Photo-Crosslinkable Hydrogels for 3D Bioprinting in the Repair of Osteochondral Defects: A Review of Present Applications and Future Perspectives
Abstract
:1. Introduction
2. 3D Printing Photo-Crosslinked Hydrogels and Repair of Cartilage Defects
2.1. Materials for Photo-Crosslinkable Hydrogels
2.1.1. Hyaluronic Acid
2.1.2. Silk Fibroin
2.1.3. Alginate
2.1.4. Chitosan
2.1.5. Gelatin
2.1.6. Synthetic Materials
2.2. Advantages of Photo-Crosslinked Hydrogels
2.3. Applications of 3D Bioprinted Photo-Crosslinkable Hydrogels for Osteochondral Regeneration
2.3.1. Cartilage-like Tissue Hydrogels
2.3.2. Stem Cell Encapsulation Hydrogels
2.3.3. Cartilage Tissue Cell Encapsulation Hydrogels
3. Problems with 3D Bioprinting Photo-Crosslinked Hydrogels
3.1. Cell Viability and Loaded Cells
3.1.1. Bioink and Cell Viability
3.1.2. 3D Bioprinting Methods, Parameters, and Cell Viability
3.2. Cytotoxicity of Photo-Crosslinked Hydrogels
3.2.1. Free Radical Toxicity
3.2.2. Phototoxicity
3.2.3. Cytotoxicity of Photoinitiators
4. Solutions and Future Horizons
- i.
- Developing and exploring more biocompatible photoinitiators for visible light to minimize the damage to cells by the photoinitiators. Small doses of photoinitiators promote the crosslinking of hydrogels at very low concentrations without affecting the rate of the crosslinking reaction and the mechanical strength of the hydrogels. In addition, developing more hydrogels without external photoinitiators such as hydrogel-based photoinitiator systems that contain crosslinkable polymers to crosslink and form hydrogels. Atom transfer radical polymerization (ATRP) is a controllable radical synthesis technology catalyzed by transition metal complexes. Vinyl monomers are initiated by the initiator R-X and polymerized to form macromolecules in a reversible oxidation-reduction process [137]. Compared with traditional radical polymerization, ATRP can improve the homogeneity of hydrogels, and different structures and properties of hydrogels can be obtained by using different initiators.
- ii.
- iii.
- Optimizing printing methods and parameters [143,144,145], such as microencapsulation and nanoencapsulation that can encapsulate the cells in a protective shell with good biocompatibility and isolate them from the surrounding environment to reduce the stimulation of the external environment to the cells. Optimizing the diameter of the printing needle, jet speed, pore diameter, shape, and porosity of the structure to optimize the printing structure [146].
- iv.
- Combining different gelation methods of hydrogel printing strategies to optimize the performance of composites in the process. Several hydrogels with different gelation methods were combined to optimize the performance of hydrogels. For example, the thermal crosslinking material is combined with the photo-crosslinking material to form a hydrogel composite for rapid crosslinking into a hydrogel [147,148,149]. The dual-responsive hydrogel constructs demonstrated higher resolution and shape fidelity as well as better cell viability and proliferation than the thermal responsive control.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | articular cartilage |
| ECM | extracellular matrix |
| CTE | cartilage tissue engineering |
| HA | hyaluronic acid |
| 3D | three-dimensional |
| 2D | two-dimensional |
| HAMA | methacryloylated hyaluronic acid |
| NorHA | norbornene-modified hyaluronic acid |
| MSCs | marrow mesenchymal stem cells |
| SF | silk fibroin |
| SilMA | methacryloylated silk fibroin |
| CS | chitosan |
| CSMA | methacrylamide chitosan |
| TPVA | thiol terminated polyvinyl alcohol |
| PEGDA | polyethylene glycol diacrylate |
| GelMA | gelatin methacryloyl hydrogels |
| BMSCs | Bone marrow mesenchymal stem cells |
| PEG | polyethylene glycol |
| pMHMGCL/PCL | Polymethylacryloyl poly (hydroxymethylhexyl ester -ε- Caprolactone)-Poly(ε- Caprolactone) |
| HAP | hydroxyapatite |
| PVA | polyvinyl alcohol |
| ICRS | International Cartilage Repair Association cartilage damage classification system |
| cdECM | cartilage-derived ECM |
| cdECMMA | methacrylation cdECM |
| GAGs | glycosaminoglycans |
| SerMA | sericin methacryloyl |
| hASCs | human adipose-derived stem cells |
| Ca-AM | Calcein Acetoxymethyl Ester |
| PI | Pyridine iodide |
| DLP | digital light processing |
| hMSCs | human MSCs |
| Sil-MA | methacrylated SF solutions |
| SDCM | solubilized decellularized cartilage matrix |
| PVA-A | PVA/amine |
| PVA-Nb | PVA/cis-5-norbornene-endo-2,3-dicarboxylic anhydride |
| HGC | methacrylated hexanoyl glycol CS |
| GC | glycol CS |
| GM | glycidyl methacrylate |
| CPDs | cyclobutane pyrimidine dimers |
| LAP | lithium phenyl-2,4,6-trimethylbenzoylphosphinate |
References
- Wasyłeczko, M.; Sikorska, W.; Chwojnowski, A. Review of synthetic and hybrid scaffolds in cartilage tissue engineering. Membranes 2020, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Radzi, M.A. Scaffolds for cartilage regeneration: To use or not to use? Adv. Exp. Med. Biol. 2020, 1249, 97–114. [Google Scholar]
- Campos, Y.; Almirall, A.; Fuentes, G.; Bloem, H.L.; Kaijzel, E.L.; Cruz, L.J. Tissue engineering: An alternative to repair cartilage. Tissue Eng. Part B Rev. 2019, 25, 357–373. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Gatenholm, B.; Lindahl, C.; Brittberg, M.; Simonsson, S. Collagen 2A type B induction after 3D bioprinting chondrocytes in situ into osteoarthritic chondral tibial lesion. Cartilage 2020, 13, 1755S–1769S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernecke, C.; Braun, H.J.; Dragoo, J.L. The Effect of intra-articular corticosteroids on articular cartilage: A systematic review. Orthop. J. Sports Med. 2015, 3, 2325967115581163. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of damaged articular cartilage: Current approaches and future directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Kundra, R. Platelet-rich plasma (PRP) for knee disorders. EFORT Open Rev. 2017, 2, 28–34. [Google Scholar] [CrossRef]
- Steadman, J.R.; Rodkey, W.G.; Rodrigo, J.J. Microfracture: Surgical technique and rehabilitation to treat chondral defects. Clin. Orthop. Relat. Res. 2001, 391, S362–S369. [Google Scholar] [CrossRef]
- Torrie, A.M.; Kesler, W.W.; Elkin, J.; Gallo, R.A. Osteochondral allograft. Curr. Rev. Musculoskelet. Med. 2015, 8, 413–422. [Google Scholar] [CrossRef] [Green Version]
- Devitt, B.M.; Bell, S.W.; Webster, E.K.; Feller, A.J.; Whitehead, T.S. Surgical treatments of cartilage defects of the knee: Systematic review of randomised controlled trials. Knee 2017, 24, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Recker, D.; Ilgenfritz, J.; Saris, D.B.; the SUMMIT Extension Study Group. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: Five-year follow-up of a prospective randomized trial. Am. J. Sports Med. 2018, 46, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Derrett, S.; Stokes, E.A.; James, M.; Bartlett, W.; Bentley, G. Cost and health status analysis after autologous chondrocyte implantation and mosaicplasty: A retrospective comparison. Int. J. Technol. Assess. Health Care 2005, 21, 359–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.; Metcalfe, A.; Waugh, N. Autologous chondrocyte implantation in the knee: Systematic review and economic evaluation. Health Technol. Assess. 2017, 21, 1–294. [Google Scholar] [CrossRef] [Green Version]
- Lammi, M.J.; Piltti, J.; Prittinen, J.; Qu, C.J. Challenges in fabrication of tissue-engineered cartilage with correct cellular colonization and extracellular matrix assembly. Int. J. Mol. Sci. 2018, 19, 2700. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, G.; Cao, Y. Recent progress in cartilage tissue engineering—Our experience and future directions. Engineering 2017, 3, 28–35. [Google Scholar] [CrossRef]
- Eftekhari, A.; Dizaj, S.M.; Sharifi, S.; Salatin, S.; Saadat, Y.R.; Vahed, S.Z.; Samiei, M.; Ardalan, M.; Rameshrad, M.; Ahmadian, E.; et al. The use of nanomaterials in tissue engineering for cartilage regeneration; Current approaches and future perspectives. Int. J. Mol. Sci. 2020, 21, 536. [Google Scholar] [CrossRef] [Green Version]
- Rana, M.; Siegler, H.D.L.H. Tuning the properties of PNIPAm-based hydrogel scaffolds for cartilage tissue engineering. Polymers 2021, 13, 3154. [Google Scholar] [CrossRef]
- Choi, S.K.; Goodnow, R.A.; Kalivretenos, A.; Chiles, G.W.; Fushiya, S.; Nakanishi, K. Synthesis of novel and photolabile philanthotoxin analogs: Glutamate receptor antagonists. Tetrahedron 1992, 48, 4793–4822. [Google Scholar] [CrossRef]
- Ying, G.; Jiang, N.; Parra-Cantu, C.; Tang, G.; Zhang, J.; Wang, H.; Chen, S.; Huang, N.; Xie, J.; Zhang, Y. Bioprinted injectable hierarchically porous gelatin methacryloyl hydrogel constructs with shape-memory properties. Adv. Funct. Mater. 2020, 30, 2003740. [Google Scholar] [CrossRef]
- Ying, G.L.; Jiang, N.; Maharjan, S.; Yin, Y.; Chai, R.; Cao, X.; Yang, J.; Miri, A.; Hassan, S.; Zhang, Y. Aqueous two-phase emulsion bioink-enabled 3D bioprinting of porous hydrogels. Adv. Mater. 2018, 30, 1805460. [Google Scholar] [CrossRef] [PubMed]
- Gallastegui, A.; Spesia, M.B.; Dell’Erba, I.E.; Chesta, C.A.; Previtali, C.M.; Palacios, R.E.; Gómez, M.L. Controlled release of antibiotics from photopolymerized hydrogels: Kinetics and microbiological studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 896–905. [Google Scholar] [CrossRef]
- Mei, Q.; Rao, J.; Bei, H.P.; Liu, Y.; Zhao, X. 3D Bioprinting photo-crosslinkable hydrogels for bone and cartilage repair. Int. J. Bioprint. 2021, 7, 367. [Google Scholar] [CrossRef]
- Ifkovits, J.L.; Burdick, J.A. Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007, 13, 2369–2385. [Google Scholar] [CrossRef]
- Hou, P.; Zhang, N.; Wu, R.; Xu, W.; Hou, Z. Photo-cross-linked biodegradable hydrogels based on n-arm-poly (ethylene glycol), poly (ε-caprolactone) and/or methacrylic acid for controlled drug release. J. Biomater. Appl. 2017, 32, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, K.; Zhao, S.; Zhang, C.; Li, J.; Yang, H.; Liu, X.; Yin, X.; Chen, D.; Xu, W.; et al. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2018, 108, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, C.; Liu, J.; Jin, Y.; Xu, L.; Wang, G.; Wang, Z.; Wang, L. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials 2018, 163, 89–104. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.; Zhang, Y.; Zan, Y.; Ni, T.; Liu, M.; Pei, R. Photo-crosslinkable, bone marrow-derived mesenchymal stem cells-encapsulating hydrogel based on collagen for osteogenic differentiation. Colloids Surf. B Biointerfaces 2019, 174, 528–535. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Suo, H.; Yan, M.; Yin, J.; Fu, J. 3D printing of biomimetic multi-layered GelMA/nHA scaffold for osteochondral defect repair. Mater. Des. 2019, 171, 107708. [Google Scholar] [CrossRef]
- Tytgat, L.; Vagenende, M.; Declercq, H.; Martins, J.; Thienpont, H.; Ottevaere, H.; Dubruel, P.; Van Vlierberghe, S. Synergistic effect of κ-carrageenan and gelatin blends towards adipose tissue engineering. Carbohydr. Polym. 2018, 189, 1–9. [Google Scholar] [CrossRef]
- Tytgat, L.; Van Damme, L.; Van Hoorick, J.; Declercq, H.; Thienpont, H.; Ottevaere, H.; Blondeel, P.; Dubruel, P.; van Vlierberghe, S. Additive manufacturing of photo-crosslinked gelatin scaffolds for adipose tissue engineering. Acta Biomater. 2019, 94, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, L.; Van Damme, L.; Arevalo MD, P.O.; Declercq, H.; Thienpont, H.; Otteveare, H.; Blondeel, P.; Dubruel, P.; van Vlierberghe, S. Extrusion-based 3D printing of photo-crosslinkable gelatin and κ-carrageenan hydrogel blends for adipose tissue regeneration. Int. J. Biol. Macromol. 2019, 140, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Kumar, H.; Tian, Z.; Jin, X.; Holzman, J.F.; Menard, F.; Kim, K. Visible light photoinitiation of cell-adhesive gelatin methacryloyl hydrogels for stereolithography 3D bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 26859–26869. [Google Scholar] [CrossRef] [PubMed]
- Seeto, W.J.; Tian, Y.; Pradhan, S.; Kerscher, P.; Lipke, E.A. Rapid production of cell-laden microspheres using a flexible microfluidic encapsulation platform. Small 2019, 15, 1902058. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zheng, Z.; Liu, Y.; Ruan, C.; Pan, H.; Zhao, X. A shear-thinning adhesive hydrogel reinforced by photo-initiated crosslinking as a fit-to-shape tissue sealant. J. Mater. Chem. B 2019, 7, 6488–6499. [Google Scholar] [CrossRef]
- Monteiro, N.; Thrivikraman, G.; Athirasala, A.; Tahayeri, A.; Franca, C.; Ferracane, J.L.; Bertassoni, L.E. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent. Mater. 2018, 34, 389–399. [Google Scholar] [CrossRef]
- Han, G.D.; Kim, J.W.; Noh, S.H.; Kim, S.W.; Jang, E.C.; Nah, J.W.; Lee, Y.-G.; Kim, M.-K.; Ito, Y.; Son, T.-I. Potent anti-adhesion agent using a drug-eluting visible-light curable hyaluronic acid derivative. J. Ind. Eng. Chem. 2019, 70, 204–210. [Google Scholar] [CrossRef]
- Sherman, L.; Sleeman, J.; Herrlich, P.; Ponta, H. Hyaluronate receptors: Key players in growth, differentiation, migration and tumor progression. Curr. Opin. Cell Biol. 1994, 6, 726–733. [Google Scholar] [CrossRef]
- Schuurmans, C.; Mihajlovic, M.; Hiemstra, C.; Ito, K.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and chondroitin sulfate (meth)acrylate-based hydrogels for tissue engineering: Synthesis, characteristics and pre-clinical evaluation. Biomaterials 2020, 268, 120602. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.; Ryu, J.H. Adhesive catechol-conjugated hyaluronic acid for biomedical applications: A mini review. Appl. Sci. 2020, 11, 21. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, A.X.; Overholt, K.J.; Yu, G.Z.; Fritch, M.R.; Alexander, P.G.; Shen, H.; Tuan, R.S.; Lin, H. Enhancing chondrogenesis and mechanical strength retention in physiologically relevant hydrogels with incorporation of hyaluronic acid and direct loading of TGF-β. Acta Biomater. 2018, 83, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Alemdar, C.; Yücel, I.; Erbil, B.; Erdem, H.; Atiç, R.; Özkul, E. Effect of insulin-like growth factor-1 and hyaluronic acid in experimentally produced osteochondral defects in rats. Indian J. Orthop. 2016, 50, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wang, C.; Trippel, S.B. Hyaluronic acid-binding insulin-like growth factor-1: Creation of a gene encoding a bifunctional fusion protein. Mol. Biol. Rep. 2020, 47, 9749–9756. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Rodriguez, B.; Diaz-Vidal, T.; Rosales-Rivera, L.; García-González, C.; Alvarez-Lorenzo, C.; Al-Modlej, A.; Domínguez-Arca, V.; Prieto, G.; Barbosa, S.; Martínez, J.S.; et al. Hybrid methacrylated gelatin and hyaluronic acid hydrogel scaffolds. Preparation and systematic characterization for prospective tissue engineering applications. Int. J. Mol. Sci. 2021, 22, 6758. [Google Scholar] [CrossRef]
- Zeng, M.; Johnson, M.; Creagh-Flynn, J.; Xu, Q.; Tai, H.; Wang, W. Green synthetic approach for photo-cross-linkable methacryloyl hyaluronic acid with a tailored substitution degree. Biomacromolecules 2020, 21, 2229–2235. [Google Scholar] [CrossRef]
- Guan, G.; Lv, Q.; Liu, S.; Jiang, Z.; Zhou, C.; Liao, W. 3D-bioprinted peptide coupling patches for wound healing. Mater. Today Bio 2021, 13, 100188. [Google Scholar] [CrossRef]
- Rakin, R.H.; Kumar, H.; Rajeev, A.; Natale, G.; Menard, F.; Li, I.T.S.; Kim, K. Tunable metacrylated hyaluronic acid-based hybrid bioinks for stereolithography 3D bioprinting. Biofabrication 2021, 13, 044109. [Google Scholar] [CrossRef]
- Nedunchezian, S.; Wu, C.-W.; Wu, S.-C.; Chen, C.-H.; Chang, J.-K.; Wang, C.-K. Characteristic and chondrogenic differentiation analysis of hybrid hydrogels comprised of Hyaluronic Acid Methacryloyl (HAMA), Gelatin Methacryloyl (GelMA), and the acrylate-functionalized nano-silica crosslinker. Polymers 2022, 14, 2003. [Google Scholar] [CrossRef]
- Galarraga, J.H.; Locke, R.C.; Witherel, E.C.; Stoeckl, B.D.; Castilho, M.; Mauck, R.L.; Malda, J.; Levato, R.; Burdick, A.J. Fabrication of MSC-laden composites of hyaluronic acid hydrogels reinforced with MEW scaffolds for cartilage repair. Biofabrication 2021, 14, 014106. [Google Scholar] [CrossRef]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: A review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [Green Version]
- Applegate, M.B.; Partlow, B.P.; Coburn, J.; Marelli, B.; Pirie, C.; Pineda, R.; Kaplan, D.L.; Omenetto, F.G. Photo crosslinking of silk fibroin using riboflavin for ocular prostheses. Adv. Mater. 2016, 28, 2464. [Google Scholar] [CrossRef]
- Ravichandran, V.; Jayakrishnan, A. Synthesis and evaluation of anti-fungal activities of sodium alginate-amphotericin B conjugates. Int. J. Biol. Macromol. 2018, 67, 4317–4322. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Tsuji, Y.; Kuriki, I.; Kakimoto, A.; Nikaido, Y.; Ninomiya, R.; Iyoda, T.; Fukai, F.; Hashizume, M. Control of cell adhesion and proliferation utilizing polysaccharide composite film scaffolds. Colloids Surf. B Biointerfaces 2017, 160, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Xu, F.; Ding, H.; Tan, F.; Song, F.; Wang, J. Incorporation of magnesium ions into photo-crosslinked alginate hydrogel enhanced cell adhesion ability. J. Tissue Eng. Regen. Med. 2015, 9, 1088–1092. [Google Scholar] [CrossRef]
- Yuan, N.; Jia, L.; Geng, Z.; Wang, R.; Li, Z.; Yang, X.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y. The Incorporation of strontium in a sodium alginate coating on titanium surfaces for improved biological properties. Biomed. Res. Int. 2017, 2017, 9867819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-based biomaterials for regeneration and therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef]
- Tan, F.; Liu, J.; Liu, M.; Wang, J. Charge density is more important than charge polarity in enhancing osteoblast-like cell attachment on poly(ethylene glycol)-diacrylate hydrogel. Mater. Sci. Eng. C 2017, 76, 330–339. [Google Scholar] [CrossRef]
- Yuan, H.; Zheng, X.; Liu, W.; Zhang, H.; Shao, J.; Yao, J.; Mao, C.; Hui, J.; Fan, D. A novel bovine serum albumin and sodium alginate hydrogel scaffold doped with hydroxyapatite nanowires for cartilage defects repair. Colloids Surf. B Biointerfaces 2020, 192, 111041. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef]
- Cerrutti, B.M.; Lamas, J.C.; Campana-Filho, S.P.; Frollini, E. Carboxymethyl chitosan: Preparation and use in colloidal ceramic processing. J. Polym. Environ. 2013, 21, 816–825. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y.; Wang, X.; Huang, L.; Chen, Y.; Bao, C. Glucose-sensitive delivery of metronidazole by using a photo-crosslinked chitosan hydrogel film to inhibit Porphyromonas gingivalis proliferation. Int. J. Biol. Macromol. 2018, 122, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Xu, J.; Wang, Z.; Nie, J.; Ma, G. Preparation and properties of photo-crosslinkable hydrogel based on photopolymerizable chitosan derivative. Int. J. Biol. Macromol. 2012, 53, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, S.; Zhang, C.; Liang, K.; Li, J.; Yang, H.; Gu, S.; Bai, Z.; Ye, D.; Xu, W. Photopolymerized maleilated chitosan/thiol-terminated poly (vinyl alcohol) hydrogels as potential tissue engineering scaffolds. Carbohydr. Polym. 2018, 184, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Wu, J.; Reinhart-King, C.; Chu, C. Synthesis, characterization and cytotoxicity of photo-crosslinked maleic chitosan–polyethylene glycol diacrylate hybrid hydrogels. Acta Biomater. 2010, 6, 3908–3918. [Google Scholar] [CrossRef]
- Yoo, H.S. Photo-cross-linkable and thermo-responsive hydrogels containing chitosan and Pluronic for sustained release of human growth hormone (hGH). J. Biomater. Sci. Polym. Ed. 2007, 18, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Rickett, T.A.; Amoozgar, Z.; Tuchek, C.A.; Park, J.; Yeo, Y.; Shi, R. Rapidly photo-cross-linkable chitosan hydrogel for peripheral neurosurgeries. Biomacromolecules 2010, 12, 57–65. [Google Scholar] [CrossRef]
- Lee, J.I.; Kim, H.S.; Yoo, H.S. DNA nanogels composed of chitosan and Pluronic with thermo-sensitive and photo-crosslinking properties. Int. J. Pharm. 2009, 373, 93–99. [Google Scholar] [CrossRef]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Li, Y.-T. Functional assessment of cross-linked porous gelatin hydrogels for bioengineered cell sheet carriers. Biomacromolecules 2010, 11, 1387–1397. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- van den Steen, P.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–536. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [Green Version]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, L.; Gu, Z.; Wu, J. Red jujube-incorporated Gelatin Methacryloyl (GelMA) hydrogels with anti-oxidation and immunoregulation activity for wound healing. J. Biomed. Nanotechnol. 2019, 15, 1357–1370. [Google Scholar] [CrossRef]
- Rebers, L.; Granse, T.; Tovar, G.E.; Southan, A.; Borchers, K. Physical interactions strengthen chemical gelatin Methacryloyl gels. Gels 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hoorick, J.; Tytgat, L.; Dobos, A.; Ottevaere, H.; Van Erps, J.; Thienpont, H.; Ovsianikov, A.; Dubruel, P.; Van Vlierberghe, S. (Photo-)crosslinkable gelatin derivatives for biofabrication applications. Acta Biomater. 2019, 97, 46–73. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lu, C.; Wang, L.; Chen, M.; White, J.; Hao, X.; McLean, K.M.; Chen, H.; Hughes, T.C. Gelatin-based photocurable hydrogels for corneal wound repair. ACS Appl. Mater. Interfaces 2018, 10, 13283–13292. [Google Scholar] [CrossRef]
- García-Astrain, C.; Peña-Rodriguez, C.; Retegi, A.; Eceiza, A.; Corcuera, M.; Gabilondo, N. Green chemistry for the cross-linking of photo-sensitive furan modified gelatin. Mater. Lett. 2015, 160, 142–145. [Google Scholar] [CrossRef]
- Greene, T.; Lin, C.-C. Modular cross-linking of gelatin-based thiol–norbornene hydrogels for in Vitro 3D culture of hepatocellular carcinoma cells. ACS Biomater. Sci. Eng. 2015, 10, 1314–1323. [Google Scholar] [CrossRef]
- AnilKumar, S.; Allen, S.C.; Tasnim, N.; Akter, T.; Park, S.; Kumar, A.; Chattopadhyay, M.; Ito, Y.; Suggs, L.J.; Joddar, B. The applicability of furfuryl-gelatin as a novel bioink for tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 314–323. [Google Scholar] [CrossRef]
- Levett, P.A.; Melchels, F.P.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suo, H.; Xu, K.; Zheng, X. Using glucosamine to improve the properties of photocrosslinked gelatin scaffolds. J. Biomater. Appl. 2014, 29, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Levett, P.A.; Moller, N.T.; Besems, J.; Boere, K.W.; Van Rijen, M.H.; De Grauw, J.C.; Dhert, W.; Van Weeren, P.R.; Malda, J. Crosslinkable hydrogels derived from cartilage, meniscus, and tendon tissue. Tissue Eng. Part A 2015, 21, 1195–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.; Schilling, A.F.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015, 37, 2349–2355. [Google Scholar] [CrossRef]
- Boere, K.W.; Visser, J.; Seyednejad, H.; Rahimian, S.; Gawlitta, D.; Van Steenbergen, M.J.; Dhert, W.; Hennink, W.E.; Vermonden, T.; Malda, J. Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomater. 2014, 10, 2602–2611. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Akkineni, A.R.; Gelinsky, M.; Woodruff, M.A.; Klein, T.J. A hydrogel model incorporating 3D-plotted hydroxyapatite for osteochondral tissue engineering. Materials 2016, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- de la Vega, L.; Lee, C.; Sharma, R.; Amereh, M.; Willerth, S.M. 3D bioprinting models of neural tissues: The current state of the field and future directions. Brain Res. Bull. 2019, 150, 240–249. [Google Scholar] [CrossRef]
- Zhang, Y.; An, D.; Pardo, Y.; Chiu, A.; Song, W.; Liu, Q.; Zhou, F.; McDonough, S.P.; Ma, M. High-water-content and resilient PEG-containing hydrogels with low fibrotic response. Acta Biomater. 2017, 53, 100–108. [Google Scholar] [CrossRef]
- Li, H.; Zheng, H.; Zhang, Y.; Zhang, W.; Tong, W.; Gao, C. Preparation of photo-responsive poly(ethylene glycol) microparticles and their influence on cell viability. J. Colloid Interface Sci. 2018, 514, 182–189. [Google Scholar] [CrossRef]
- Bal, T.; Nazli, C.; Okcu, A.; Duruksu, G.; Karaöz, E.; Kizilel, S. Mesenchymal stem cells and ligand incorporation in biomimetic poly(ethylene glycol) hydrogels significantly improve insulin secretion from pancreatic islets. J. Tissue Eng. Regen. Med. 2014, 11, 694–703. [Google Scholar] [CrossRef]
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijaya Venkata Raman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y.H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef] [PubMed]
- Duchi, S.; Onofrillo, C.; O’Connell, C.D.; Blanchard, R.; Augustine, C.; Quigley, A.F.; Kapsa, R.M.I.; Pivonka, P.; Wallace, G.; Di Bella, C.; et al. Handheld co-axial bioprinting: Application to in situ surgical cartilage repair. Sci. Rep. 2017, 7, 5837. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A.; De La Guardia, M. Hydrogel-based 3d bioprinting for bone and cartilage tissue engineering. Biotechnol. J. 2020, 15, 2000095. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Tomaschke, A.; Kleinjan, E.; Muralidharan, A.; Pascual-Garrido, C.; Mcleod, R.; Ferguson, V.L.; Bryant, S.J. A Stereolithography-based 3D printed hybrid scaffold for in situ cartilage defect repair. Macromol. Biosci. 2017, 18, 1700267. [Google Scholar] [CrossRef]
- Shen, T.; Dai, Y.; Li, X.; Xu, S.; Gou, Z.; Gao, C. Regeneration of the osteochondral defect by a wollastonite and macroporous fibrin biphasic scaffold. ACS Biomater. Sci. Eng. 2017, 4, 1942–1953. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Liu, S.; Feng, Z.; Wang, H.; Yang, D.; Guo, W.; Yuan, Z.; Gao, S.; Zhang, Y.; et al. Hierarchical macro-microporous WPU-ECM scaffolds combined with microfracture promote in situ articular cartilage regeneration in rabbits. Bioact. Mater. 2020, 6, 1932–1944. [Google Scholar] [CrossRef]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Xiongfa, J.; Hao, Z.; Liming, Z.; Jun, X. Recent advances in 3D bioprinting for the regeneration of functional cartilage. Regen. Med. 2018, 13, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Apelgren, P.; Karabulut, E.; Amoroso, M.; Mantas, A.; Ávila, H.M.; Kölby, L.; Kondo, T.; Toriz, G.; Gatenholm, P. In vivo human cartilage formation in three-dimensional bioprinted constructs with a novel bacterial nanocellulose bioink. ACS Biomater. Sci. Eng. 2019, 5, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chang, S.; Zhang, M.; Bian, X.; Li, C.; Li, D. Advances of hydrogel-based bioprinting for cartilage tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 746564. [Google Scholar] [CrossRef] [PubMed]
- Visscher, D.O.; Lee, H.; van Zuijlen, P.P.; Helder, M.N.; Atala, A.; Yoo, J.J.; Lee, S.J. A photo-crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering. Acta Biomater. 2020, 121, 193–203. [Google Scholar] [CrossRef] [PubMed]
- De Moor, L.; Fernandez, S.; Vercruysse, C.; Tytgat, L.; Asadian, M.; De Geyter, N.; Van Vlierberghe, S.; Dubruel, P.; Declercq, H. Hybrid bioprinting of chondrogenically induced human mesenchymal stem cell spheroids. Front. Bioeng. Biotechnol. 2020, 8, 484. [Google Scholar] [CrossRef]
- Van Damme, L.; Van Hoorick, J.; Blondeel, P.; Van Vlierberghe, S. Toward adipose tissue engineering using thiol-norbornene photo-crosslinkable gelatin hydrogels. Biomacromolecules 2021, 22, 2408–2418. [Google Scholar] [CrossRef]
- Van Hoorick, J.; Dobos, A.; Markovic, M.; Gheysens, T.; Van Damme, L.; Gruber, P.; Tytgat, L.; Van Erps, J.; Thienpont, H.; Dubruel, P.; et al. Thiol-norbornene gelatin hydrogels: Influence of thiolated crosslinker on network properties and high definition 3D printing. Biofabrication 2020, 13, 015017. [Google Scholar] [CrossRef]
- Van Hoorick, J.; Gruber, P.; Markovic, M.; Rollot, M.; Graulus, G.-J.; Vagenende, M.; Tromayer, M.; Van Erps, J.; Thienpont, H.; Martins, J.C.; et al. Highly reactive thiol-norbornene photo-click hydrogels: Toward improved processability. Macromol. Rapid Commun. 2018, 39, e1800181. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Schacht, E.; Dubruel, P. Reversible gelatin-based hydrogels: Finetuning of material properties. Eur. Polym. J. 2011, 47, 1039–1047. [Google Scholar] [CrossRef]
- Levato, R.; Lim, K.S.; Li, W.; Asua, A.U.; Peña, L.B.; Wang, M.; Falandt, M.; Bernal, P.N.; Gawlitta, D.; Zhang, Y.S.; et al. High-resolution lithographic biofabrication of hydrogels with complex microchannels from low-temperature-soluble gelatin bioresins. Mater. Today Bio 2021, 12, 100162. [Google Scholar] [CrossRef]
- Custódio, C.A.; Reis, R.L.; Mano, J.F. Photo-cross-linked laminarin-based hydrogels for biomedical applications. Biomacromolecules 2016, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, D.Y.; Lim, T.H.; Park, C.H. Correction to: Silk fibroin bioinks for Digital Light Processing (DLP) 3D bioprinting. Bioinspired Biomater. 2020, 1249, 53–66. [Google Scholar] [CrossRef]
- Galarraga, J.H.; Kwon, M.Y.; Burdick, J.A. 3D bioprinting via an in situ crosslinking technique towards engineering cartilage tissue. Sci. Rep. 2019, 9, 19987. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.-Y.; Chang, W.-C.; Wei, L.-J.; Huang, Y.-H.; Chen, C.-H.; Shih, C.-T.; Chen, Y.-W.; Shen, Y.-F. 3D Printing of cytocompatible water-based light-cured polyurethane with hyaluronic acid for cartilage tissue engineering applications. Materials 2017, 10, 136. [Google Scholar] [CrossRef] [PubMed]
- Setayeshmehr, M.; Hafeez, S.; van Blitterswijk, C.; Moroni, L.; Mota, C.; Baker, M. Bioprinting via a dual-gel bioink based on poly(vinyl alcohol) and solubilized extracellular matrix towards cartilage engineering. Int. J. Mol. Sci. 2021, 22, 3901. [Google Scholar] [CrossRef]
- Cho, I.S.; Cho, M.O.; Li, Z.; Nurunnabi, M.; Park, S.Y.; Kang, S.-W.; Huh, K.M. Synthesis and characterization of a new photo-crosslinkable glycol chitosan thermogel for biomedical applications. Carbohydr. Polym. 2016, 144, 59–67. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D bioprinting for tissue and organ fabrication. Ann. Biomed. Eng. 2016, 45, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Koch, L.; Deiwick, A.; Franke, A.; Schwanke, K.; Haverich, A.; Zweigerdt, R.; Chichkov, B.N. Laser bioprinting of human induced pluripotent stem cells—The effect of printing and biomaterials on cell survival, pluripotency, and differentiation. Biofabrication 2018, 10, 035005. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Vetsch, J.R.; Paul, G.R.; Rubert, M.; Mueller, R. Alginate dependent changes of physical properties in 3D bioprinted cell-laden porous scaffolds affect cell viability and cell morphology. Biomed. Mater. 2019, 14, 065009. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.J.; Chung, S.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, A.S. Cell-laden 3D bioprinting hydrogel matrix depending on different compositions for soft tissue engineering: Characterization and evaluation. Mater. Sci. Eng. C 2017, 71, 678–684. [Google Scholar] [CrossRef]
- Dubbin, K.; Hori, Y.; Lewis, K.K.; Heilshorn, S.C. Dual-Stage Crosslinking of a gel-phase bioink improves cell viability and homogeneity for 3D bioprinting. Adv. Health Mater. 2016, 5, 2488–2492. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; O’Connell, C.D.; Onofrillo, C.; Choong, P.F.M.; Di Bella, C.; Duchi, S. Human articular cartilage repair: Sources and detection of cytotoxicity and genotoxicity in photo-crosslinkable hydrogel bioscaffolds. Stem Cells Transl. Med. 2019, 9, 302–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hached, F.; Vinatier, C.; Le Visage, C.; Gondé, H.; Guicheux, J.; Grimandi, G.; Billon-Chabaud, A. Biomaterial-assisted cell therapy in osteoarthritis: From mesenchymal stem cells to cell encapsulation. Best Pract. Res. Clin. Rheumatol. 2017, 31, 730–745. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.J.; Lin, P.; Caplan, A.; Dennis, J.E. MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013, 2013, 732742. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Hasan, A.; Kaarela, O.; Byambaa, B.; Sheikhi, A.; Gaharwar, A.K.; Khademhosseini, A. Advancing frontiers in bone bioprinting. Adv. Health Mater. 2019, 8, e1801048. [Google Scholar] [CrossRef]
- Deo, K.A.; Singh, K.A.; Peak, C.W.; Alge, D.L.; Gaharwar, A.K. Bioprinting 101: Design, fabrication, and evaluation of cell-laden 3D bioprinted scaffolds. Tissue Eng. Part A 2020, 26, 318–338. [Google Scholar] [CrossRef]
- Ji, S.; Guvendiren, M. Recent advances in bioink design for 3D bioprinting of tissues and organs. Front. Bioeng. Biotechnol. 2017, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Chan, V.; Zorlutuna, P.; Jeong, J.H.; Kong, H.; Bashir, R. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab A Chip 2010, 10, 2062–2070. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Oudshoorn, M.H.; van Geemen, D.; Hennink, W.E.; Alblas, J.; Dhert, W.J. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials 2009, 30, 344–353. [Google Scholar] [CrossRef]
- Atsumi, T.; Murata, J.; Kamiyanagi, I.; Fujisawa, S.; Ueha, T. Cytotoxicity of photosensitizers camphorquinone and 9-fluorenone with visible light irradiation on a human submandibular-duct cell line in vitro. Arch. Oral Biol. 1998, 43, 73–81. [Google Scholar] [CrossRef]
- Okada, H.; Suh, W.-K.; Jin, J.; Woo, M.; Du, C.; Elia, A.; Duncan, G.S.; Wakeham, A.; Itie, A.; Lowe, S.W.; et al. Generation and characterization of Smac/DIABLO-deficient mice. Mol. Cell. Biol. 2002, 22, 3509–3517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Sage, E.; Douki, T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. Mol. Mech. Mutagen. 2005, 571, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Godar, D.E.; Gurunathan, C.; Ilev, I. 3D Bioprinting with UVA1 radiation and photoinitiator irgacure 2959: Can the ASTM standard L929 cells predict human stem cell cytotoxicity? Photochem. Photobiol. 2018, 95, 581–586. [Google Scholar] [CrossRef]
- O’Connell, C.D.; Zhang, B.; Onofrillo, C.; Duchi, S.; Blanchard, R.; Quigley, A.; Bourke, J.; Gambhir, S.; Kapsa, R.; Di Bella, C.; et al. Tailoring the mechanical properties of gelatin methacryloyl hydrogels through manipulation of the photocrosslinking conditions. Soft Matter 2018, 14, 2142–2151. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Datta, S.; Das, A.; Chowdhury, A.R.; Datta, P. Bioink formulations to ameliorate bioprinting-induced loss of cellular viability. Biointerphases 2019, 14, 051006. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Han, J.; Oh, J.; Park, S.; Ryu, S.; Jung, S.; Shin, J.-Y.; Lee, B.S.; Hong, B.H.; et al. Graphene oxide flakes as a cellular adhesive: Prevention of reactive oxygen species mediated death of implanted cells for cardiac repair. ACS Nano 2015, 9, 4987–4999. [Google Scholar] [CrossRef]
- Koons, G.L.; Mikos, A.G. Progress in three-dimensional printing with growth factors. J. Control. Release 2018, 295, 50–59. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, W.; Holmes, B.; Zhang, L.G. Biologically inspired smart release system based on 3D bioprinted perfused scaffold for vascularized tissue regeneration. Adv. Sci. 2016, 3, 1600058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, R.; Stevens, L.; Thompson, B.C.; Gilmore, K.J.; Gorkin, R.; Stewart, E.M.; Panhuis, M.I.H.; Romero-Ortega, M.; Wallace, G.G. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials 2015, 67, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, O.; Kaplan, D.L. Cell armor for protection against environmental stress: Advances, challenges and applications in micro- and nanoencapsulation of mammalian cells. Acta Biomater. 2018, 95, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Kamperman, T.; Henke, S.; Berg, A.V.D.; Shin, S.R.; Tamayol, A.; Khademhosseini, A.; Karperien, H.B.J.; Leijten, J.C.H. Single cell microgel based modular bioinks for uncoupled cellular micro- and macroenvironments. Adv. Health Mater. 2016, 6, 1600913. [Google Scholar] [CrossRef]
- Vossoughi, A.; Matthew, H.W.T. Encapsulation of mesenchymal stem cells in glycosaminoglycans-chitosan polyelectrolyte microcapsules using electrospraying technique: Investigating capsule morphology and cell viability. Bioeng. Transl. Med. 2018, 3, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Pages, E.; Ducom, A.; Fontaine, A.; Guillemot, F. Controlling laser-induced jet formation for bioprinting mesenchymal stem cells with high viability and high resolution. Biofabrication 2014, 6, 045001. [Google Scholar] [CrossRef]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef]
- Abbadessa, A.; Blokzijl, M.; Mouser, V.; Marica, P.; Malda, J.; Hennink, W.; Vermonden, T. A thermo-responsive and photo-polymerizable chondroitin sulfate-based hydrogel for 3D printing applications. Carbohydr. Polym. 2016, 149, 163–174. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Hsu, S.-H. Synthesis and characterization of dual stimuli-sensitive biodegradable polyurethane soft hydrogels for 3D cell-laden bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 29273–29287. [Google Scholar] [CrossRef]
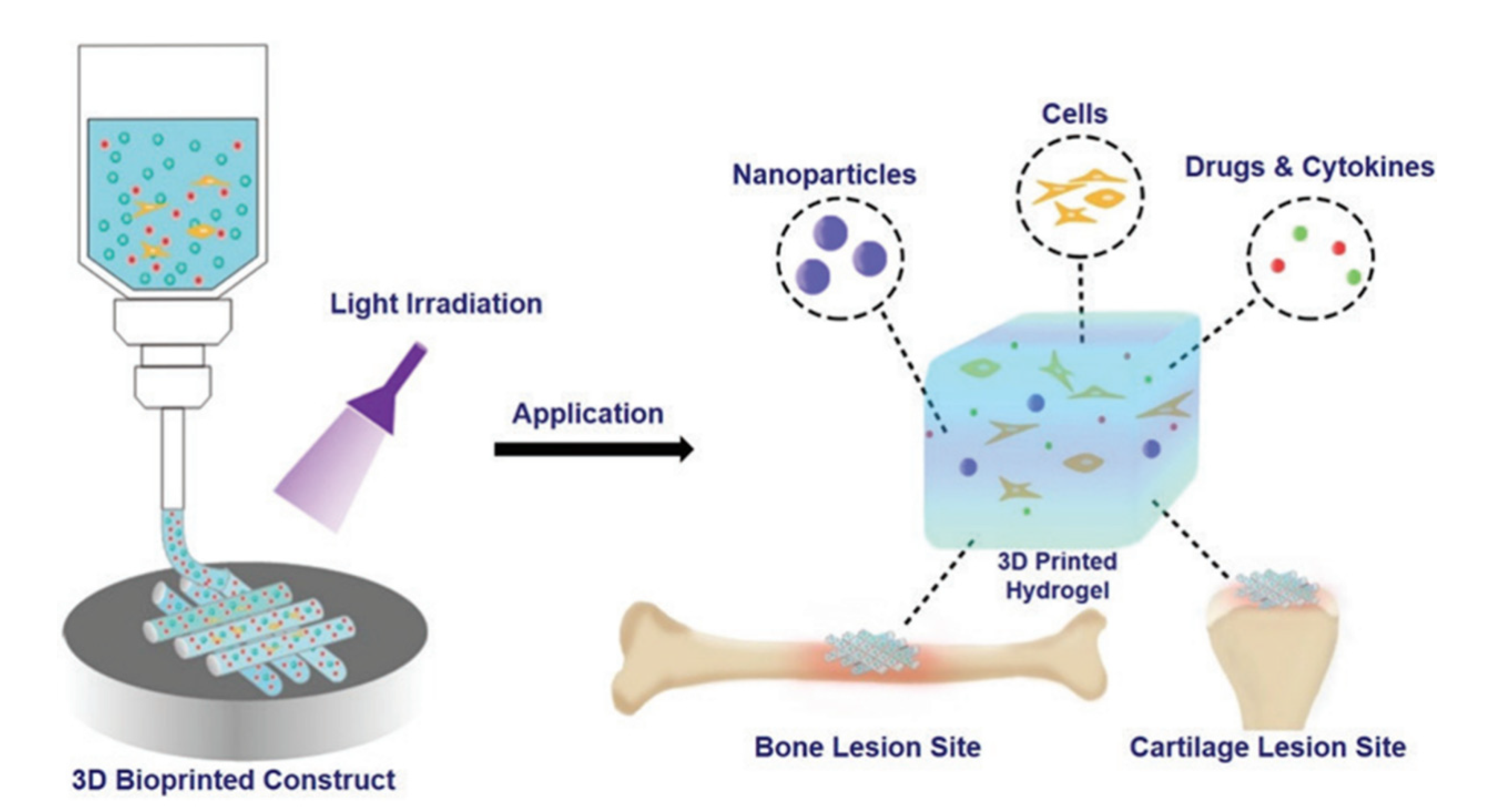
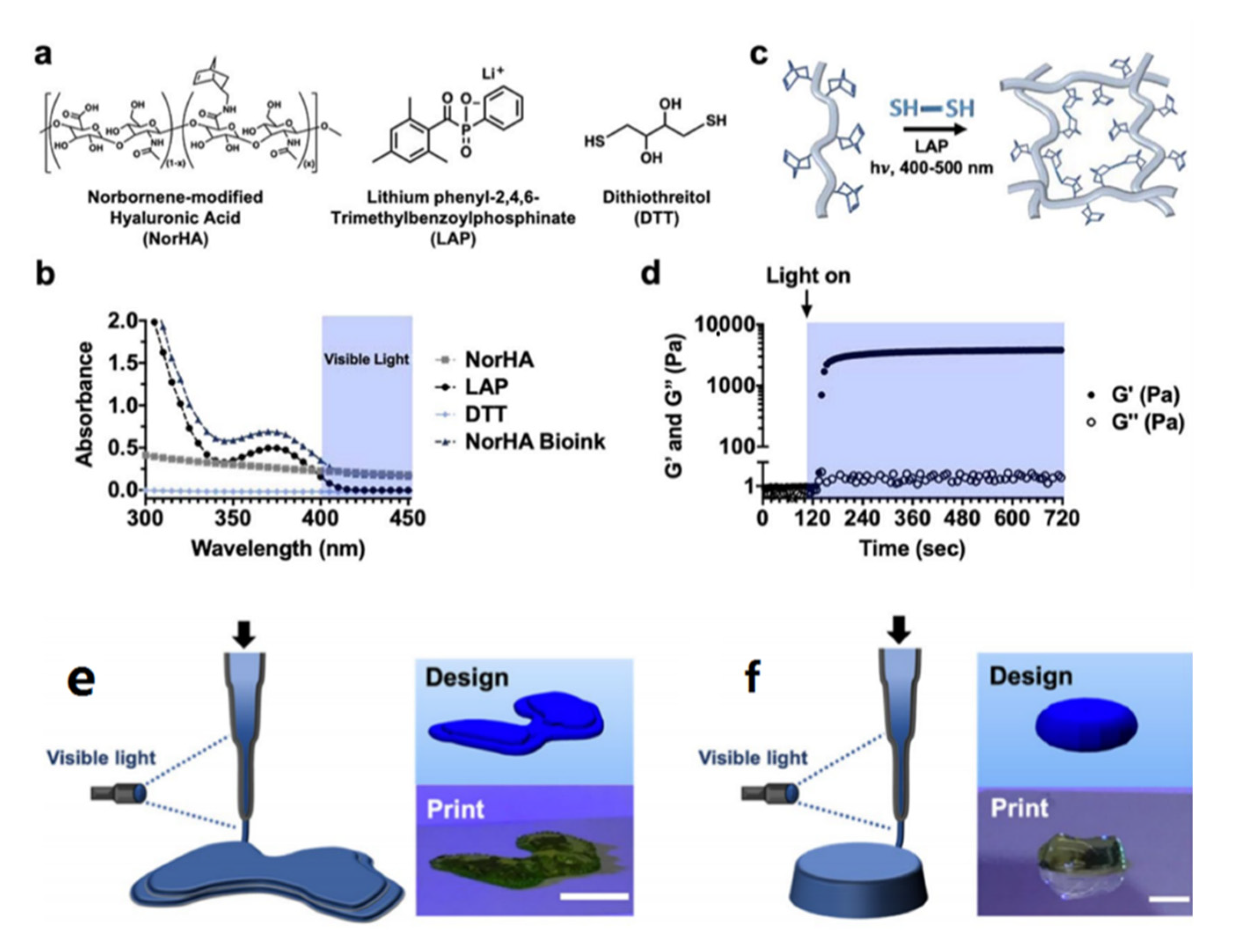

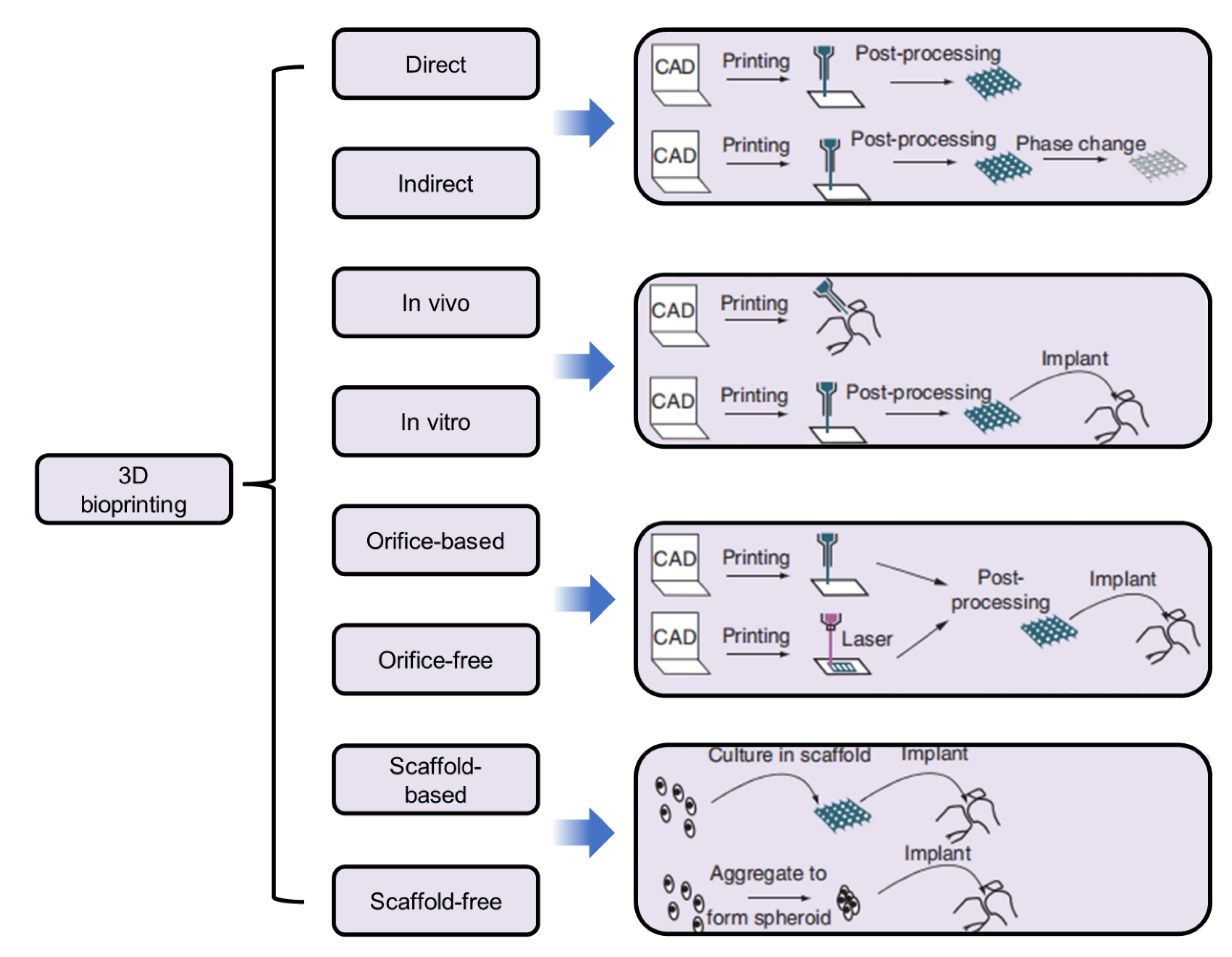

| Name | Abbreviation | Light | Ref |
|---|---|---|---|
| 1-[4-(2-hydroxyethoxy) phenyl]-2-hydroxyl-2-methyl-1-acetone | Irgacure 2959 | UV | [25,26,27,28] |
| Lithium phenyl-2,4,6-trimethylbenzoyl phosphinate | LAP | UV visible light | [29,30,31,32] |
| 2, 4, 5, 7-tetrabromofluorescein disodium salts | Eosin Y | visible light | [33,34] |
| 2-Hydroxy-2-Methylphenylacetone | Irgacure 1173 | UV | [35,36] |
| lactochrome | riboflavin | visible light | [37] |
| Samples | Composition | Crosslinking Mechanism | Advantages | Disadvantages |
|---|---|---|---|---|
| HA | D-glucuronic acid and N-acetyl-D-glucosamine as disaccharide structural units | Cured by vinyl polymerization with the introduction of methacrylates | Abundant active sites, Machinability, Adapt to multiple printing methods | Complex modification process |
| SF | A variety of amino acids | Dehydration condensation of amino acids | Spatial structural controllability, High orientation, High tensile strength | Variability affected by storage conditions |
| Alginate | Polysaccharide carbohydrate | Introduction of cations and induction of crosslinking | Good biocompatibility, Low immunogenicity, Easy access | Poor cell adhesion, Lack of osteogenic induction |
| Gelatin | Heterogeneous mixture | Methacrylic acid modification induced photo-crosslinking | Non-toxicity after degradation, Promote cell migration, Proliferation and differentiation, Trigger cell-mediated enzymatic degradation | Susceptible to bacterial contamination |
| Synthetic materials | Polymer monomer | Polymerization of monomers | Adjustable performance, Repeatability, Suitable for production | Poor biocompatibility |
| Research Status | Existing Problems | Optimization |
|---|---|---|
| The requirement of photoinitiator | Destruction of UV light for encapsulated cells, Biotoxicity of photoinitiators | Develop visible light photo-crosslinking method, Search for low-toxicity photoinitiators |
| Material selection for application environment | Insufficient functionality of materials | Physical mixing or chemical modification imparts multifunctional properties on materials |
| Hydrogels encapsulate cells directly | Direct exposure to the external environment is destructive to cells | Build cell protective shells to keep cells alive |
| Photocurable hydrogel strategies are limiting | Printing method is limited | Optimize the printing process, Combine various cross-linking processes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, G.; Xu, J.; Yu, Q.; Zhang, J.; Hu, X.; Sun, C.; Zhang, H. Photo-Crosslinkable Hydrogels for 3D Bioprinting in the Repair of Osteochondral Defects: A Review of Present Applications and Future Perspectives. Micromachines 2022, 13, 1038. https://doi.org/10.3390/mi13071038
Tan G, Xu J, Yu Q, Zhang J, Hu X, Sun C, Zhang H. Photo-Crosslinkable Hydrogels for 3D Bioprinting in the Repair of Osteochondral Defects: A Review of Present Applications and Future Perspectives. Micromachines. 2022; 13(7):1038. https://doi.org/10.3390/mi13071038
Chicago/Turabian StyleTan, Gang, Jing Xu, Qin Yu, Jieyu Zhang, Xuefeng Hu, Chenwei Sun, and Hui Zhang. 2022. "Photo-Crosslinkable Hydrogels for 3D Bioprinting in the Repair of Osteochondral Defects: A Review of Present Applications and Future Perspectives" Micromachines 13, no. 7: 1038. https://doi.org/10.3390/mi13071038
APA StyleTan, G., Xu, J., Yu, Q., Zhang, J., Hu, X., Sun, C., & Zhang, H. (2022). Photo-Crosslinkable Hydrogels for 3D Bioprinting in the Repair of Osteochondral Defects: A Review of Present Applications and Future Perspectives. Micromachines, 13(7), 1038. https://doi.org/10.3390/mi13071038






