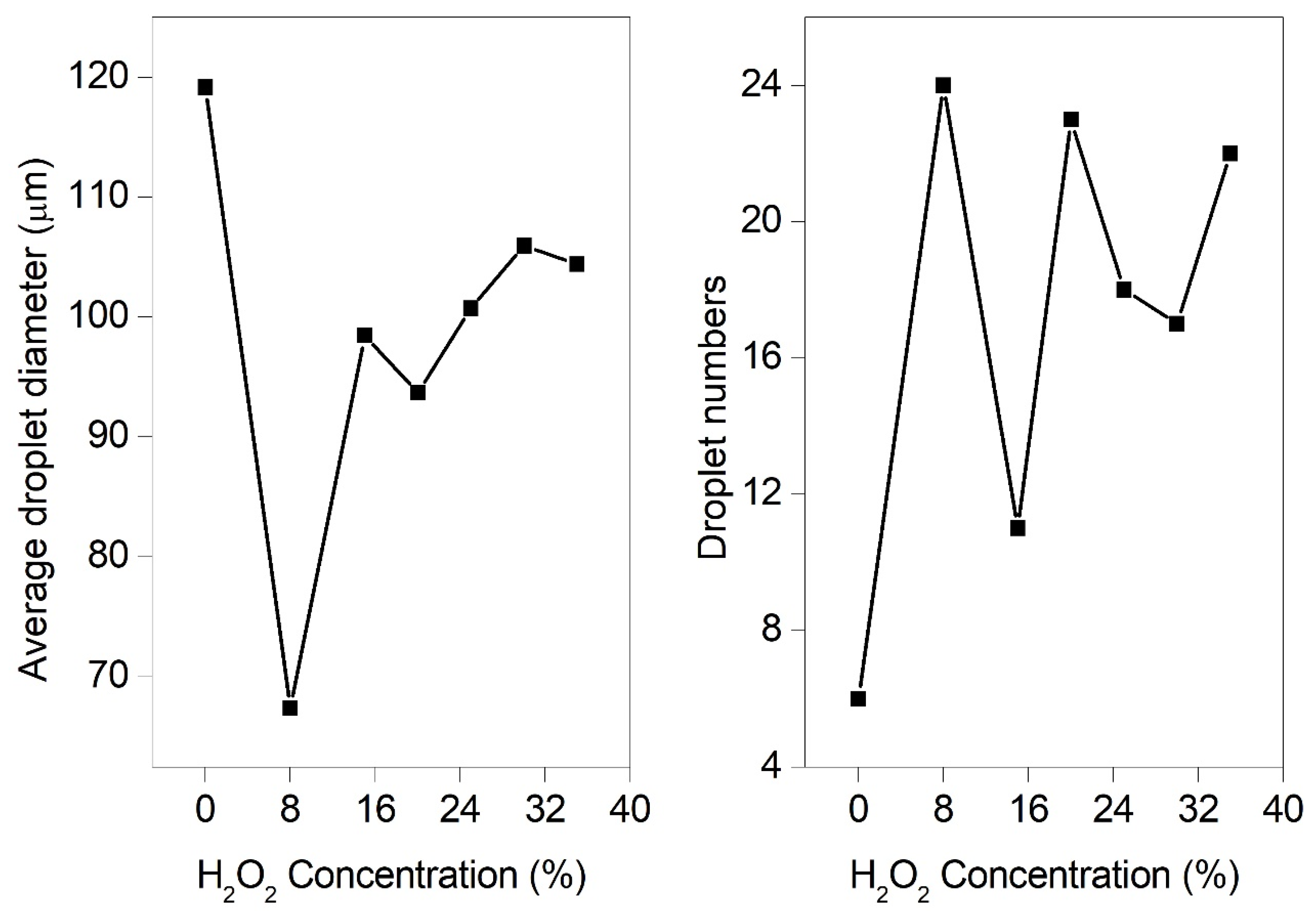
3.4. Effect of H2O2 Concentration on the Breakup Performance
3.5. Mechanism Analysis on Electrostatic Atomization of H2O2 Solution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gatineau, M.; El-Warrak, A.O.; Bolliger, C.; Mourez, M.; Berthiaume, F. Effects of sterilization with hydrogen peroxide gas plasma, ethylene oxide, and steam on bioadhesive properties of nylon and polyethylene lines used for stabilization of canine stifle joints. Am. J. Vet. Res. 2012, 73, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, B.; Rowan, N.J. Terminal sterilization of medical devices using vaporized hydrogen peroxide: A review of current methods and emerging opportunities. J. Appl. Microbiol. 2019, 127, 1403–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, I.A.; Datta, A.K. An overview of sterilization methods for packaging materials used in aseptic packaging systems. Food Bioprod. Process. 2003, 81, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Rohatgi, N.; Schubert, W.; Koukol, R.; Foster, T.L.; Stabekis, P.D. Certification of vapor phase hydrogen peroxide sterilization process for spacecraft application. In Proceedings of the 32nd ICES Conference, San Antonio, TX, USA, 15 July 2002. [Google Scholar]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen peroxide: A key chemical for today’s sustainable development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Mullens, P. The use of hydrogen peroxide for propulsion and power. In Proceedings of the 35th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Los Angeles, CA, USA, 20–24 June 1999. [Google Scholar]
- Pasini, A.; Torre, L.; Romeo, L.; Cervone, A.; d’Agostino, L. Testing and Characterization of a Hydrogen Peroxide Monopropellant Thruster. J. Propuls. Power 2008, 24, 507–515. [Google Scholar] [CrossRef]
- Zaseck, C.R.; Son, S.F.; Pourpoint, T.L. Combustion of micron-aluminum and hydrogen peroxide propellants. Combust. Flame 2013, 160, 184–190. [Google Scholar] [CrossRef]
- Woschnak, A.; Krejci, D.; Schiebl, M.; Scharlemann, C. Development of a green bipropellant hydrogen peroxide thruster for attitude control on satellites. Prog. Propuls. Phys. 2013, 4, 689–706. [Google Scholar]
- Nagiev, T.M. The coupled reactions of oxidation by hydrogen peroxide. Russ. Chem. Rev. 1985, 54, 974–985. [Google Scholar] [CrossRef]
- Quinn, J. Oxidizer selection for the ISTAR program (liquid oxygen versus hydrogen peroxide). In Proceedings of the 38th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Indianapolis, IN, USA, 7–10 July 2002. [Google Scholar]
- Low, A.; Lay, M.; Verbeek, J.; Swan, J. Decoloring hemoglobin as a feedstock for second-generation bioplastics. Prep. Biochem. Biotechnol. 2012, 42, 29–43. [Google Scholar] [CrossRef]
- Cramer, J.; Greene, M.; Pal, S.; Santoro, R. RBCC ejector mode operating characteristics for single and twin thruster configurations. In Proceedings of the 37th Joint Propulsion Conference and Exhibit, Salt Lake City, UT, USA, 8–11 July 2001. [Google Scholar]
- Scharlemann, C. Development of Miniaturized Green Propellant Based Mono-and Bipropellant Thrusters. In Proceedings of the 43rd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Cincinnati, OH, USA, 8–11 July 2007. [Google Scholar]
- Resendel, M.G.; Ribeiro, C.C. GRASP with path-relinking: Recent advances and applications. In Metaheuristics: Progress as Real Problem Solvers; Springer: Boston, MA, USA, 2005; pp. 29–63. [Google Scholar]
- Lehmann, A.; Overton, J.M.C.; Leathwick, J.R. GRASP: Generalized regression analysis and spatial prediction. Ecol. Model. 2002, 157, 189–207. [Google Scholar] [CrossRef]
- Krejci, D.; Woschnak, A.; Scharlemann, C.; Ponweiser, K. Hydrogen peroxide decomposition for micro propulsion: Simulation and experimental verification. In Proceedings of the 47th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Diego, CA, USA, 31 July–3 August 2011. [Google Scholar]
- Ahmed, T.; Kourmatzis, A.; Masri, A.R. Atomization behaviour of a hybrid air-blast-electrostatic atomizer for spray combustion. Fuel 2021, 288, 119716. [Google Scholar] [CrossRef]
- Zeng, W.; Guo, P.; Jiang, P.; Liu, W.; Hong, T.; Chen, C. Combination of microfluidic chip and electrostatic atomization for the preparation of drug-loaded core–shell nanoparticles. Nanotechnology 2020, 31, 145301. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Li, J. Experimental Study on Particle Removal of a Wet Electrostatic Precipitator with Atomization of Charged Water Drops. Energy Fuels 2020, 34, 7257–7268. [Google Scholar] [CrossRef]
- Hara, M.; Ishibe, S.; Sumiyoshitani, S.; Akazaki, M. Electrical corona and specific charge on water drops from a cylindrical conductor with high d.c. voltage. J. Electrost. 1980, 8, 239–270. [Google Scholar] [CrossRef]
- Maski, D.; Durairaj, D. Effects of electrode voltage, liquid flow rate, and liquid properties on spray chargeability of an air-assisted electrostatic-induction spray-charging system. J. Electrost. 2010, 68, 152–158. [Google Scholar] [CrossRef]
- Chen, G.; Gomez, A. Counterflow diffusion flames of quasi-monodisperse electrostatic sprays. Symp. Combust. 1992, 24, 1531–1539. [Google Scholar] [CrossRef]
- Kreitzer, P.; Kuhlman, J.; Mehra, D.; Gray, D.; Yerkes, K. Effects of contact charging on spray impingement heat transfer performance and spray behavior. In Proceedings of the 39th AIAA Thermophysics Conference, Miami, FL, USA, 25–28 June 2007. [Google Scholar]
- William, B.M. Hydrogen peroxide-ACS Monograph Series: By Walter C. Schumb, Charles N. Satterfield and Ralph L. Wentworh. J. Frankl. Inst. 1956, 261, 380. [Google Scholar]
- Li, S.; Zhuo, Z.; He, L.; Huang, X. Atomization characteristics of nano-Al/ethanol nanofluid fuel in electrostatic field. Fuel 2019, 236, 811–819. [Google Scholar] [CrossRef]
- Zhuo, Z.; Li, S.; Lu, Y.; Huang, X. Synergetic effects of nanoparticle concentration and electrification on the breakup performance of nanofluid fuel. Int. J. Heat Mass Transf. 2019, 137, 940–950. [Google Scholar] [CrossRef]
- Cloupeau, M.; Prunet-Foch, B. Electrostatic spraying of liquids in cone-jet mode. J. Electrostat 1989, 22, 135–159. [Google Scholar] [CrossRef]
- Yoon, S.S.; Heister, S.D. Categorizing linear theories for atomizing round jets. At. Sprays 2003, 13, 499–516. [Google Scholar] [CrossRef]
- Kourmatzis, A.; Ergene, E.L.; Shrimpton, J.S.; Kyritsis, D.C.; Mashayek, F.; Huo, M. Combined aerodynamic and electrostatic atomization of dielectric liquid jets. Exp. Fluids 2012, 53, 221–235. [Google Scholar] [CrossRef]
- Rigit, A.R.H.; Shrimpton, J.S. Electrical performance of charge injection electrostatic atomizers. At. Sprays 2006, 16, 401–419. [Google Scholar] [CrossRef]
- Rigit, A.R.H.; Shrimpton, J.S. Spray characteristics of charge injection electrostatic atomizers with small orifice diameter. At. Sprays 2006, 16, 421–442. [Google Scholar]
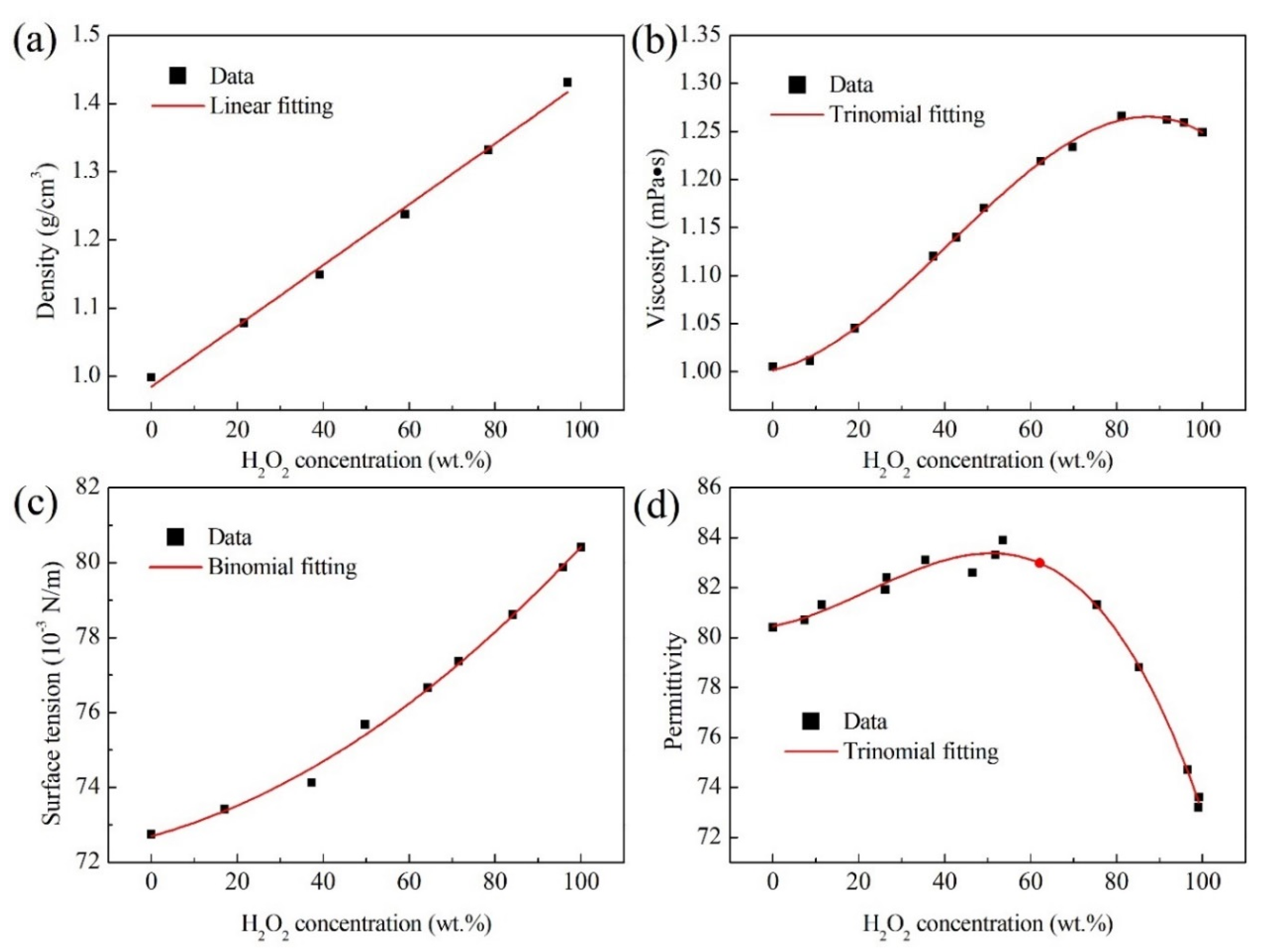

| Physical Properties | Model Formula |
|---|---|
| Density (ρ) | ; R2 = 0.994 |
| Viscosity (μ) | ; R2 = 0.998 |
| Surface tension (γ) | ; R2 = 0.994 |
| Permittivity (ε) | ; R2 = 0.990 |
| Nozzle Types | Inner Diameter (di, mm) | Outer Diameter (do, mm) |
|---|---|---|
| 10G | 3.00 | 3.50 |
| 11G | 2.50 | 3.00 |
| 12G | 2.20 | 2.80 |
| 13G | 1.90 | 2.40 |
| 14G | 1.55 | 2.10 |
| 15G | 1.35 | 1.80 |
| 16G | 1.16 | 1.60 |
| 17G | 1.07 | 1.47 |
| 18G | 0.86 | 1.26 |
| 19G | 0.67 | 1.07 |
| 20G | 0.60 | 0.90 |
| 21G | 0.50 | 0.80 |
| 22G | 0.40 | 0.70 |
| 23G | 0.33 | 0.63 |
| 24G | 0.30 | 0.55 |
| 25G | 0.26 | 0.50 |
| 26G | 0.24 | 0.45 |
| 27G | 0.21 | 0.40 |
| 28G | 0.19 | 0.35 |
| 30G | 0.16 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Sheng, L.; Lu, Y.; Li, S. Atomization Characteristics of Hydrogen Peroxide Solutions in Electrostatic Field. Micromachines 2022, 13, 771. https://doi.org/10.3390/mi13050771
Huang X, Sheng L, Lu Y, Li S. Atomization Characteristics of Hydrogen Peroxide Solutions in Electrostatic Field. Micromachines. 2022; 13(5):771. https://doi.org/10.3390/mi13050771
Chicago/Turabian StyleHuang, Xuefeng, Ling Sheng, Yibin Lu, and Shengji Li. 2022. "Atomization Characteristics of Hydrogen Peroxide Solutions in Electrostatic Field" Micromachines 13, no. 5: 771. https://doi.org/10.3390/mi13050771






