1. Introduction
Optofluidics have emerged in recent years as a new discipline able to combine fluidic and optical functions on a miniaturized scale in a unique and unprecedented manner [
1,
2,
3]. Thanks to the recent advances in the field of microfluidics, simpler and precise handling of fluids can be achieved on a microscale, offering new opportunities to realize optofluidic devices [
4]. In addition to the advantage of reducing the overall device size, microfluidic integration can improve the repeatability of the device response by allowing for fine control of the liquid flow.
In the field of optical sensing, microfluidic integration presents the relevant implication of reducing sample and reagent volumes and of preventing evaporation of the sample under analysis during tests. Due to the enormous benefits provided by the optofluidic approach in the realization of miniaturized optical sensors, a huge number of sensor architectures have been proposed in recent years for the realization of self-containing sensors for liquid sensing, the so-called optical “lab-on-a-chip” (LOC) [
1,
5,
6].
The prospect of lowering the cost and simplifying the fabrication procedure has made polymer materials the favorite choice for building microfluidic systems, in turn, inspiring the development of optofluidic devices based on hybrid integration of polymer microfluidic and silicon optical modules assembled with a multilayered modular approach [
7,
8,
9,
10].
Waveguides integrated with microfluidic channels often form the basis of microfluidic networks for optofluidic device design [
11,
12,
13,
14]. Integrated devices which combine both fluidic channels and optical waveguides on a planar platform have been demonstrated [
15,
16,
17].
The introduction of integrated optofluidic liquid-core waveguides represents a step forward in the development of optofluidic devices as they intrinsically provide devices with microfluidic capability [
1,
18]. However, microfluidic components such as the inlet and outlet and channels for injecting and guiding fluids need to be interconnected with optofluidic channels, without affecting the optical performance of the waveguides themselves.
Optofluidic waveguides can serve as sensors. Because the waveguide itself acts as a channel for transporting both the liquid sample and the optical probing mode, a high level of compactness and increased sensitivity can be achieved by implementing an optofluidic waveguide as a building block in sensor design.
Silicon-based optofluidic waveguides such as slot, photonic crystal waveguides and ARROWs have shown great potential for planar photonics and novel optofluidic devices for sensing applications [
10,
18,
19,
20].
In the study by Measor et al. [
21], an architecture of solid-core and optofluidic waveguides, comprising fluidic reservoirs for sample storage connected to the optofluidic waveguide, has been integrated on a planar optofluidic platform for sensing applications. In the study by Testa et al. [
9], a planar optofluidic platform for sensing applications has been realized with a modular hybrid approach, with a microfluidic inlet, outlet and a micromixer fabricated in the top polymeric layers and fluidically connected with the underneath optofluidic waveguide.
The integration of microfluidic connections has become more difficult in the case of micron-sized and high performance interferometric photonic devices, such as optofluidic ring resonators, where an unsuitable device design could cause a strong degradation of the device performance.
In the study by Chandrahalim et al. [
22], fluidic channels for sample delivery in a planar silica-based hollow core ring resonator were inserted on the inner side of the hollow waveguides shaping the cavity where virtually no WGM is excited, without significantly affecting the optical performance of the resonator.
In previous work, we presented a hybrid planar optofluidic ring resonator sensor [
23], where each of the photonic functional elements (the cavity, the bus waveguide and the coupler) was formed by liquid-core waveguides (hybrid ARROW) [
24]. The proposed ring did not integrate any microfluidic channels for sample delivery; thus, the liquid core was filled by capillary action from the open-ended bus waveguide, and the sensing performance was evaluated by thermally induced changes in the core refractive index.
In a planar optofluidic ring resonator, microfluidic channels must be properly placed along the ring path in order to ensure uniform and rapid liquid replacement. However, inserting microfluidic channels across the optofluidic waveguides can increase the propagation losses by disrupting the optical confinement in the waveguides and decreasing the Q-factor.
In this study, we design, simulate and experimentally demonstrate that noninvasive substrate integration of a planar network of microfluidic channels in an ARRROW-based hybrid optofluidic ring resonator can be accomplished by carefully designing the device geometry. In particular, the integration of microfluidic channels is achieved by exploiting the interference pattern created by the self-imaging effect in multimode interference waveguide couplers (MMI).
2. Design and Simulations
Figure 1a shows the basic ring layout. Optical design optimization of the ring has been performed by following the general rules as reported in [
25]. Hybrid ARROW waveguides with four antiresonant cladding layers of alternating high and low refractive indexes shape the ring resonator (
Figure 1b) [
24]. The waveguide, core width d
c = 3 μm and depth h = 12 μm, is designed to support linearly polarized, quasi-single mode operation at the working wavelength of 780 nm.
The ring resonator comprises the bus waveguides, the MMI coupler (MMIc), bend-shaped waveguides with a curvature radius of R = 100 μm and a single mode straight waveguide (SW). The MMIc has a width of WMMI = 10.24 μm and a length of LMMI = 354 μm, and it is designed to equally split the input power into the two output waveguides (balanced splitter). The SW has a length of L = LMMI.
For microfluidic channels (MCs) integration, two important points have been considered: (1) from a fluidic point of view, inlet and outlet channels should be opportunely positioned in order to ensure rapid and complete liquid replacement in the cavity, and (2) from an optical point of view, the channels should be positioned in order to minimize the induced disturbance on the propagating mode.
Regarding the last requirement, fluidic connections should be positioned on the input and output bus waveguides in order to avoid any perturbation of the ring cavity (see
Figure 2a). However, from a fluidic point of view, this configuration can be modeled as two hydraulic resistances in parallel, R
MMI and R
Ring, that are strongly unbalanced (R
Ring ≈ 60 R
MMI) [
26]. This implies a very slow liquid flow rate in the ring that, in turn, can cause inefficient replacement of the liquid. By placing the inlet and outlet channels on opposite sides of the ring loop (
Figure 2b) it is possible to balance the fluidic resistances, R
L and R
R, and ensure a fast and complete replacement of the liquid in the cavity. In this configuration we have estimated R
R ≈ 0.96 R
L.
In order to compare the fluidic schemes, finite element method (FEM) microfluidics simulations using COMSOL Multiphysics software were performed. Three-dimensional steady-state velocity distribution of the solution is shown in
Figure 3 for both the unbalanced and balanced fluidic ring scheme. In both schemes, the flow rate of the inlet fluid stream was set to 0.1 m/s.
Figure 3a reports the simulation result for the unbalanced configuration. The ratio R
Ring/R
MMI can be calculated by taking the flow rate ratio FRR
U = Q
MMIc/Q
SW between the flow rate in the MMIc (Q
MMIc) and in the SW (Q
SW) [
26], obtaining the FRR
U = 77.3. In
Figure 3b) the simulation result for the balanced configuration is reported. In this case, the ratio R
R/R
L can be calculated as the flow rate ratio FRR
B = Q
L/Q
R = 1.3. These results confirm that in the unbalanced fluidic scheme the liquid flows substantially in the MMIc, causing an inefficient liquid replacement in the ring loop. In the balanced fluidic scheme, instead, the inlet fluid flows along the whole ring cavity. Moreover, because the flow rate in the input and output bus waveguides is one order of magnitude lower than the rate of the flow in the ring cavity, the balanced ring configuration prevents the problem of liquid leakage from the open-ended bus waveguides.
On the basis of the fluidic analysis, the layout of the optofluidic ring resonator has been designed with microfluidic channels placed on the opposite side of the ring loop, following the scheme of
Figure 2b. However, this configuration requires an accurate optical design to avoid an increase in the optical losses at the intersection point of the microfluidic channels with the waveguide wall of the ring cavity.
Figure 4 shows the proposed layout. As can be observed from
Figure 4, an additional multimode interference device (MMI
SW) has been integrated to assist microfluidic channel insertion on the SW, as will be discussed in the following section.
The effect on the propagation losses due to the removal of the waveguide boundary in correspondence with the MCs insertions has been investigated by numerical simulations. Numerical simulations have been performed by using a two-dimensional finite-difference time-domain method (2D FDTD) (OMNISIM, © Photon Design, Oxford, UK). The optical behavior of the MMI coupler (MMIc) and of the straight optofluidic waveguide channel has been studied.
First, the unperturbed MMI
c (without integrated MCs) has been simulated. The field of a single mode ARROW waveguide at λ = 780 nm is considered the exciting optical source on the input waveguide (i1).
Figure 5 displays the interference pattern created by the multimode interference effect in the MMI
c. From simulations, we have estimated the normalized power transmitted on output channel 1 (O
1),
, and output channel 2 (O
2),
, of the unperturbed MMI.
and
are the power transmitted from port O
1 and port O
2 and
is the reference input power, transmitted from the input port i1 and acting as the exciting field. The transmitted power is referred to the fundamental ARROW mode. We obtained
and
. The discrepancy with the theoretical values of
P1t = 0.50 and
P2t = 0.50 has already been described elsewhere [
25] and can be explained by taking into account the optical losses due to the leaky propagation of the h-ARROW modes. Because the MMI effect is the result of the co-propagation of leaky higher order modes, interferential output images undergo degradation to some extent. From the
Figure 5, the interference produces an intensity profile along the MMI
c length with a series of very low (virtually null) intensity regions in the proximity of the waveguide walls where microfluidic channels can be inserted to minimize their influence on the light propagation. It should be noted that during the light circulation in the ring cavity the MMI
c is alternately excited from both the input ports (O
1, O
2) and, in particular, by the bus waveguide (port O
1) and the by the ring loop (port O
2), in the former case producing an interference pattern that is mirrored with respect to the propagation axis z as compared with the pattern shown in the
Figure 5. Based on this consideration, in order to reduce the perturbation on waveguide propagation, the microfluidic channel can be placed where a null intensity region appears in the proximity of both opposite MMI
c walls (shaded box in the figure).
Hence, the MMI
c has been simulated by inserting a 14 μm wide microfluidic channel which is located at z = 305 µm, where the field intensity decreases substantially near the ARROW waveguide wall. Numerical results give, in this case, transmitted powers
and
. These values are very close to the ones calculated for the unperturbed MMI
c and demonstrate that the integration of the MC does not introduce additional losses, and the modal propagation is substantially unaffected by the integration of the microfluidic channel. In order to confirm the effectiveness of our design approach, the MMI
c has been simulated by including a 14 μm wide MC placed at z = 205 µm, where the field is high near the waveguide wall. In this case, we obtained
and
, resulting in a power drop of about 10.6% and 6.8% on channels O
1 and O
2, respectively. The results are summarized in
Table 1.
Concerning the insertion of the microchannel MC
2 along the ring loop, we evaluated the impact of this integration along the straight waveguide (SW), that is a single mode h-ARROW waveguide, on the mode propagation. We preliminarily evaluated the normalized power transmitted by the unperturbed waveguide (no MC
2), obtaining a value of
.
P0,ch is the reference power, referred to the fundamental input ARROW mode. This result was then compared with the normalized power transmitted by the same waveguide with an integrated 10 µm-wide MC, which is centered with respect to the propagation axis of the SW (see
Figure 2), obtaining
. Hence, a power drop of about 13% should be expected due to microfluidic integration.
Using the same argument, the insertion of the outlet microfluidic channel was accomplished by adding an MMI device (MMISW), properly designed and incorporated along the straight waveguide section SW. The MMISW is constituted by a couple of 1 × 1 MMI placed in a cascade along the propagation axis.
A 1 × 1 MMI device transfers light from the input to the output waveguide with minimal loss. Because an ARROW-based MMI is intrinsically a lossy optical element, we considered the minimum width and length to create a suitable interference pattern. Starting from the waveguide width d
c and considering the fabrication constraints, 1 × 1 MMI sections with dimensions of width = 5 µm and length L
1 = 42.6 µm could be considered. The interference pattern created by a 1 × 1 MMI section of length 2L
1 is shown in
Figure 6. As it can be observed from the figure, the interference created by a single 1 × 1 MMI section of length L
1 does not exhibit very low-intensity regions near the waveguide walls, which are wide enough to locate the microfluidic channel. Instead, by doubling the MMI sections length it is possible to exploit the reimaging effect created by the first section of length L
1 in the center of the MMI
SW to produce a strong decrease in the intensity near the waveguide wall and where the MC
2 can be placed (shaded box of
Figure 6). For this reason, the length of the MMI
SW has been set to L
MMISW = 2L
1.
By numerical simulations we found that the introduction of the MMI
SW causes a power drop of about 1.6% along the SW. The subsequent integration of the microfluidic channel MC
2 leads to a total power drop of 2.4% as compared with the simple straight waveguide channel. By comparing this value with the power loss caused by mere integration of MC
2 on the straight waveguide, equal to 13%, it could be observed that the proposed introduction of the MMI
SW allows a great enhancement of the optical performance of the considered ring section, as proposed. The simulation results of the MC
2 integration are summarized in
Table 2.
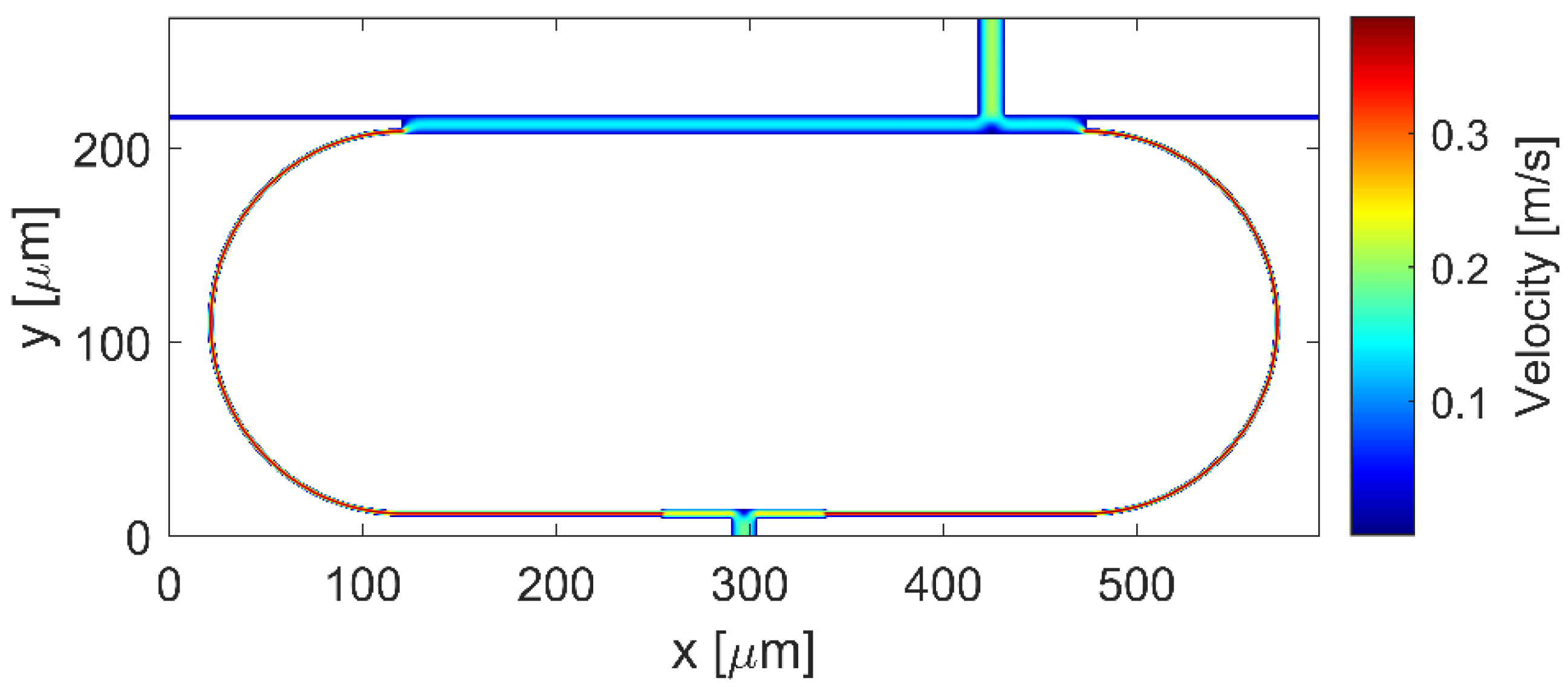
Finally, we simulated the flow profile of the ring layout shown in
Figure 4, optimized according to the obtained numerical results. The MC
1 with a width of 14 µm is located at z = 305 along the z axis of the MMIc, and the MC
2 with a width of 10 µm is in the center of the MMI
SW.
Figure 7 displays the velocity distribution, numerically simulated.
Moreover, in this case we have estimated the flow rate ratio between the flows in the opposite curved sections, obtaining the FRR = QL/QR = 1.3.
Hence, fluidic and optical simulation results suggest that by exploiting the presence of the MMIc and introducing an additional MMI device (MMISW), we could successfully integrate microfluidic channels while keeping the induced propagation losses as low as possible.