Application of 3D Bioprinting in Urology
Abstract
:1. Introduction
2. The Methods of 3D Bioprinting
2.1. Micro-Extrusion 3D Bioprinting
2.2. Inkjet 3D Bioprinting
2.3. Laser-Assisted Bioprinting
3. The Bioinks of 3D Bioprinting
3.1. Decellularized ECM
3.2. Hydrogel
3.3. D Porous Bioscaffolds
4. Three-Dimensional Printing in Each Genitourinary Organ
5. Three-Dimensional Bio-Printing Applications in Urological Tissue Engineering
5.1. Three-Dimensional Bioprinting of the Bladder
5.2. Three-Dimensional Bioprinting of the Urethra
5.3. Three-Dimensional Bioprinting of the Testis
5.4. Three-Dimensional Bioprinting of the Vagina
5.5. Three-Dimensional Bioprinting of the Kidney
6. Challenges and Perspectives
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Youssef, R.F.; Spradling, K.; Yoon, R.; Dolan, B.; Chamberlin, J.; Okhunov, Z.; Clayman, R.; Landman, J. Applications of three-dimensional printing technology in urological practice. BJU Int. 2015, 116, 697–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.Y.; Skewes, J.; Desselle, M.; Wong, C.; Woodruff, M.A.; Dasgupta, P.; Rukin, N.J. Current applications of three-dimensional printing in urology. BJU Int. 2020, 125, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abaci, A.; Guvendiren, M. Designing Decellularized Extracellular Matrix-Based Bioinks for 3D Bioprinting. Adv. Healthc. Mater. 2020, 9, 2000734. [Google Scholar] [CrossRef]
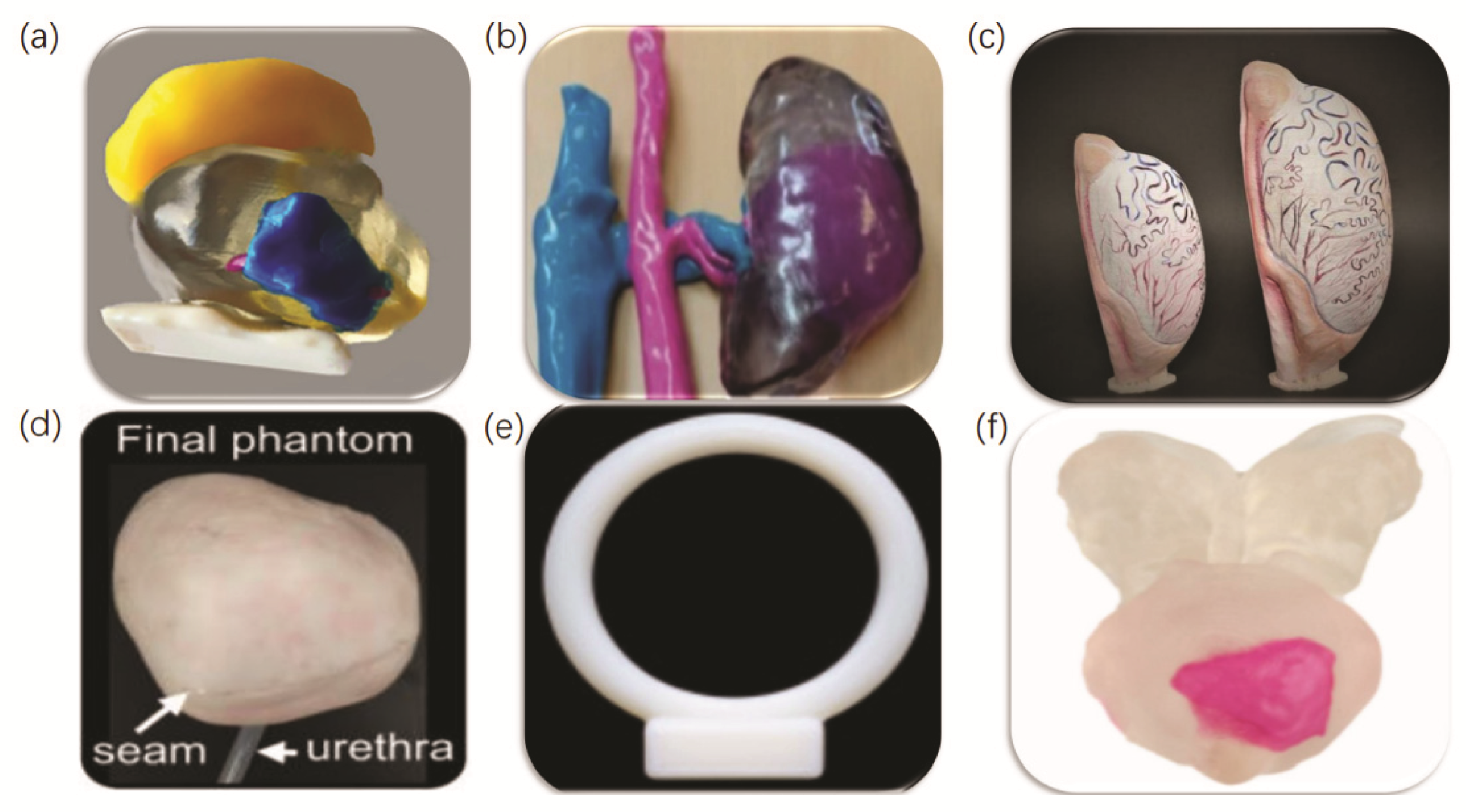
- Agung, N.P.; Nadhif, M.H.; Irdam, G.A.; Mochtar, C.A. The Role of 3D-Printed Phantoms and Devices for Organ-specified Appliances in Urology. Int. J. Bioprint. 2021, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.A.P.; Baird, A.; Lucky, M. Innovation in Urology: Three Dimensional Printing and Its Clinical Application. Front. Surg. 2020, 7, 29. [Google Scholar] [CrossRef]
- Song, D.; Xu, Y.; Liu, S.; Wen, L.; Wang, X. Progress of 3D Bioprinting in Organ Manufacturing. Polymers 2021, 13, 3178. [Google Scholar] [CrossRef]
- Wake, N.; Rosenkrantz, A.B.; Huang, R.; Park, K.U.; Wysock, J.S.; Taneja, S.S.; Huang, W.C.; Sodickson, D.K.; Chandarana, H. Patient-specific 3D printed and augmented reality kidney and prostate cancer models: Impact on patient education. 3d Print. Med. 2019, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Nguyen, N.H.; Hwang, S.I.; Lee, H.J.; Hong, S.K.; Byun, S. Personalized 3D kidney model produced by rapid prototyping method and its usefulness in clinical applications. Int. Braz. J. Urol. 2018, 44, 952–957. [Google Scholar] [CrossRef]
- Kocyigit, A.; Narlicay, S. The production of testis biomodels using three-dimensional (3D) technologies. Andrologia 2021, 53, e14171. [Google Scholar] [CrossRef]
- Lurie, K.L.; Smith, G.T.; Khan, S.A.; Liao, J.C.; Ellerbee, A.K. Three-dimensional, distendable bladder phantom for optical coherence tomography and white light cystoscopy. J. Biomed. Opt. 2014, 19, 36009. [Google Scholar] [CrossRef] [Green Version]
- Koutsamanis, I.; Paudel, A.; Alva Zúñiga, C.P.; Wiltschko, L.; Spoerk, M. Novel polyester-based thermoplastic elastomers for 3D-printed long-acting drug delivery applications. J. Control. Release 2021, 335, 290–305. [Google Scholar] [CrossRef] [PubMed]
- Ebbing, J.; Jäderling, F.; Collins, J.W.; Akre, O.; Carlsson, S.; Höijer, J.; Olsson, M.J.; Wiklund, P.N. Comparison of 3D printed prostate models with standard radiological information to aid understanding of the precise location of prostate cancer: A construct validation study. PLoS ONE 2018, 13, e0199477. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.P.; Bhuiyan, D.B.; Ogle, B.M. Solid organ fabrication: Comparison of decellularization to 3D bioprinting. Biomater. Res. 2016, 20, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozbolat, I.; Moncal, K.; Gudapati, H. Evaluation of bioprinter technologies. Addit. Manuf. 2017, 13, 179–200. [Google Scholar] [CrossRef] [Green Version]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M.; Buzanska, L. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef] [Green Version]
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 10, 1–3. [Google Scholar] [CrossRef]
- Biazar, E.; Najafi, S.M.; Heidari, K.S.; Yazdankhah, M.; Rafiei, A.; Biazar, D. 3D bio-printing technology for body tissues and organs regeneration. J. Med. Eng. Technol. 2018, 42, 187–202. [Google Scholar] [CrossRef]
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol. Bioeng. 2010, 106, 963–969. [Google Scholar] [CrossRef]
- Saunders, R.E.; Gough, J.E.; Derby, B. Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials 2008, 29, 193–203. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Yan, W.C.; Lu, W.F.; Wang, C.H.; Fuh, J. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef]
- Laurent, J.; Blin, G.; Chatelain, F.; Vanneaux, V.; Fuchs, A.; Larghero, J.; Thery, M. Convergence of microengineering and cellular self-organization towards functional tissue manufacturing. Nat. Biomed. Eng. 2017, 1, 939–956. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Remy, M.; Bordenave, L.; Amedee, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef]
- Guillemot, F.; Souquet, A.; Catros, S.; Guillotin, B. Laser-assisted cell printing: Principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine 2010, 5, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Mollica, P.A.; Booth-Creech, E.N.; Reid, J.A.; Zamponi, M.; Sullivan, S.M.; Palmer, X.; Sachs, P.C.; Bruno, R.D. 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels. Acta Biomater. 2019, 95, 201–213. [Google Scholar] [CrossRef]
- Baiguera, S.; Del Gaudio, C.; Di Nardo, P.; Manzari, V.; Carotenuto, F.; Teodori, L. 3D Printing Decellularized Extracellular Matrix to Design Biomimetic Scaffolds for Skeletal Muscle Tissue Engineering. Biomed. Res. Int. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kilian, K.A. Bridging the Gap: From 2D Cell Culture to 3D Microengineered Extracellular Matrices. Adv. Healthc. Mater. 2015, 4, 2780–2796. [Google Scholar] [CrossRef] [Green Version]
- Urbanczyk, M.; Layland, S.L.; SchenkeLayland, K. The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues. Matrix Biol. J. Int. Soc. Matrix Biol. 2020, 85–86, 1–14. [Google Scholar] [CrossRef]
- Aleksander, S.; Mahesh, D.; Hyun-Wook, K.; Ivy, M.; Colin, B.; Thomas, S.; Sang, J.L.; John, J.; James, Y.; Shay, S.; et al. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015, 25, 24–34. [Google Scholar]
- Pati, F.; Ha, D.; Jang, J.; Han, H.H.; Rhie, J.; Cho, D. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 2015, 62, 164–175. [Google Scholar] [CrossRef]
- Jinah, J.; Taek, G.K.; Byoung, S.K.; Seok-Won, K.; Sang-Mo, K.; Dong-Woo, C. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016, 33, 88–95. [Google Scholar]
- Jean, W.W.; Roberto, G.; Julian, J.G.; Rebecca, L.B.; Colin, G.L.; Diane, H.; Anthony, N.D.; Jeffrey, H.O.; Karen, L.C. Evidence for Mechanisms Underlying the Functional Benefits of a Myocardial Matrix Hydrogel for Post-MI Treatment. J. Am. Coll. Cardiol. 2016, 67, 1074–1086. [Google Scholar]
- Lee, J.S.; Shin, J.; Park, H.; Kim, Y.; Kim, B.; Oh, J.; Cho, S. Liver extracellular matrix providing dual functions of two-dimensional substrate coating and three-dimensional injectable hydrogel platform for liver tissue engineering. Biomacromolecules 2013, 15, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Shiri, U.; Jung-Ju, H.; Monica, L.M.; Megan, E.F.; Rui, W.; Shu-ying, C.; Ming-Huei, C.; Eric, M.B. The role of adipose protein derived hydrogels in adipogenesis. Biomaterials 2008, 29, 3712–3719. [Google Scholar]
- Takeuchi, Y.; Patience, C.; Magre, S.; Weiss, R.A.; Banerjee, P.T.; Le Tissier, P.; Stoye, J.P. Host range and interference studies of three classes of pig endogenous retrovirus. J. Virol. 1998, 72, 9986–9991. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Song, Z.; Nie, X.; Yang, J.; Zhu, C.; Guo, K.; Gu, Y. Characteristic properties of muscular-derived extracellular matrix and its application in rat abdominal wall defects. Regen. Med. 2018, 13, 503–517. [Google Scholar] [CrossRef]
- Dae-Woon, K.; Sung-Chan, S.; Jeon-Yeob, J.; Hee-Young, P.; Jin-Choon, L.; Soo-Geun, W.; Byung-Joo, L. Decellularization of Human Nasal Septal Cartilage for the Novel Filler Material of Vocal Fold Augmentation. J. Voice 2017, 31, 127-e1. [Google Scholar]
- Jian, Z.; Zhi, Q.H.; Neill, J.T.; Shi, F.T.; Wen, Y.C.; Hai, Y.Z.; Li, Z.; Hong, W.H.; Qiang, W.; Stephen, F.B. Perfusion-decellularized skeletal muscle as a three-dimensional scaffold with a vascular network template. Biomaterials 2016, 89, 114–126. [Google Scholar]
- Dzobo, K.; Senthebane, D.A.; Pillay, M.; Ssemakalu, C.; Mkhumbeni, N.; Motaung, K.S.C.M. The Future of Tissue Engineering and Regenerative Medicine in Africa. Tissue Eng. Part A 2017, 23, 1023–1025. [Google Scholar] [CrossRef]
- Adamski, M.; Fontana, G.; Gershlak, J.R.; Gaudette, G.R.; Le, H.D.; Murphy, W.L. Two Methods for Decellularization of Plant Tissues for Tissue Engineering Applications. J. Vis. Exp. 2018, e57586. [Google Scholar] [CrossRef]
- Peter, M.C.; Thomas, W.G.; Stephen, F.B. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar]
- Badylak, S.F.; Taylor, D.; Uygun, K. Whole-Organ Tissue Engineering: Decellularization and Recellularization of Three-Dimensional Matrix Scaffolds. Annu. Rev. Biomed. Eng. 2011, 13, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Byoung, S.K.; Hyeonji, K.; Ge, G.; Jinah, J.; Dong-Woo, C. Decellularized extracellular matrix: A step towards the next generation source for bioink manufacturing. Biofabrication 2017, 9, 034104. [Google Scholar]
- Brown, B.N.; Buckenmeyer, M.J.; Prest, T.A. Preparation of Decellularized Biological Scaffolds for 3D Cell Culture. In 3D Cell Culture; Methods in molecular biology (Clifton, N.J.); Humana Press: New York, NY, USA, 2017; Volume 1612, pp. 15–27. [Google Scholar]
- Keane, T.J.; Swinehart, I.T.; Badylak, S.F. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 2015, 84, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Dzobo, K.; Motaung, K.S.C.M.; Adesida, A. Recent Trends in Decellularized Extracellular Matrix Bioinks for 3D Printing: An Updated Review. Int. J. Mol. Sci. 2019, 20, 4628. [Google Scholar] [CrossRef] [Green Version]
- Marta, C.C.; Luca, F.; Dalila, D.F.; Martina, R.; Francesca, B. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 1–10. [Google Scholar]
- Byoung, S.K.; Yang, W.K.; Jeong-Sik, K.; Gyu, T.P.; Ge, G.; Wonil, H.; Moon-Bum, K.; Hyungseok, L.; Jae, H.K.; Dong-Woo, C. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: A step towards advanced skin tissue engineering. Biomaterials 2018, 168, 38–53. [Google Scholar]
- Ren, Y.; Zhang, D.; He, Y.; Chang, R.; Guo, S.; Ma, S.; Yao, M.; Guan, F. Injectable and Antioxidative HT/QGA Hydrogel for Potential Application in Wound Healing. Gels 2021, 7, 204. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, A.; Yuan, C.; Chen, X.; Liu, Y. Recent trends on burn wound care: Hydrogel dressings and scaffolds. Biomater. Sci. 2021, 9, 4523–4540. [Google Scholar] [CrossRef]
- Govindaraj, P.; Boopalan, R.; Maya, N.A.; Mukesh, D. Nanostructure coated AZ31 magnesium cylindrical mesh cage for potential long bone segmental defect repair applications. Colloids Surf. B Biointerfaces 2018, 172, 690–698. [Google Scholar]
- Qiu, P.; Li, M.; Chen, K.; Fang, B.; Chen, P.; Tang, Z.; Lin, X.; Fan, S. Periosteal matrix-derived hydrogel promotes bone repair through an early immune regulation coupled with enhanced angio- and osteogenesis. Biomaterials 2020, 227, 119552. [Google Scholar] [CrossRef] [PubMed]
- Anada, T.; Pan, C.C.; Stahl, A.M.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized Bone-Mimetic Hydrogel Constructs by 3D Bioprinting to Promote Osteogenesis and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2017, 33, 59–75. [Google Scholar] [CrossRef]
- Zhang, F.X.; Liu, P.; Ding, W.; Meng, Q.B.; Su, D.H.; Zhang, Q.C.; Lian, R.X.; Yu, B.Q.; Zhao, M.D.; Dong, J.; et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials 2021, 278, 121169. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, K.; McClure, S.R.; Bonassar, L.J. Polyacrylamide hydrogel lubricates cartilage after biochemical degradation and mechanical injury. J. Orthop. Res. 2022, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.K.; Ranjan, P.; Dutta, R.K.; Verma, S.K. Management of inflammation in cardiovascular diseases. Pharmacol. Res. 2021, 173, 105912. [Google Scholar] [CrossRef]
- Shvartz, V.; Kanametov, T.; Sokolskaya, M.; Petrosyan, A.; Le, T.; Bockeria, O.; Bockeria, L. Local Use of Hydrogel with Amiodarone in Cardiac Surgery: Experiment and Translation to the Clinic. Gels 2021, 7, 29. [Google Scholar] [CrossRef]
- Zhu, D.; Li, Z.; Huang, K.; Caranasos, T.G.; Rossi, J.S.; Cheng, K. Minimally invasive delivery of therapeutic agents by hydrogel injection into the pericardial cavity for cardiac repair. Nat. Commun. 2021, 12, 1412. [Google Scholar] [CrossRef]
- Desireé, A.G.; Lorena, D.C.; José, O.C.S.; Roseane, M.R. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar]
- An, S.; Choi, S.; Min, S.; Cho, S.W. Hyaluronic Acid-based Biomimetic Hydrogels for Tissue Engineering and Medical Applications. Biotechnol. Bioproc. E 2021, 26, 503–516, (prepublish). [Google Scholar] [CrossRef]
- Advincula, R.C.; Dizon, J.R.C.; Caldona, E.B.; Viers, R.A.; Siacor, F.D.C.; Maalihan, R.D.; Espera, A.H. On the progress of 3D-printed hydrogels for tissue engineering. MRS Commun. 2021, 11, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Aditya, P.; Shantanu, M.; Sivakumar, C.; Rama, S.R.T.; Venkataramana, R. Modeling drug release through stimuli responsive polymer hydrogels. Int. J. Pharmaceut. 2017, 532, 502–510. [Google Scholar]
- Nanotechnology Applications for Tissue Engineering; Elsevier Inc.: Amsterdam, The Netherlands, 2015.
- Imdad, A.; Li, X.; Chen, X.; Jiao, Z.; Mohammad, P.; Yang, W.; Li, H.; Mohini, S. A review of electro-stimulated gels and their applications: Present state and future perspectives. Mater. Sci. Eng. C 2019, 103, 109852. [Google Scholar]
- Baillet, J.; Gaubert, A.; Bassani, D.M.; Verget, J.; Latxague, L.; Barthélémy, P. Supramolecular gels derived from nu-cleoside based bolaamphiphiles as a light-sensitive soft material. Chem. Commun. (Camb. Engl.) 2020, 56, 3397–3400, Erratum in Chem. Commun. (Camb. Engl.)2020, 56, 9569. [Google Scholar]
- Xuexia, L.; Qiaoqiao, M.; Jianlong, S.; Cui, W.; Ranjith, K.K.; Mingrong, Z.; Honggui, L.; Shu-Feng, Z. Dual-Responsive Alginate Hydrogels for Controlled Release of Therapeutics. Molecules 2019, 24, 2089. [Google Scholar]
- Zhu, Y.; Wang, L.; Li, Y.; Huang, Z.; Luo, S.; He, Y.; Han, H.; Raza, F.; Wu, J.; Ge, L. Injectable pH and redox dual responsive hydrogels based on self-assembled peptides for anti-tumor drug delivery. Biomater. Sci. 2020, 8, 5415–5426. [Google Scholar] [CrossRef]
- Saini, G.; Segaran, N.; Mayer, J.L.; Saini, A.; Albadawi, H.; Oklu, R. Applications of 3D Bioprinting in Tissue Engineering and Regenerative Medicine. J. Clin. Med. 2021, 10, 4966. [Google Scholar] [CrossRef]
- Li, W.; Sheng, K.; Ran, Y.; Zhang, J.; Li, B.; Zhu, Y.; Chen, J.; He, Q.; Chen, X.; Wang, J.; et al. Transformation of acellular dermis matrix with dicalcium phosphate into 3D porous scaffold for bone regeneration. J. Biomater. Sci. Polym. Ed. 2021, 32, 2071–2087. [Google Scholar] [CrossRef]
- Samadian, H.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Bit, A.; Alam, M.; Goodarzi, A.; Darya, G.; Salehi, M. A tailored polylactic acid/polycaprolactone biodegradable and bioactive 3D porous scaffold containing gelatin nanofibers and Taurine for bone regeneration. Sci. Rep. 2020, 10, 13366. [Google Scholar] [CrossRef]
- Jiang, S.; Lyu, C.; Zhao, P.; Li, W.; Kong, W.; Huang, C.; Genin, G.M.; Du, Y. Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wanasekara, N.D.; Ghosh, S.; Chen, M.; Chalivendra, V.B.; Bhowmick, S. Effect of stiffness of micron/sub-micron electrospun fibers in cell seeding. J. Biomed. Mater. Res. Part A 2015, 103, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Rubod, C.; Brieu, M.; Cosson, M.; Rivaux, G.; Clay, J.C.; de Landsheere, L.; Gabriel, B. Biomechanical properties of human pelvic organs. Urology 2012, 79, 968.e17–968.e22. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vidal, L.; Murdica, V.; Venegoni, C.; Pederzoli, F.; Bandini, M.; Necchi, A.; Salonia, A.; Alfano, M. Causal contributors to tissue stiffness and clinical relevance in urology. Commun. Biol. 2021, 4, 1011. [Google Scholar] [CrossRef] [PubMed]
- Pederzoli, F.; Joice, G.; Salonia, A.; Bivalacqua, T.J.; Sopko, N.A. Regenerative and engineered options for urethroplasty. Nat. Rev. Urol. 2019, 16, 453–464. [Google Scholar] [CrossRef]
- Barbosa, M.Z.; Zylbersztejn, D.S.; de Mattos, L.A.; Carvalho, L.F. Three-dimensionally-printed models in reproductive surgery: Systematic review and clinical applications. Minerva Ginecol. 2019, 71, 235–244. [Google Scholar] [CrossRef]
- Cheung, C.L.; Looi, T.; Lendvay, T.S.; Drake, J.M.; Farhat, W.A. Use of 3-dimensional printing technology and silicone modeling in surgical simulation: Development and face validation in pediatric laparoscopic pyeloplasty. J. Surg. Educ. 2014, 71, 762–767. [Google Scholar] [CrossRef]
- Kaouk, J.; Bertolo, R. Editorial Comment. Urology 2018, 116, 227–228. [Google Scholar] [CrossRef]
- Bendre, H.H.; Rajender, A.; Barbosa, P.V.; Wason, S.E.L. Robotic dismembered pyeloplasty surgical simulation using a 3D-printed silicone-based model: Development, face validation and crowdsourced learning outcomes assessment. J. Robot. Surg. 2020, 14, 897–902. [Google Scholar] [CrossRef]
- Barsky, M.; Kelley, R.; Bhora, F.Y.; Hardart, A. Customized Pessary Fabrication Using Three-Dimensional Printing Technology. Obstet Gynecol. 2018, 131, 493–497. [Google Scholar] [CrossRef]
- Hu, X.; Man, Y.; Li, W.; Li, L.; Xu, J.; Parungao, R.; Wang, Y.; Zheng, S.; Nie, Y.; Liu, T.; et al. 3D Bio-Printing of CS/Gel/HA/Gr Hybrid Osteochondral Scaffolds. Polymers 2019, 11, 1601. [Google Scholar] [CrossRef] [Green Version]
- Angulo, J.C.; Gómez, R.G.; Nikolavsky, D. Reconstruction of Membranous Urethral Strictures. Curr. Urol. Rep. 2018, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.; Fukunishi, T.; Bai, Y.; Bedja, D.; Pitaktong, I.; Mattson, G.; Jeyaram, A.; Lui, C.; Ong, C.S.; Inoue, T.; et al. Cardiac regeneration using human-induced pluripotent stem cell-derived biomaterial-free 3D-bioprinted cardiac patch in vivo. J. Tissue Eng. Regen. Med. 2019, 13, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Darzi, S.; McPhee, G.; Del, B.M.; Werkmeister, J.A.; Gargett, C.E.; Mukherjee, S. 3D bioprinted endometrial stem cells on melt electrospun poly epsilon-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta Biomater. 2019, 97, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Hao, J.; Lu, Y.; Wang, L.; Wallace, G.G.; Zhou, Q. Three-dimensional bio-printing. Sci. China Life Sci. 2015, 58, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.H.; Lee, J.; Park, S.A.; Kim, W.D. Three-dimensional bio-printing equipment technologies for tissue engineering and regenerative medicine. Tissue Eng. Regen. Med. 2016, 13, 663–676. [Google Scholar] [CrossRef]
- Atala, A.; Bauer, S.B.; Soker, S.; Yoo, J.J.; Retik, A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006, 367, 1241–1246. [Google Scholar] [CrossRef]
- Raya-Rivera, A.; Esquiliano, D.R.; Yoo, J.J.; Lopez-Bayghen, E.; Soker, S.; Atala, A. Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet 2011, 377, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
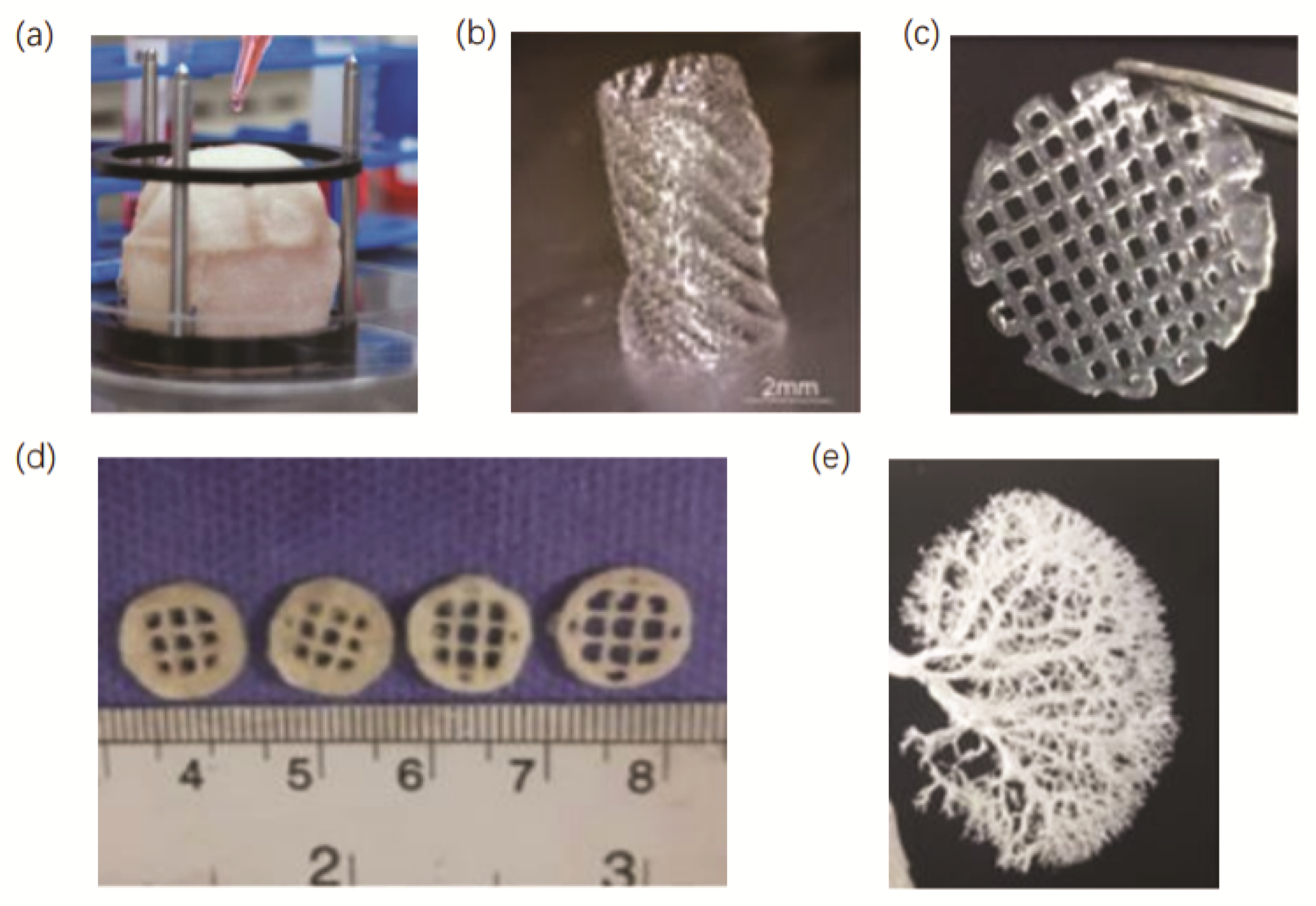
- Huang, J.W.; Lv, X.G.; Li, Z.; Song, L.J.; Feng, C.; Xie, M.K.; Li, C.; Li, H.B.; Wang, J.H.; Zhu, W.D.; et al. Urethral reconstruction with a 3D porous bacterial cellulose scaffold seeded with lingual keratinocytes in a rabbit model. Biomed. Mater. 2015, 10, 055005. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef]
- Versteegden, L.R.; van Kampen, K.A.; Janke, H.P.; Tiemessen, D.M.; Hoogenkamp, H.R.; Hafmans, T.G.; Roozen, E.A.; Lomme, R.M.; van Goor, H.; Oosterwijk, E.; et al. Tubular collagen scaffolds with radial elasticity for hollow organ regeneration. Acta Biomater. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Poels, J.; Abou-Ghannam, G.; Decamps, A.; Leyman, M.; Rieux, A.; Wyns, C. Transplantation of testicular tissue in alginate hydrogel loaded with VEGF nanoparticles improves spermatogonial recovery. J. Control. Release 2016, 234, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Zheng, J.; Li, Z.; Qi, X.; Tian, Y.; Zhang, M.; Zhang, J.; Huang, X. Printing 3D vagina tissue analogues with vagina decellularized extracellular matrix bioink. Int. J. Biol. Macromol. 2021, 180, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.A.; Kolesky, D.B.; Skylar-Scott, M.A.; Herrmann, J.; Obuobi, H.; Moisan, A.; Lewis, J.A. Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci. Rep. 2016, 6, 34845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huling, J.; Min, S.I.; Kim, D.S.; Ko, I.K.; Atala, A.; Yoo, J.J. Kidney regeneration with biomimetic vascular scaffolds based on vascular corrosion casts. Acta Biomater. 2019, 95, 328–336. [Google Scholar] [CrossRef]
- Wake Forest School of Medicine. Replacement Organs and Tissue. Available online: https://school.wakehealth.edu/Research/Institutes-and-Centers/Wake-Forest-Institute-for-Regenerative-Medicine/Research/Replacement-Organs-and-Tissue (accessed on 16 March 2022).
- Chae, S.; Kim, J.; Yi, H.G.; Cho, D.W. 3D Bioprinting of an In Vitro Model of a Biomimetic Urinary Bladder with a Contract-Release System. Micromachines 2022, 13, 277. [Google Scholar] [CrossRef]
- Bashiri, Z.; Amiri, I.; Gholipourmalekabadi, M.; Falak, R.; Asgari, H.; Maki, C.B.; Moghaddaszadeh, A.; Koruji, M. Artificial testis: A testicular tissue extracellular matrix as a potential bio-ink for 3D printing. Biomater. Sci. 2021, 9, 3465–3484. [Google Scholar] [CrossRef]
- Orabi, H.; Bouhout, S.; Morissette, A.; Rousseau, A.; Chabaud, S.; Bolduc, S. Tissue engineering of urinary bladder and urethra: Advances from bench to patients. Sci. World J. 2013, 2013, 154564. [Google Scholar] [CrossRef] [Green Version]
- Baumert, H.; Simon, P.; Hekmati, M.; Fromont, G.; Levy, M.; Balaton, A.; Molinie, V.; Malavaud, B. Development of a seeded scaffold in the great omentum: Feasibility of an in vivo bioreactor for bladder tissue engineering. Eur. Urol. 2007, 52, 884–892. [Google Scholar] [CrossRef]
- Wan, Q.; Xiong, G.; Liu, G.; Shupe, T.D.; Wei, G.; Zhang, D.; Liang, D.; Lu, X.; Atala, A.; Zhang, Y. Urothelium with barrier function differentiated from human urine-derived stem cells for potential use in urinary tract reconstruction. Stem Cell Res. Ther. 2018, 9, 304. [Google Scholar] [CrossRef]
- Yang, H.; Chen, B.; Deng, J.; Zhuang, G.; Wu, S.; Liu, G.; Deng, C.; Yang, G.; Qiu, X.; Wei, P.; et al. Characterization of rabbit urine-derived stem cells for potential application in lower urinary tract tissue regeneration. Cell Tissue Res. 2018, 374, 303–315. [Google Scholar] [CrossRef]
- Ali, M.; Pr, A.K.; Yoo, J.J.; Zahran, F.; Atala, A.; Lee, S.J. A Photo-Crosslinkable Kidney ECM-Derived Bioink Accelerates Renal Tissue Formation. Adv. Healthc. Mater. 2019, 8, e1800992. [Google Scholar] [CrossRef] [PubMed]
- Little, M.H.; Hale, L.J.; Howden, S.E.; Kumar, S.V. Generating Kidney from Stem Cells. Annu. Rev. Physiol. 2019, 81, 335–357. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.; Patel, N.R.; Ashammakhi, N.; Nguyen, K. 3-Dimensional Bioprinting of Cardiovascular Tissues. JACC Basic Transl. Sci. 2021, 6, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Liao, L. Current Applications and Future Directions of Bioengineering Approaches for Bladder Augmentation and Reconstruction. Front. Surg. 2021, 8, 214. [Google Scholar] [CrossRef]



| Bioprinting Methods | Cell Viability | Ink Viscosity | Printing Speed | Related Costs | Resolution |
|---|---|---|---|---|---|
| Micro-extrusion | 40–95% | Wide range | Low | Moderate | Low |
| Inkjet | >85% | Very low | High | Low | Moderate |
| Laser-assisted | >95% | Low | Moderate | High | High |
| Type | Composition | Classification | Characteristics |
|---|---|---|---|
| dECM | The fraction obtained by removing the cellular components and some small molecules or antigens from the natural ECM | Animal-derived; Human-derived; Plant-derived | Excellent biocompatibility; Good degradability and immunogenicity; nutrient-rich |
| Hydrogel | Extremely hydrophilic three-dimensional network structure gel | Natural polymer hydrogels; Synthetic hydrogels | High biocompatibility; Low immunogenicity; Long-term stability; Responsive hydrogels |
| 3D porous bioscaffold | A novel scaffold with micron or even nanopore structure | Natural sources; Synthetic | Large surface area; Greatly facilitates material transport and cell attachment |
| Field | Research Goal | 3D Bioprinting Technique | Scaffold Biomaterial | Cell Type | Reference |
|---|---|---|---|---|---|
| Bladder | Development of an alternative approach using autologous engineered bladder tissues for reconstruction | Multicellular spheroid formation | Collagen; Polyglycolic acid | Human uroepithelial and muscle cells | [88] |
| Urethra | Assessment of the effectiveness of tissue-engineered urethras using patients’ own cells in patients who needed urethral reconstruction | Multicellular spheroid formation | Lactide-co-glycolide acid | Human smooth muscle and urothelial cells | [89] |
| Urethra | Evaluation of the effects of urethral reconstruction with a three-dimensional (3D) porous bacterial cellulose (BC) scaffold seeded with lingual keratinocytes in a rabbit model | Multicellular spheroid formation | 3D porous bacterial cellulose | Rabbit lingual keratinocytes | [90] |
| Urethra | Construction of 3D bioprinting urethral using PCL, PLCL and different rabbit cell types | Inkjet | PCL; PLCL | Rabbit urothelial cells and smooth muscle cells | [91] |
| Urethra | Construction of a new type I collagen-based tubular scaffold is presented that possesses intrinsic radial elasticity | Extrusion-based | Insoluble type I collagen; Carbodiimide crosslinking | SCaBER cells | [92] |
| Testis | Development of the potential of alginate hydrogel loaded with nanoencapsulated growth factors to Improve cryopreserved tissue engraftment | Tissue encapsulation | VEGF nanoparticles Alginate; Fibrin | Spermatogoni-al | [93] |
| Vagina | Reconstruction of the biomimetic 3D vagina tissue with AVM bioink encapsulating BMSCs | Inkjet | Acellular vagina matrix; Sodium; Gelatin | Bone marrow mesenchymal stem cells | [94] |
| Kidney | Construction of a bioprinting method for creating 3D human renal proximal tubules in vitro | Inkjet | Fibrinogen; Gelatin | PTEC-TERT1 cells | [95] |
| Kidney | Kidney regeneration with biomimetic vascular scaffolds based on vascular corrosion casts | Embedding and coating | Hollow collagen vascular scaffold | MS1 cells, Human renal cells | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liu, Y.; Dai, Y.; Yang, L.; Chen, G. Application of 3D Bioprinting in Urology. Micromachines 2022, 13, 1073. https://doi.org/10.3390/mi13071073
Zhao Y, Liu Y, Dai Y, Yang L, Chen G. Application of 3D Bioprinting in Urology. Micromachines. 2022; 13(7):1073. https://doi.org/10.3390/mi13071073
Chicago/Turabian StyleZhao, Yue, Yuebai Liu, Yi Dai, Luo Yang, and Guo Chen. 2022. "Application of 3D Bioprinting in Urology" Micromachines 13, no. 7: 1073. https://doi.org/10.3390/mi13071073





